
Association of serum cystatin C level and major adverse cardiovascular events in patients with percutaneous coronary intervention
Highlight box
Key findings
• Our study identified serum cystatin C as a risk factor for unplanned cardiac percutaneous coronary intervention (PCI) again in acute coronary syndrome (ACS) patients following PCI, moreover, which displayed a U-shaped dose-response relationship.
What is known and what is new?
• At present, some studies have shown that high serum cystatin C level is a risk factor for the development and prognosis of cardiovascular diseases, but it is still controversial.
• Our study was the first to identify low serum cystatin C as a risk factor for unplanned cardiac PCI again in ACS patients following PCI.
What is the implication, and what should change now?
• Serum cystatin C level may be considered a biomarker to improve the prognosis of ACS patients after PCI.
Introduction
Globally, acute coronary syndrome (ACS), the initial manifestation of coronary artery disease (CAD), is the primary cause of death and morbidity, especially in low- and middle-income earners (1,2). The percutaneous coronary intervention (PCI) was considered the most effective treatment for patients with ACS, however, patients with ACS did not have satisfactory long-term clinical outcomes with PCI, which may be attributed to the fact that PCI only increases blood flow to the coronary arteries without eliminating the cause of thrombosis (3). Therefore, guideline-directed secondary prevention, e.g., statin and aspirin administration, was critical to prevent major adverse cardiovascular events (MACEs) for patients with ACS after PCI (4). Clinical research showed that the MACE of patients with ACS following PCI was composed of all-cause mortality, acute myocardial infarction, stroke, and unplanned coronary revascularization, which was an essential cause of all-cause mortality in patients with ACS (5). Understanding mechanisms and screening for biomarkers with predictive and diagnostic value for MACE of patients with ACS after PCI is important.
Cystatin C, a cysteine protease inhibitor, has been used as a biomarker for estimating the glomerular filtration rate (6). The study revealed that almost all nucleated body cells could express cystatin C (7). As an inhibitor of cysteine proteases, cystatin C played a critical role in a variety of biological processes, including apoptosis, major histocompatibility complex-II (MHC-II)-mediated antigen presentation, activation of precursor proteins (including enzymes and prohormone), control of innate immune cells, and protein turnover (8,9). In addition, as a marker of kidney function for over 25 years, a growing number of studies has shown that, in groups at high cardiovascular risk, cystatin C levels may be related to mortality and the risk of cardiovascular events, however, this is still controversial (10,11). Some studies showed that serum cystatin C level was an independent predictor of MACE in patients with ACS after PCI, but it has also been reported that serum cystatin C level was not significantly different between with and without MACE for patients with ACS after PCI (12-15). Moreover, based on the correlation between serum cystatin C level and renal function, present studies have focused more on the effect of serum cystatin C level elevation on poor prognosis after PCI for ACS patients. Whether a reduction in plasma cystatin C levels has some effect on prognosis after PCI for ACS patients remains unclear. Therefore, a retrospective study at a single center was conducted to evaluate the ssociation between serum cystatin C and MACE in ACS patients after PCI. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-482/rc).
Methods
Patients
Our current study was approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University (No. WZ2024001). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since this was a retrospective study, no informed consent was required from participants. Data were retrospectively reviewed from 479 consecutive patients with ACS who underwent PCI between January 2011 and December 2011 in the Department of Cardiology of the Affiliated Hospital of Inner Mongolia Medical University. The inclusion criteria were as follows: ≥18 years old, received PCI at our hospital, and had follow-up records and clinical data. The exclusion criteria were as follows: had documented familial dyslipidemia, and had severe complications including acute coronary artery stent thrombosis or coronary artery dissection during indexed PCI, other complications, such as cerebral infarction and hemorrhage, cancer, asthma, pulmonary hypertension, and metabolic diseases other than diabetes, and kidney dysfunction, lack of clinical data.
All patients enrolled in the study underwent PCI for primary ACS by qualified cardiologists at the Affiliated Hospital of Inner Mongolia Medical University and at least one stent was successfully implanted. A total of 330 patients with a definite principal diagnosis of ST-elevation myocardial infarction, and non-ST-elevation myocardial infarction were diagnosed according to respective guidelines issued by the Chinese Society of Cardiology (16,17). Data from all patients were collected from the hospital information system by one author and checked by another author, including demographic information, medical history, blood pressure, liver and kidney function, lipid parameters, medication history, and serum cystatin C levels, etc.
Follow-up and clinical evaluation
Follow-up was performed by phone, face-to-face clinic visits, and re-hospitalization. MACE of patients with ACS after PCI was recorded, and MACE was defined by the need for a PCI for ACS again, moreover, the patient’s coronary angiography showed smooth blood flow found at the first coronary stent. In the end, 330 patients were enrolled in our study, the study schematic diagram is presented in Figure 1.
Statistical methods
Statistical analysis was performed using SPSS version 20.0 software (IBM, Chicago, IL, USA) and R language (version 4.2). Clinical data with normal distribution in the two groups were expressed as mean ± standard deviation and analyzed by Student’s t-test, and those with skewed distribution were compared using the Kruskal-Wallis test. Comparisons of categorical variables were conducted using the chi-squared (χ2) test.
Univariate and multivariate logistic regression were used to analyze the association between serum cystatin C level and MACE. To explore the association between serum cystatin C level and MACE, the cumulative incidences were estimated using Kaplan-Meier curves and detected by the Log-Rank test. Unadjusted hazard ratios (HRs) for primary outcomes were calculated by a univariate Cox proportional hazards model. Patient baseline differential factors as covariates, including taking copidogrel, sex, and age, were included in the multivariate Cox proportional hazards model. Adjusted HRs were calculated by a multivariate Cox proportional hazards model. Restricted cubic spline (RCS) analysis was applied to evaluate the dose-response relationship between serum cystatin C level and MACE in patients with PCI for ACS, with four knots located at the 5th, 35th, 65th, and 95th percentiles as per Harrell’s recommendations (18). Two-side P<0.05 was considered statistically significant.
Results
Baseline characteristics of the selected participants
A total of 330 participants were selected for the final data analysis. The average age of the selected participants was 65.33±9.59 years, and 72.12% of the participants were men. After a median follow-up of 63 months (range, 1–148 months), the overall prevalence of MACE was 36.67% in the study population, defined as 121 patients with ACS following PCI who underwent a need for a PCI for ACS again (Figure 1). Compared to patients who did not have MACE, patients who had MACE during the follow-up were younger (62.53±9.72 vs. 66.96±9.14 years, P<0.001), and were more likely to have lower serum cystatin C level (0.99±0.32 vs. 1.15±0.78 mg/L, P=0.03) and taking clopidogrel (22.31% vs. 35.41%, P=0.01). No significant differences in other baseline characteristics were observed between the two groups, which is shown in Table 1.
Table 1
| Characteristics | MACE (n=121) | Without MACE (n=209) | P value |
|---|---|---|---|
| Men | 87 (71.90) | 151 (72.25) | 0.95 |
| Age (years) | 62.53±9.72 | 66.96±9.14 | <0.001 |
| SBP (mmHg) | 132.91±22.52 | 136.36±23.63 | 0.19 |
| DBP (mmHg) | 84.39±11.66 | 81.82±11.98 | 0.06 |
| TC (mmol/L) | 3.98±1.01 | 3.95±1.03 | 0.83 |
| TG (mmol/L) | 1.56±0.91 | 1.53±0.92 | 0.81 |
| HDL-C (mmol/L) | 0.97±0.19 | 1.01±0.22 | 0.14 |
| LDL-C (mmol/L) | 2.1±0.7 | 2.1±0.74 | 0.94 |
| Glucose (mmol/L) | 5.47±1.95 | 5.73±2.38 | 0.39 |
| Cre (μmol/L) | 70.25±17.71 | 79.03±51.10 | 0.07 |
| Urea (μmol/L) | 6.27±2.24 | 6.97±4.47 | 0.11 |
| Cystatin C (mg/L) | 0.99±0.32 | 1.15±0.78 | 0.03 |
| Cigarette smoking | 0.77 | ||
| Never | 70 (57.85) | 132 (63.16) | |
| Former | 14 (11.57) | 21 (10.05) | |
| Current | 37 (30.58) | 56 (26.79) | |
| Alcohol intake | 0.18 | ||
| Never | 102 (84.30) | 170 (81.34) | |
| Former | 0 (0.00) | 8 (3.83) | |
| Current | 19 (15.70) | 31 (14.83) | |
| Hypertension history | 79 (65.29) | 133 (63.64) | 0.76 |
| DM history | 44 (36.36) | 70 (33.49) | 0.60 |
| Medications | |||
| Aspirin | 58 (47.93) | 117 (55.98) | 0.16 |
| Clopidogrel | 27 (22.31) | 74 (35.41) | 0.01 |
| NOACs | 11 (9.09) | 21 (10.05) | 0.78 |
| ACEI/ARB | 37 (30.58) | 60 (28.71) | 0.72 |
| CCB | 36 (29.75) | 57 (27.27) | 0.63 |
| Beta-blocker | 38 (31.40) | 73 (34.93) | 0.51 |
| Statins | 53 (43.80) | 104 (49.76) | 0.30 |
| Oral anti-diabetic medication | 33 (27.27) | 42 (20.10) | 0.13 |
| Insulin | 13 (10.74) | 19 (9.09) | 0.63 |
Age, SBP, DBP, TC, TG, HDL-C, LDL-C, Cre, urea, cystatin C, and glucose values are as means ± standard deviation, continuous variables with normal distribution were compared using the Student’s t-test, those with skewed distribution were compared using the Kruskal-Wallis test; the number of individuals (n) with percentage (n/N) are indicated and were compared using the chi-squared test. MACE: a major adverse cardiovascular event, is defined as the need for repeat revascularization in the heart. SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, triglyceride; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Cre, creatinine; DM, diabetes mellitus; NOACs, new oral anticoagulants; ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; CCB, calcium channel blocker.
Independent risk factors for MACE
Univariate and multivariate regression analyses were performed to evaluate the independent risk factors for MACE. In the univariate regression analysis, serum cystatin C levels were all independently associated with MACE for patients with PCI for ACS [odds ratio (OR) =0.460; 95% confidence interval (CI): 0.226–0.938; P=0.03]. Adjustment for taking clopidogrel, serum cystatin C was a significant independent predictive factor for MACE in patients who underwent PCI for ACS (OR =0.472; 95% CI: 0.229–0.972; P=0.042). However, in the multivariate analysis adjusted for taking clopidogrel and age, serum cystatin C was no longer an independent risk factor for MACE (Table 2).
Table 2
| Model | OR | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | 0.460 | 0.226–0.938 | 0.03 |
| Adjust model 1 | 0.472 | 0.229–0.972 | 0.042 |
| Adjust model 2 | 0.658 | 0.344–1.257 | 0.21 |
Model 1 adjusted for taking clopidogrel. Model 2 adjusted for taking clopidogrel and age. MACE: a major adverse cardiovascular event, is defined as the need for repeat revascularization in the heart. OR, odds ratio; CI, confidence interval.
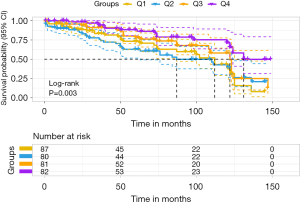
The participants were classified into four groups according to the interquartile value of serum cystatin C levels. Accordingly, the fourth quartile of serum cystatin C was designated as the reference in the multivariable Cox proportional hazards regression analysis (Cox regression). The Cox result showed that the lowest and second quartile of serum cystatin C indicated an increased risk of MACE in patients with ACS after PCI (unadjusted model, model 1 and model 2, Table 3). Moreover, the second quartile of serum cystatin C indicated an increased risk of MACE in patients with ACS after PCI (adjusted HR =2.109; 95% CI: 1.193–3.727; model 3). It remained statistically significant after further adjustment for taking clopidogrel, sex, and age (Table 2). Kaplan-Meier curves indicated the likelihood of MACE-free in patients with PCI for ACS. The risk of developing MACE was significantly different between the four groups (P=0.003). As serum cystatin C levels increased, the likelihood of surviving without MACE gradually increased. However, the result showed that the group with the second-level serum cystatin C (Q2) had the greatest risk of developing MACE for patients with PCI for ACS (Figure 2).
Table 3
| Quartiles of serum cystatin C (mg/L) | Unadjusted model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MACE HR (95% CI) | P value | MACE HR (95% CI) | P value | MACE HR (95% CI) | P value | MACE HR (95% CI) | P value | ||||
| Q1 ≤0.84 | 2.153 (1.218–3.806) | 0.008 | 2.144 (1.213–3.790) | 0.009 | 2.148 (1.214–3.799) | 0.009 | 1.542 (0.850–2.798) | 0.15 | |||
| 0.84< Q2 ≤0.96 | 2.249 (1.275–3.967) | 0.005 | 2.244 (1.272–3.960) | 0.005 | 2.241 (1.269–3.956) | 0.005 | 2.109 (1.193–3.727) | 0.01 | |||
| 0.96< Q3 ≤1.12 | 1.383 (0.749–2.551) | 0.30 | 1.382 (0.750–2.550) | 0.30 | 1.387 (0.750–2.564) | 0.30 | 1.272 (0.686–2.356) | 0.45 | |||
| Q4 >1.12 | 1.00 (Reference) | – | 1.00 (Reference) | – | 1.00 (Reference) | – | 1.00 (Reference) | – | |||
Model 1 adjusted for taking clopidogrel. Model 2 adjusted for sex above model 1. Model 3 adjusted for sex and age above model 1. MACE: a major adverse cardiovascular event, is defined as the need for repeat revascularization in the heart. HR, hazard ratio; CI, confidence interval; Q, quartile.
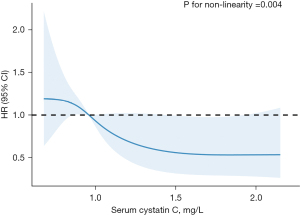
Analysis of nonlinear relationship between serum cystatin C and MACE in patients with PCI for ACS
Our results displayed that the relationship between serum cystatin C and MACE in patients with PCI for ACS was nonlinear after adjusting for taking clopidogrel. We fitted a proportional hazards model with a RCS function with 4 knots to visualize potential nonlinear associations. The tests for nonlinear association between serum cystatin C and MACE in patients with ACS after PCI were highly significant (P=0.004). We found that the ACS patients with serum cystatin C from 0.86 to 0.95 mg/L had a significantly increased risk of MACE, while patients with serum cystatin C from 1.44 to 1.92 mg/L were significantly lower in risk of MACE than others (Figure 3).
Discussion
This retrospective study assessed the prognostic role of serum cystatin C in ACS patients undergoing PCI. Our findings indicated that serum cystatin C was related to MACE for patients with PCI for ACS. The patients with low serum cystatin C level had a significantly increased risk of MACE at a median follow-up of 63 months when compared with patients with a higher serum cystatin C level. RCS analysis showed a significant U-shaped dose-response association of cystatin C level with MACE in ACS patients after PCI.
At present, there is an ongoing debate on the association between serum cystatin C levels and the risk of cardiovascular disease (CVD). Some reports have shown that serum cystatin C has been thoroughly investigated as a risk indicator for CVD or a bad prognosis in some at-risk populations, including CVD, and heart failure. Moreover, with an increase in serum cystatin C levels, the risk of that disease and poor prognosis (all-cause and cardiovascular mortality) were significantly increased (19). Prospective Mendelian randomization analyses, however, did not corroborate that cystatin C is causally linked to the development of CVD, which includes ischemic stroke, heart failure, and coronary heart disease (20). In our study, we first identified a U-shaped dose-response correlation between MACE and cystatin C level in ACS patients receiving PCI, which was illustrated by a significantly decreased risk of unplanned repeat PCI for ACS patients with PCI, whose serum cystatin C levels were from 1.44 to 1.92 mg/L compared to others. Therefore, maintaining a stable and appropriate concentration of serum cystatin C was critical to improving the prognosis of patients with PCI for ACS.
There are several reasons why the U-shaped correlation was found between cystatin C level and MACE in patients with PCI for ACS. Excessive levels of cystatin C (>1.44 mg/L) in the serum suggested renal dysfunction, while renal dysfunction was closely associated with increased coronary heart disease risk in the general population (21,22). A previous study showed that serum cystatin C could interact with adiponectin (APN), an adipocyte-derived bioactive molecule with antiatherogenic properties, to form cystatin C-APN complex, which led to abolishing the anti-atherogenic effects of APN (23). Subsequent investigation revealed that in patients with CVD, serum cystatin C-APN complex levels were favorably correlated with unstable necrotic or lipidic plus necrotic coronary plaque components and negatively associated with stable fibrotic coronary plaque components (24). Besides, some studies suggested serum cystatin C as an inflammatory marker to regulate inflammation reactions and various immune processes, which was closely correlated to atherosclerotic plaque formation (25-27). However, does a lower serum cystatin C level provide a benefit for patients with CVD and normal humans? The answer is still no. Li’s study showed that cystatin C deficiency resulted in autophagy dysfunction and apoptosis in macrophages and apoE-deficient mice, while autophagy dysfunction and apoptosis played a critical role in plaque rupture and thrombosis of atherosclerotic lesions (28). Cystatin C as an inhibitor of the elastin-degrading cysteine proteases of the cathepsin family affected regulating plaque stability by matrix remodeling, therefore, leukocyte-specific over- and under-expression of cystatin C led to a change in plaque regression (29). In summary, maintaining an appropriate concentration of serum cystatin C was considered an effective approach to decrease the risk of MACE for patients with PCI for ACS.
The concentration of serum cystatin C from 0.63–1.44 mg/L was recommended as a reference value for the Chinese Han population. However, our results showed that patients with serum cystatin C from 1.44 to 1.92 mg/L were significantly lower in risk of MACE than others, which was paradoxical to clinical practice. This could be attributed to the old patients with ACS as selected participants in our study. On the one hand, age-related physiological change in the kidney was found in the old population, which led to increased serum cystatin C levels (30). On the other hand, a previous study illustrated that serum cystatin C had a significant increase in patients with coronary heart disease (CHD) compared to the normal population (31). Therefore, a more individualized management of serum cystatin C has been suggested for patients with PCI for ACS, especially for old patients.
The current investigation has the following shortcomings. Firstly, this was a single-center retrospective study that was limited by the temporal relationship regarding the association between exposure and outcomes; secondly, the design of the observational study did not allow us to infer a causal relationship; third, the baseline serum cystatin C levels were used in our study, its dynamic change was not obtained during follow-up, we did not know whether serum cystatin C levels were affected by lifestyle habits and medication use. In the future prospective study, it is necessary to focus on the relationship between the dynamic change of serum cystatin C and in MACE different racial/ethnic patients with PCI for ACS.
Conclusions
Our study was the first to identify low serum cystatin C as a risk factor for unplanned cardiac PCI again in ACS patients following PCI, moreover, further analyses showed a significant U-shaped dose-response association of cystatin C level with MACE in ACS patients after PCI. A multicentre prospective study is needed to further validate our findings, which have potential clinical significance for improving the prognosis of ACS patients after PCI.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-482/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-482/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-482/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-482/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Inner Mongolia Medical University (No. WZ2024001). Since this was a retrospective study, no informed consent was required from participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Musunuru K, Kathiresan S. Genetics of Common, Complex Coronary Artery Disease. Cell 2019;177:132-45. [Crossref] [PubMed]
- Liang B, Cai XY, Gu N. Marine Natural Products and Coronary Artery Disease. Front Cardiovasc Med 2021;8:739932. [Crossref] [PubMed]
- Wang C, Kong Y, Ding Y, et al. Serum Calprotectin Levels and Outcome Following Percutaneous Coronary Intervention in Patients with Diabetes and Acute Coronary Syndrome. Med Sci Monit 2019;25:9517-23. [Crossref] [PubMed]
- Park Y, Franchi F, Rollini F, et al. Antithrombotic Therapy for Secondary Prevention in Patients With Diabetes Mellitus and Coronary Artery Disease. Circ J 2016;80:791-801. [Crossref] [PubMed]
- Berwanger O, Santucci EV, de Barros E, Silva PGM, et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA 2018;319:1331-40. [Crossref] [PubMed]
- Feng B, Lu Y, Ye L, et al. Mendelian randomization study supports the causal association between serum cystatin C and risk of diabetic nephropathy. Front Endocrinol (Lausanne) 2022;13:1043174. [Crossref] [PubMed]
- Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens 2020;29:591-8. [Crossref] [PubMed]
- Hartmann S, Lucius R. Modulation of host immune responses by nematode cystatins. Int J Parasitol 2003;33:1291-302. [Crossref] [PubMed]
- Bagshaw SM, Bellomo R. Cystatin C in acute kidney injury. Curr Opin Crit Care 2010;16:533-9. [Crossref] [PubMed]
- Luo J, Wang LP, Hu HF, et al. Cystatin C and cardiovascular or all-cause mortality risk in the general population: A meta-analysis. Clin Chim Acta 2015;450:39-45. [Crossref] [PubMed]
- Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis 2013;62:595-603. [Crossref] [PubMed]
- Fracassi F, Niccoli G, Scalone G, et al. Prognostic role of multiple biomarkers in stable patients undergoing fractional flow reserve-guided coronary angioplasty. J Cardiovasc Med (Hagerstown) 2016;17:687-93. [Crossref] [PubMed]
- Correa S, Morrow DA, Braunwald E, et al. Cystatin C for Risk Stratification in Patients After an Acute Coronary Syndrome. J Am Heart Assoc 2018;7:e009077. [Crossref] [PubMed]
- Sun Y, Lu Q, Cheng B, et al. Prognostic value of cystatin C in patients with acute coronary syndrome: A systematic review and meta-analysis. Eur J Clin Invest 2021;51:e13440. [Crossref] [PubMed]
- Sun TW, Xu QY, Yao HM, et al. The predictive value of plasma cystatin C for acute coronary syndrome treated with percutaneous coronary intervention. Heart Lung 2012;41:456-62. [Crossref] [PubMed]
- Chinese Society of Cardiology of Chinese Medical Association. 2019 Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2019;47:766-83. [PubMed]
- Chinese Society of Cardiology of Chinese Medical Association. Guideline and consensus for the management of patients with non-ST-elevation acute coronary syndrome(2016). Zhonghua Xin Xue Guan Bing Za Zhi 2017;45:359-76. [PubMed]
- Harrell FE Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988;80:1198-202. [Crossref] [PubMed]
- Lassus J, Harjola VP. Cystatin C: a step forward in assessing kidney function and cardiovascular risk. Heart Fail Rev 2012;17:251-61. [Crossref] [PubMed]
- van der Laan SW, Fall T, Soumaré A, et al. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol 2016;68:934-45. [Crossref] [PubMed]
- Benoit SW, Ciccia EA, Devarajan P. Cystatin C as a biomarker of chronic kidney disease: latest developments. Expert Rev Mol Diagn 2020;20:1019-26. [Crossref] [PubMed]
- Di Angelantonio E, Danesh J, Eiriksdottir G, et al. Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med 2007;4:e270. [Crossref] [PubMed]
- Fujita M, Yamamoto H, Yoshida N, et al. Atheroprotective Roles of Adiponectin via CCL2 Inhibition. J Atheroscler Thromb 2021;28:1204-13. [Crossref] [PubMed]
- Matsumoto A, Yamamoto H, Matsuoka T, et al. Cystatin C-Adiponectin Complex in Plasma Associates with Coronary Plaque Instability. J Atheroscler Thromb 2017;24:970-9. [Crossref] [PubMed]
- Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett 2018;196:80-90. [Crossref] [PubMed]
- Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res 2021;117:2525-36. [Crossref] [PubMed]
- Sharma M, Schlegel MP, Afonso MS, et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ Res 2020;127:335-53. [Crossref] [PubMed]
- Li W, Sultana N, Siraj N, et al. Autophagy dysfunction and regulatory cystatin C in macrophage death of atherosclerosis. J Cell Mol Med 2016;20:1664-72. [Crossref] [PubMed]
- Bengtsson E, To F, Grubb A, et al. Absence of the protease inhibitor cystatin C in inflammatory cells results in larger plaque area in plaque regression of apoE-deficient mice. Atherosclerosis 2005;180:45-53. [Crossref] [PubMed]
- Raman M, Middleton RJ, Kalra PA, et al. Estimating renal function in old people: an in-depth review. Int Urol Nephrol 2017;49:1979-88. [Crossref] [PubMed]
- Fluschnik N, Ojeda F, Zeller T, et al. Predictive value of long-term changes of growth differentiation factor-15 over a 27-year-period for heart failure and death due to coronary heart disease. PLoS One 2018;13:e0197497. [Crossref] [PubMed]



