Lower extremity venous reflux
Introduction
Venous return from the lower extremity to the heart must overcome gravitational forces in the upright position. In order to counter this gravitational force, biological adaptations such as muscle pump and venous valves and supportive fascial structure have evolved. However, over time, these can fail and lead to venous incompetence, a common problem affecting at least 25% of women and 15% of men (1,2). Several treatment options including endovenous techniques and surgery are available for the venous reflux. These treatment options are largely palliative, and recurrence is common. However, imaging plays a central role in the evaluation of reflux, treatment selection, and monitoring for recurrence. This article reviews the current understanding of lower extremity reflux and highlights the role of imaging in its management.
Venous anatomy
The venous system can be divided into three major components: the superficial venous system, the deep venous system, and the perforating veins.
The superficial venous system
The superficial venous system has two parts: the thin-walled collecting veins and the thick-walled truncal veins such as Great and Short Saphenous veins. The great saphenous vein (GSV) is a continuation of the dorsal venous arch in the foot. It travels anterior to the medial malleolus and ascends in the superficial fascia along the medial aspect of the lower extremity and drains into the deep system via the saphenofemoral junction (SFJ) (3-5). Near the SFJ, three major tributaries drain into the GSV—the external pudendal, inferior epigastric, and external circumflex iliac veins (Figure 1). The GSV can be congenitally duplicated in approximately 1% of cases (6).
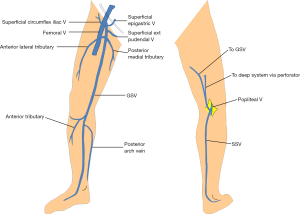
The short saphenous vein (SSV) is the other major truncal superficial vein, which begins on the lateral aspect of the foot. It travels posterior to the lateral malleolus and ascends along the posterior midline superficial to the deep muscular fascia. In approximately two-thirds of patients, the SSV terminates at popliteal fossa by forming the saphenopopliteal junction (SPJ). In the remaining one-third of patients, its course is variable: it may drain into a posterior medial tributary of the GSV, directly into the GSV as the thigh extension of the SSV, or into a perforator (Figure 1) (7,8). A standard SPJ may co-exist in many of these cases. The mid-portion of the SSV may be duplicated in as many 4% of individuals (9).
The great saphenous vein courses in a deep plane of the hypodermis just outside the muscular fascia, covered by a connective tissue lamina extending from the inguinal ligament to the ankle. This fascia has been termed the “saphenous fascia” (4,10). A similar fascial covering has been described in relation with the short saphenous vein (9). This fascial tissue has been implicated in providing the muscular squeeze during muscle contraction to enhance blood flow within them (10). The saphenous fascia also restricts venous dilation and prevents the development of varicose veins (11).
The deep veins of the lower extremity
The veins of the deep venous system include the plantar vein (foot), the paired peroneal and anterior and posterior tibial veins (leg), and the popliteal and femoral veins (thigh). Numerous venous sinusoids within the muscles, particularly in the soleal and gastrocnemius veins, also form an important component of this system.
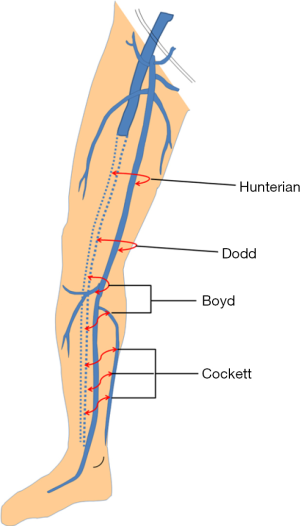
Perforating veins (perforators)
Perforators are bridging channels between the superficial and deep venous systems (Figure 2). These veins obliquely perforate the deep fascia and play an important role in equilibrating blood-flow during calf muscle contraction. Four clinically important perforator groups have been identified: upper thigh (Hunterian), lower thigh (Dodd’s), at knee level (Boyd’s) and in the calf region (Cockett’s).
Hemodynamics affecting venous circulation
The uphill task
Gravity/hydrostatic pressure
The venous flow towards the heart at rest is provided mainly by resting heart energy beyond the capillaries, i.e., vis a tergo (force acting from behind). The pressure difference between venules and right atrium is approximately 15 mmHg. While this pressure gradient can sustain venous return to the heart in the supine position, it is not sufficient to counter gravity when upright. Hydrostatic pressure at the right atrium is conventionally defined as 0 mmHg. Inferior to this point, the hydrostatic pressure rises by approximately 0.73 mmHg per centimeter in upright position, totalling approximately 90 mmHg at the level of the ankle. This pressure is then transmitted equally across the walls of the deep and superficial veins (10,12).
Intra-abdominal pressure
Increased intra-abdominal pressure during physical activity gets transmitted into the venous system, and values as high as 200 mmHg have been reported (10).
Venous compression
Another important contributing factor to lower extremity venous hypertension is extrinsic venous compression. Iliac vein compression is commonly encountered as an incidental finding on cross-sectional imaging. While this can be an important contributing factor to venous stasis, greater than 50% compression has been seen in up to 25% of asymptomatic individuals (13,14). The most common of these syndromes is left iliac vein compression by the right iliac artery, known as May-Thurner syndrome (MTS). Other variants exist, such as compression of right iliac vein by the right iliac artery or compression of the left iliac vein by the left iliac artery. All these variants can contribute to stasis and endothelial injury from adjacent pulsation microtrauma leading to deep venous thrombosis in the extremities, which can lead to venous scarring and further chronic venous insufficiency in the long term (15,16).
Venous hemodynamics in lower extremity
Venous return in the lower extremity involves a complex interplay of the calf muscle pump, venous valves, and perforating veins.
Pressure provided by calf muscle squeeze is the primary force for venous return against gravity from the lower extremity in an upright position. It works in both vertical and horizontal directions—across the central and perforating veins—and generates an ambulatory pressure gradient across the knee. Calf muscle contraction elevates the pressure of the deep venous compartment of leg to approximately 140 mmHg, propelling venous blood into the popliteal and femoral veins (10,12). During muscle relaxation, the pressure gradient is reversed and causes physiological reflux, lasting approximately 200 to 300 milliseconds in veins with competent valves.
Venous valves play a crucial role in preventing pathologic reflux and maintaining net vertically directed flow by preventing reflux. The valves also divide the hydrostatic column of blood into segments and prevent the full pressure of the fluid column from exerting force on the distal veins (17,18). Valves are most densely distributed in the infrapopliteal segment which implies their critical functional importance in this area. Venous valves are also present in the femoro-popliteal segment, at the common femoral vein (CFV) near the inguinal ligament, superficial femoral vein (SFV) just distal to the deep femoral vein (DFV) tributary, and in the popliteal vein (PV) near the adductor hiatus (19). Hydrostatic pressure can be significantly improved by the correction of femoral or popliteal vein incompetence; however, the relative importance of proximal versus distal valves has not been established (20).
Previously, perforating veins were thought to have a unidirectional centripetal flow in healthy people. However, the veins were later found to have physiologic bi-directional flow, with both centripetal and centrifugal components, depending on the phase of calf muscle pump activity (20-22). As a result, “reflux” in the perforating veins is no longer considered a cause of venous hypertension, since it has been documented in healthy subjects by duplex ultrasonography (21) and in varicose vein patients by electromagnetic flow measurements (20). The pressure curves of the two vessels are nearly identical in healthy people and patients with varicose veins (23-25).
However, when reflux is present, such as from an incompetent GSV, blood re-enters the deep system through perforating veins. The perforating veins upstream to incompetent segment may dilate secondarily because of the volume overload due to re-entry. While these veins may meet diagnostic criteria for venous incompetence, these perforators can regain their competence after successful treatment of an incompetent GSV, indicating that their dilation is secondary to reflux rather than the primary cause. Similarly, it is through the perforating veins that high deep venous pressure is transmitted to superficial veins, causing superficial varicosities, stasis dermatitis and venous ulcers.
Lower extremity reflux/incompetence
Pathophysiology
Primary valvular incompetence arises from progressive venous remodeling from chronic hemodynamic stress or valve agenesis. Secondary incompetence may occur after deep venous thrombosis (17). There is evidence suggesting that varicose changes precede the development of overt valvular incompetence (26,27). Dilated saphenous veins undergo valvular remodelling with increased collagen and reduced elastin content (26). However, decreased venous elasticity has been demonstrated in patients without varices but who are at high risk for development of venous incompetence (28). Similar connective tissue abnormalities have been identified in upper limb veins of patients with varicosities (29). These findings suggest that abnormalities in venous architecture precede the development of both varicosities and valvular reflux (30).
Secondary valvular insufficiency develops during venous recanalization after DVT. Valvular leaflet fusion has been demonstrated in 50% of cases with chronic venous insufficiency. Other abnormalities contributing to valvular incompetence include thrombus in the valve sinus, endothelial erosions, and basement membrane thickening (31). Despite these pathologic findings, valvular destruction is not an inevitable consequence of acute DVT; 33% to 59% of thrombosed segments show reflux on duplex ultrasonography on 1 year follow up (32), implying that up to one-third of patients may recover from DVT without long-term sequelae.
Imaging
The goal of duplex ultrasonographic imaging in patients with venous insufficiency is mapping venous anatomy, identifying anatomic variants, and finding the sources of venous insufficiency. Duplex ultrasound is the most accurate tool for evaluation of venous insufficiency, since it is noninvasive, non-ionizing, reproducible, and gives dynamic information. Duplex US is indicated for evaluation of patients with suspected venous insufficiency who are contemplating therapy and for monitoring response after therapy (33,34).
Equipment requirements include a probe capable of grayscale imaging at 7.5–10 MHz and pulsed-wave Doppler imaging. The examination of venous incompetence should preferably be assessed by the interventionalists performing corrective procedures, as this facilitates optimal patient and treatment selection (7).
Technique
Patients are evaluated in the standing position to ensure maximum venous distention. The patient will need to be able to support their weight on the opposite leg to participate in maneuvers to elicit reflux.
Slight limb flexion and outward rotation provides optimal visualisation of the great saphenous vein. The entire length of the GSV is first examined using axial grayscale technique, noting the maximal vein diameter (normally <4 mm). Any varicose tributaries are then identified and traced distally. Next, the SFJ is assessed for reflux. Color or power doppler imaging are used in combination with sudden compression and release of distal venous segments (35) to identify sites of reflux. Since color Doppler imaging often underestimates the degree of venous reflux, pulsed-wave doppler imaging is preferred while performing compression and release (36).
For assessment of the short saphenous vein, the knee is slightly flexed and the muscles of the thigh are relaxed. Using axial grayscale technique, the SSV is serially examined from the calf upwards until its termination at the SPJ, again noting the maximal diameter, and assessing for venous competence of the SPJ. A thigh extension of the SSV is also assessed for reflux if present. Comprehensive deep venous evaluation must also be performed for detection of DVT and reflux. Chronic DVT findings may be subtle and manifest as webs, focal wall thickening, or calcification. Persistent or repeated venous obstructions can contribute to venous hypertension. Perforating veins in the thigh and the leg are lastly examined in transverse and oblique planes to identify the longitudinal axis of perforator.
Identification of incompetent segment
The normal limit of the calibre of GSV and SSV in upright position is 4 and 3 mm respectively (7). Sudden caliber change of the vessels is an important marker of regurgitant flow within that segment, as incompetent veins are dilated and tortuous. The diameter often changes abruptly at the level of the incompetent valves in the superficial system (e.g., SFJ) or at the level of perforating veins communicating with an incompetent deep venous segment. There are several common tributaries in the thigh region that can contribute to GSV reflux, including antero-lateral and the posterior-medial tributaries in the thigh region. Pudendal veins can also contribute to GSV reflux in pregnant women.
Imaging criteria for reflux
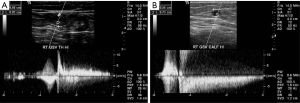
While reflux can be evaluated using both color or pulsed-wave Doppler, pulsed-wave is more accurate. A small blip of color just after release of compression is physiologic, and likely represents a small amount of retrograde flow before complete closure of valves. Reflux is generally defined as greater than 0.5 seconds of flow reversal (Figure 3) (37). There are numerous norms reported in the literature, with some investigators reporting a limit of 1 second for the deep venous system and 0.3 seconds for perforators (38), and others define a 0.5 seconds for perforating veins as well (39). Hemodynamically significant perforators are usually located central to incompetent venous channels. Perforating veins with diameters greater than 3.5 mm can also be taken as a sign of significant reflux (40). Pathological perforators are a newly described entity of incompetent perforating veins near venous ulcers that do not normalize after successful treatment of other pathways of reflux with the use of compression stockings (41).
Duplex ultrasound after treatment
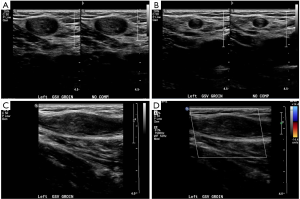
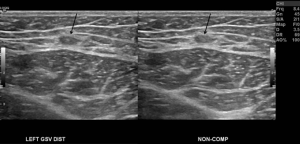
Duplex ultrasound is essential for monitoring of post-procedural complications and recurrence after endovenous ablation. Early post-procedure duplex ultrasound ensures satisfactory closure of ablated segments and to identify thrombotic complications. Evaluation 1–2 weeks after endovenous ablation of a treated segment will reveal smaller non-compressible veins with wall thickening and no flow (Figure 4). After several weeks, the venous wall undergoes fibrosis and become difficult to identify after several months (Figure 5).
Endovenous heat induced thrombosis (EHIT) refers to deep venous thrombosis after venous ablation. There are four categories, defined largely by the extent of thrombus. EHIT 1 describes thrombus up to but not inside the deep venous junction. EHIT 2 describes thrombosis of the femoral or popliteal vein occluding less than 50% of the cross-sectional diameter. EHIT 3 refers to greater than 50% occlusion of the cross-sectional diameter. EHIT 4 is complete occlusion (42). A rare complication after thermal ablation is formation of an arteriovenous fistula (43). AVFs can lead to partial patency of ablated segments with pulsatile flow on duplex ultrasound. AVFs between the proximal SSV and the sural artery or between the superficial external epigastric artery and proximal GSV have been described (44,45).
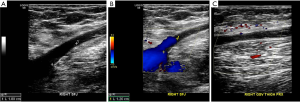
Recurrence is a common problem after endovenous therapy as recanalization of a treated segment or recruitment of minor communicating channels occurs (46,47). Duplicated veins and enlarged refluxing truncal tributaries can also result in recurrent symptoms. During duplex evaluation, the remnants of the GSV (Figure 6) must be scrutinized in patients who have had SFJ ligation with or without stripping. It may reveal collateral reconstitution and neovascularization at the refluxing saphenous vein stump.
Limitations of ultrasound
Obesity can be a limiting factor for duplex exam, especially while evaluating deep venous system. It is also important to note that open draining ulcers, severe edema with or without pain can also limit the sonographic window and hence limit the evaluation of reflux. As evaluation in an erect posture is very crucial for eliciting reflux, an inability to stand for a good length of time can result in suboptimal evaluation (48).
MR venography
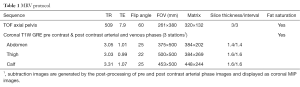
MR venography can be used for detection of deep venous thrombosis (DVT) in the lower extremity (49,50). Contrast enhanced 3D T1 weighted MR venography, specially using gadolinium-based blood-pool contrast agent (e.g., Gadofosveset) can provide good vessel visualization, signal homogeneity, and confidence level for detecting DVT (51). There is limited experience with the use of 3D MR venography in lower extremity varicose veins. However a study by Müller et al. using direct contrast-enhanced 3D MR venography (injection directly into foot vein), showed that MR venography can have a significant impact on therapeutic decision making in patients suspected of having complex varicose vein anatomy (52). This group reported a good or excellent image quality of the deep venous system and the recurrent varicose veins (including small perforators) in 89% of evaluated segments with a good inter-observer agreement. They reported that MR venography resulted in change in diagnosis of in 17 of 22 legs and resulted in change in treatment plan in these patients (52). A typical MR venography protocol for lower extremity, practiced at our institution has been described in Table 1.
Full table
Non contrast angiographic technique including TOF MRI has also been used in lower extremity venous imaging. Tamura et al used 2D TOF MRI on a 1.5 T system for the evaluation of deep venous thrombosis and reported a sensitivity of superior to conventional venography (53). There is no literature available with the use of TOF MRI for the detection of lower extremity venous reflux.
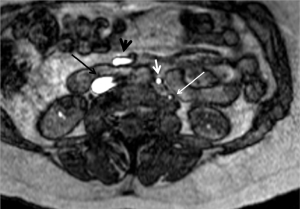
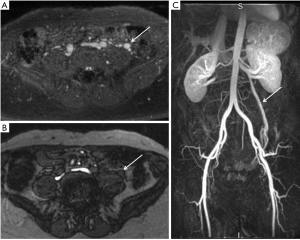
Ovarian venous reflux/pelvic congestion syndrome has a pathophysiology similar to lower extremity reflux. Incompetence of the ovarian veins leads to retrograde venous flow and progressive development of pelvic varicosities. Ovarian venous reflux has been studied with TOF MRI (Figures 7,8) (54,55). Yang et al. compared the accuracy of TOF MRI and conventional venography for detection and grading of pelvic congestion. They reported comparable detection sensitivity and an excellent agreement between the two modalities for the grading of reflux (54). These results suggest that MR may have a role for the detection of lower extremity reflux as these two entities share a similar pathophysiology. Further work is needed to explore such a possibility.
Other imaging modalities
Conventional venography has been considered the gold standard for venous imaging, particularly for the diagnosis of DVT and venous stenosis (56). The venography of the lower extremity veins has also been used for observing of post-thrombotic changes in deep veins, detection of venous malformations and preoperative imaging for saphenous venous stripping (57). However, this procedure is invasive, time-consuming, and necessitates the use of ionizing radiation and iodinated contrast material (58). In patients with secondary venous incompetence, conventional venography can identify the presence of obstructive pathology (such as May-Thurner Syndrome; Figure 9). However CT and MR examinations are preferred for this purpose as conventional venogram cannot provide additional information as to the nature of the obstruction, which is important for treatment planning (59). It is seldom used for purely diagnostic purposes e.g., in evaluation of complex venous anatomy and detection of occult perforators. It is more often used in cases where an acute venous thrombosis is suspected or in cases with venous stenosis requiring angioplasty or stenting (59).
CT venography has also been used for the evaluation of venous incompetence. Lee et al reported a sufficient image quality for evaluation of varicose veins with comprehensive anatomic information (60). Volume rendered three-dimensional images generated by CT venography can provide road map for surgical planning. However, CT venography has several disadvantages compared to duplex sonography, such as the need for iodinated contrast and ionizing radiation. Another important drawback of CT venography is the lack of functional information and inability to assess venous valve function (60).
Conclusions and summary
Venous reflux in the lower extremities is a manifestation of a degenerative process in the venous wall and supporting fascial structures, which progressively dilate over time after exposure to high physiological pressures. Veins dilate, develop incomplete valvular leaflet closure, and enter into a positive feedback loop where further dilation causes further reflux. Patients become symptomatic mostly from superficial venous hypertension, as the superficial veins lack muscular support. Duplex ultrasonography is the diagnostic modality of choice for baseline evaluation and monitoring response after therapy. A standardized and systematic evaluation is essential for the identification of the source of reflux and selection of optimal therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Evans CJ, Allan PL, Lee AJ, et al. Prevalence of venous reflux in the general population on duplex scanning: the Edinburgh vein study. J Vasc Surg 1998;28:767-76. [Crossref] [PubMed]
- Callam MJ. Epidemiology of varicose veins. Br J Surg 1994;81:167-73. [Crossref] [PubMed]
- Wendell-Smith CP. Fascia: an illustrative problem in international terminology. Surg Radiol Anat 1997;19:273-7. [Crossref] [PubMed]
- Caggiati A. Fascial relationships of the long saphenous vein. Circulation 1999;100:2547-9. [Crossref] [PubMed]
- Caggiati A, Bergan JJ, Gloviczki P, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg 2002;36:416-22. [Crossref] [PubMed]
- Ricci S, Caggiati A. Does a Double Long Saphenous Vein Exist? Phlebology 1999;14:59-64. [Crossref]
- Min RJ, Khilnani NM, Golia P. Duplex Ultrasound Evaluation of Lower Extremity Venous Insufficiency. J Vasc Interv Radiol 2003;14:1233-41. [Crossref] [PubMed]
- Caggiati A. Fascial relationships of the short saphenous vein. J Vasc Surg 2001;34:241-6. [Crossref] [PubMed]
- Caggiati A, Ricci S. The Long Saphenous Vein Compartment. Phlebology 1997;12:107-11.
- Arnoldi CC. Venous pressure in the leg of healthy human subjects at rest and during muscular exercise in the nearly erect position. Acta Chir Scand 1965;130:570-83. [PubMed]
- Caggiati A. The Saphenous compartment: The Saphenous veins are not real superficial veins. Italian Journal of Anatomy and Embryology 2013;118:40.
- Ludbrook J, Beale G. Femoral venous valves in relation to varicose veins. Lancet 1962;1:79-81. [Crossref] [PubMed]
- Oguzkurt L, Ozkan U, Ulusan S, et al. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2008;19:366-70. [Crossref] [PubMed]
- Shebel ND, Whalen CC. Diagnosis and management of iliac vein compression syndrome. J Vasc Nurs 2005;23:10-7. [Crossref] [PubMed]
- Butros SR, Liu R, Oliveira GR, et al. Venous compression syndromes: clinical features, imaging findings and management. Br J Radiol 2013;86:20130284. [Crossref] [PubMed]
- Meissner MH. Lower extremity venous anatomy. Semin Intervent Radiol 2005;22:147-56. [Crossref] [PubMed]
- Goldman MP, Fronek A. Anatomy and pathophysiology of varicose veins. J Dermatol Surg Oncol 1989;15:138-45. [Crossref] [PubMed]
- Moore HM, Gohel M, Davies AH. Number and location of venous valves within the popliteal and femoral veins - a review of the literature. J Anat 2011;219:439-43. [Crossref] [PubMed]
- Meissner MH, Moneta G, Burnand K, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg 2007;46 Suppl S:4S-24S.
- Bjordal R. Simultaneous pressure and flow recordings in varicose veins of the lower extremity. A haemodynamic study of venous dysfunction. Acta Chir Scand 1970;136:309-17. [PubMed]
- Sarin S, Scurr JH, Smith PD. Medial calf perforators in venous disease: the significance of outward flow. J Vasc Surg 1992;16:40-6. [Crossref] [PubMed]
- Recek C. Calf pump activity influencing venous hemodynamics in the lower extremity. Int J Angiol 2013;22:23-30. [Crossref] [PubMed]
- Recek C, Koudelka V. Perforating veins of the leg and their role in the pathogenesis of varicose veins. Rozhl Chir 1973;52:14-20. [PubMed]
- Recek C, Pojer H. Ambulatory pressure gradient in the veins of the lower extremity. Vasa 2000;29:187-90. [Crossref] [PubMed]
- Hojensgard IC, Sturup H. Static and dynamic pressures in superficial and deep veins of the lower extremity in man. Acta Physiol Scand 1952;27:49-67. [Crossref] [PubMed]
- Gandhi RH, Irizarry E, Nackman GB, et al. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg 1993;18:814-20. [Crossref] [PubMed]
- Rose SS, Ahmed A. Some thoughts on the aetiology of varicose veins. J Cardiovasc Surg (Torino) 1986;27:534-43. [PubMed]
- Clarke GH, Vasdekis SN, Hobbs JT, et al. Venous wall function in the pathogenesis of varicose veins. Surgery 1992;111:402-8. [PubMed]
- Vanhoutte PM, Corcaud S, de Montrion C. Venous disease: from pathophysiology to quality of life. Angiology 1997;48:559-67. [Crossref] [PubMed]
- Labropoulos N, Giannoukas AD, Delis K, et al. Where does venous reflux start? J Vasc Surg 1997;26:736-42. [Crossref] [PubMed]
- Budd TW, Meenaghan MA, Wirth J, et al. Histopathology of veins and venous valves of patients with venous insufficiency syndrome: ultrastructure. J Med 1990;21:181-99. [PubMed]
- Markel A, Manzo RA, Bergelin RO, et al. Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg 1992;15:377-82; discussion 383-4. [Crossref] [PubMed]
- Jones L, Braithwaite BD, Selwyn D, et al. Neovascularisation is the principal cause of varicose vein recurrence: results of a randomised trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg 1996;12:442-5. [Crossref] [PubMed]
- Jiang P, van Rij AM, Christie R, et al. Recurrent varicose veins: patterns of reflux and clinical severity. Cardiovasc Surg 1999;7:332-9. [Crossref] [PubMed]
- Masuda EM, Kistner RL, Eklof B. Prospective study of duplex scanning for venous reflux: comparison of Valsalva and pneumatic cuff techniques in the reverse Trendelenburg and standing positions. J Vasc Surg 1994;20:711-20. [Crossref] [PubMed]
- Araki CT, Back TL, Padberg FT, et al. Refinements in the ultrasonic detection of popliteal vein reflux. J Vasc Surg 1993;18:742-8. [Crossref] [PubMed]
- van Bemmelen PS, Bedford G, Beach K, et al. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg 1989;10:425-31. [Crossref] [PubMed]
- Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg 2003;38:793-8. [Crossref] [PubMed]
- Labropoulos N, Mansour MA, Kang SS, et al. New insights into perforator vein incompetence. Eur J Vasc Endovasc Surg 1999;18:228-34. [Crossref] [PubMed]
- Sandri JL, Barros FS, Pontes S, et al. Diameter-reflux relationship in perforating veins of patients with varicose veins. J Vasc Surg 1999;30:867-74. [Crossref] [PubMed]
- Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2011;53:2S-48S. [Crossref] [PubMed]
- Dexter D, Kabnick L, Berland T, et al. Complications of endovenous lasers. Phlebology 2012;27 Suppl 1:40-5. [Crossref] [PubMed]
- Timperman PE. Arteriovenous fistula after endovenous laser treatment of the short saphenous vein. J Vasc Interv Radiol 2004;15:625-7. [Crossref] [PubMed]
- Rudarakanchana N, Berland TL, Chasin C, et al. Arteriovenous fistula after endovenous ablation for varicose veins. J Vasc Surg 2012;55:1492-4. [Crossref] [PubMed]
- Martin EC, Todd GJ. Embolization of an arteriovenous fistula after radiofrequency ablation (RFA) of the saphenous vein. Cardiovasc Intervent Radiol 2010;33:227-8. [Crossref] [PubMed]
- Recek C. The hemodynamic paradox as a phenomenon triggering recurrent reflux in varicose vein disease. Int J Angiol 2012;21:181-6. [Crossref] [PubMed]
- Winokur RS, Khilnani NM, Min RJ. Recurrence patterns after endovenous laser treatment of saphenous vein reflux. Phlebology 2016;31:496-500. [Crossref] [PubMed]
- Lower Extremity Venous Insufficiency Evaluation. Available online: http://account.svunet.org/files/positions/LowerExtremityVenousInsufficiencyEvaluation.pdf
- Polak JF, Fox LA. MR assessment of the extremity veins. Semin Ultrasound CT MR 1999;20:36-46. [Crossref] [PubMed]
- Kluge A, Mueller C, Strunk J, et al. Experience in 207 combined MRI examinations for acute pulmonary embolism and deep vein thrombosis. AJR Am J Roentgenol 2006;186:1686-96. [Crossref] [PubMed]
- Huang SY, Kim CY, Miller MJ, et al. Abdominopelvic and lower extremity deep venous thrombosis: evaluation with contrast-enhanced MR venography with a blood-pool agent. AJR Am J Roentgenol 2013;201:208-14. [Crossref] [PubMed]
- Müller MA, Mayer D, Seifert B, et al. Recurrent lower-limb varicose veins: effect of direct contrast-enhanced three-dimensional MR venographic findings on diagnostic thinking and therapeutic decisions. Radiology 2008;247:887-95. [Crossref] [PubMed]
- Tamura K, Nakahara H. MR Venography for the Assessment of Deep Vein Thrombosis in Lower Extremities with Varicose Veins. Ann Vasc Dis 2014;7:399-403. [Crossref] [PubMed]
- Yang DM, Kim HC, Nam DH, et al. Time-resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venography. Br J Radiol 2012;85:e117-22. [Crossref] [PubMed]
- Kim CY, Miller MJ Jr, Merkle EM. Time-resolved MR angiography as a useful sequence for assessment of ovarian vein reflux. AJR Am J Roentgenol 2009;193:W458-63. [Crossref] [PubMed]
- Katz DS, Hon M. Current DVT imaging. Tech Vasc Interv Radiol 2004;7:55-62. [Crossref] [PubMed]
- Uema RT, Dezotti NR, Joviliano EE, et al. A prospective study of venous hemodynamics and quality of live at least five years after varicose vein stripping. Acta Cir Bras 2013;28:794-9. [Crossref] [PubMed]
- Bettmann MA, Robbins A, Braun SD, et al. Contrast venography of the leg: diagnostic efficacy, tolerance, and complication rates with ionic and nonionic contrast media. Radiology 1987;165:113-6. [Crossref] [PubMed]
- Kim CY and Guevara CJ. Conventional and Cross-Sectional Venography. In: Larssen EM, Desai SS, Dua A, et al. editors. Phlebology, vein surgery and venography. Switzerland: Springer International Publishing, 20014:128-9.
- Lee W, Chung JW, Yin YH, et al. Three-Dimensional CT venography of varicose veins of the lower extremity: image quality and comparison with doppler sonography. AJR Am J Roentgenol 2008;191:1186-91. [Crossref] [PubMed]