
Age-related wall shear stress changes assessed by vascular vector flow mapping in the carotid arteries of healthy adults: a cross-sectional study
Highlight box
Key findings
• Previous studies have identified differences in wall shear stress (WSS) between carotid atherosclerotic plaques and healthy populations, as well as between hypertensive and normal populations using vascular vector flow mapping (VFM) technology. However, the WSS in normal Chinese adults across different age groups remains unknown. This study preliminarily employed vascular VFM technology through a visual approach to obtain quantitative results for assessing carotid WSS parameters among healthy Chinese adults. We found that with increasing age, the carotid WSS-related parameters diminished, with significantly lower values observed in the elderly compared to the young.
What is known and what is new?
• Vascular VFM technology through a visual approach to obtain carotid WSS is known.
• This study provides additional information on the distribution of WSS among different age groups of healthy Chinese adults.
What is the implication, and what should change now?
• This means that there is currently a lack of research on the WSS parameters of the carotid artery in multicenter large-scale datasets of normal Chinese adults.
Introduction
Atherosclerosis is prevalent globally, and due to the increase in life expectancy and lifestyle changes, its incidence is increasing each year (1). Blood flow through the vascular wall exerts shear forces on endothelial cells (ECs) wall shear stress (WSS) local blood flow patterns such as turbulent flow and vortex formation at vascular bifurcations or bends, along with vascular pressure, dilation and constriction, are mechanical factors that can alter the physiological environment of the vessel wall. These factors promote EC injury, inflammation, smooth muscle cell proliferation, and plaque formation, ultimately leading to the development of atherosclerosis (2). WSS is closely related to the onset and progression of atherosclerosis, and it is affected by complex hemodynamic factors, such as blood viscosity, flow velocity gradient, flow pattern, and direction (3).
Different levels of WSS are associated with different stages of atherosclerosis pathogenesis; in early carotid atherosclerosis (CA) lesions, low WSS promotes the EC initiation of neovascular growth and the invasion of extracellular matrix (ECM) by activating membrane type-1 matrix metalloproteinase to induce EC deformation (4). Low WSS further induces alterations in cytoskeletal actin filaments (F-actin) and vascular endothelial calmodulin connections (5), inducing interactions between ECs and adjacent smooth muscle cells, resulting in an altered vascular smooth muscle cells (VSMCs) contractile phenotype and increased arterial wall stiffness (6). During CA plaque formation and progression, high WSS increases plaque instability by inducing platelet aggregation and endothelial exfoliation, making the plaque susceptible to dislodgement, causing adverse events such as stroke (7,8). Therefore, carotid WSS is clinically important in the study of CA, intima-media thickening, and plaque formation and shedding (9,10).
WSS is influenced by hemodynamic factors, differences in vessel internal diameter, and vessel geometry. Blood flow in the vessel is both forward and parallel to the longitudinal direction of the vessel, and may also have a circumferential flow that is locally perpendicular to the vessel wall; researchers have tried to find accurate and objective methods of measuring WSS (11). The accurate acquisition of data on the magnitude of carotid WSS is crucial to monitor the progression of atherosclerosis and prevent stroke.
Studies have used magnetic resonance imaging (MRI) angiography to obtain WSS values (12,13); however, MRI has limited spatial resolution. Computed tomography angiography-based WSS measurements (14) cannot be widely used in clinical practice due to the high radioactivity of this type of examination. In this study, the significant potential of computer simulations providing high temporal and spatial resolution, which are a powerful, widely accepted tool for determining WSS.
Vascular vector flow mapping (VFM) uses color Doppler and three primary pulsed Doppler crossbeams, combined with two-dimensional speckle tracking technology, to obtain the WSS and flow velocity vector of the vessel according to the geometry of the vessel (15). By obtaining the conventional longitudinal flow velocity parallel to the probe acoustic beam direction, the circumferential flow velocity perpendicular to the vessel wall direction is obtained by three primary pulsed Doppler crossbeams without angular dependence, which makes the WSS measurement objective, accurate, and intuitive (16-18). Previously, we reported initial findings using VFM, indicating that WSS parameters were lower in hypertensive populations compared to normotensive populations (19). However, to our knowledge, there have been no reports on WSS parameters in different age groups of the Chinese population. Therefore, the present study sought to preliminarily analyze the differences in carotid WSS in healthy adults of different ages and to obtain reference values for normal WSS using a novel vascular VFM technique. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-134/rc).
Methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Eighth People’s Hospital (No. 2022-018-12-02). The requirement of informed consent was waived due to the retrospective nature of the study.
Patient enrollment
In total, 118 self-reported healthy patients from different age groups were initially identified for inclusion in the study; however, 48 patients, who did not meet the inclusion criteria, were excluded from the study. Thus, ultimately, 70 healthy individuals, aged 20 to 89 years, who visited the Ultrasound Department of Shanghai Eighth People’s Hospital between February 2021 and June 2021 were retrospectively included in the study.
To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: have healthy blood pressure (≤140/90 mmHg), lipid (triglycerides <1.7 mmol/L), and glucose levels (fasting blood glucose 4.4–6.1 mmol/L), and have no CA plaques. Patients were excluded from the study if they met any of the following exclusion criteria: had a carotid intima-media thickness (IMT) >1.2 mm; and/or had a history of hypertension, diabetes mellitus, metabolic disease, abnormal liver or kidney function, cardiovascular and cerebrovascular disease, tumors, or any other major disease.
The participants were divided into the following seven groups according to their age: the 20s group (which comprised patients aged 20–29 years); the 30s group (which comprised patients aged 30–39 years); the 40s group (which comprised patients aged 40–49 years); the 50s group (which comprised patients aged 50–59 years); the 60s group (which comprised patients aged 60–69) years; the 70s group (which comprised patients aged 70–79 years); and the 80s group (which comprised patients aged 80–89 years). Due to the small number of healthy individuals in the 70s and 80s groups, only 10 participants were included in each different age group for this preliminary study (Figure 1).
Scanning protocol
In this study, the participants lay in the supine position with their necks exposed and underwent an examination that was synchronized with an electrocardiogram. We used the ALOKA LISENDO 880 color Doppler ultrasound system, manufactured by FUJIFILM Healthcare (Guangzhou) Co., Ltd., Japan. Choosing a superficial vascular probe with a frequency of 3–15 MHz. In two-dimensional ultrasound, adjust the probe to align the long-axis section of the carotid artery with the skin surface parallel. For color Doppler, the sampling frame angle between the probe and the artery was adjusted ≤30°. The image frame rate captured was greater than 10 frames per second, with the color Doppler flow mapping dynamic range set to the minimum level consistent with first-order aliasing correction. Post-measurement, the VFM vascular key on the color Doppler was active, resulting in three sound beam intersections and the fixation of the sampling frame within the middle segment of the common carotid artery’s ROIs. Three complete cardiac cycles’ dynamic images were then collected by following the intimal contours of the artery’s anterior and posterior walls (19). The WSS at each point on the carotid artery’s anterior and posterior walls was analyzed. WSS was computed automatically via an embedded analytical formula in the software:
: wall shear rate; : blood viscosity coefficient
VFM-based WSS parameters and measurements
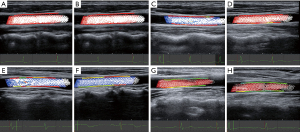
WSS was calculated in each frame and at each measured point in a complete cardiac cycle. In the complete cardiac cycle, there were approximately 10 to 15 frames images, and in each frame images, there were approximately 38 to 50 points (see Figure S1). The WSS parameters, including the WSSmaximum (max), WSSminimun (min), and WSSmean were obtained after averaging the parameters across all the frames and all the points in a cardiac cycle. The color scale on the carotid artery wall intuitively reflects the high and low levels of the WSS parameters; a red line on the vessel wall indicates a high WSS, and a green line on the vessel wall indicates a low WSS.
The carotid IMT measurements were performed using the vertical distance from the superior intima to the superior adventitia at partially enlarged longitudinal sections that were 1.0–1.5 cm below the level of the carotid bifurcation, taking the average of three measurements. The carotid internal diameter measurements were performed during systole; the vertical distance from the inner surface of the intima to the contralateral inner surface was measured in the middle of the common carotid artery. All the operations were performed by experienced and highly qualified attending physicians. Healthy blood pressure was defined as systolic blood pressure ≤140 mmHg and/or diastolic blood pressure ≤90 mmHg. Participants self-report who were previously informed that their blood lipids and blood glucose levels were normal in medical institution. The presence or absence of carotid plaque was confirmed by routine carotid ultrasound scanning of the common carotid artery, and the internal and external carotid arteries.
Statistical analysis
The statistical analysis was performed using SPSS 23.0 software (IBM Corp, Armonk, NY, USA), and R (version 4.2.2). The descriptive statistics of the patient characteristics are presented as the frequency and percentage for the categorical variables, and the mean and standard deviation (SD), or the median with the interquartile range (IQR) for the continuous variables according to the distribution of the sample. Differences in the continuous variables among the groups were analyzed by an analysis of variance or a Kruskal-Wallis test. Correlations and trends between the WSS, and the age and IMT were analyzed by a Pearson analysis and linear regression. Missing values for the baseline information data were interpolated using mean value interpolation. The P<0.05 was considered statistically significant.
Results
Demographic characteristics
In total, 118 self-reported healthy patients were initially included in the study; however, after excluding 17 patients with hypertension, 23 patients with carotid plaque, and 8 patients with intima-media thickening during the examination, a total of 70 healthy individuals were included in the study and allocated to the seven different age groups. Age, systolic blood pressure, IMT differed significantly among the age groups (P<0.001). Sex, heart rate, body surface area, and diastolic blood pressure did not differ significantly among the age groups, see Table 1.
Table 1
| General parameters | 20s group | 30s group | 40s group | 50s group | 60s group | 70s group | 80s group | P |
|---|---|---|---|---|---|---|---|---|
| Number | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Sex | 0.39 | |||||||
| Male | 5 | 6 | 5 | 5 | 6 | 4 | 5 | |
| Female | 5 | 4 | 5 | 5 | 4 | 6 | 5 | |
| Age (years) | 24.40±2.59 | 37.20±1.48 | 44.50±3.14 | 53.90±2.42 | 64.90±1.97 | 74.90±2.88 | 84.60±2.17 | <0.001* |
| Heart rate (bpm) | 63.90±4.09 | 65.00±4.55 | 66.70±6.00 | 64.10±4.65 | 64.60±5.10 | 68.30±10.26 | 70.30±8.98 | 0.25 |
| BSA (m2) | 1.68±0.23 | 1.74±0.21 | 1.70±0.14 | 1.75±0.13 | 1.81±0.15 | 1.79±0.14 | 1.74±0.11 | 0.58 |
| Systolic blood pressure (mmHg) | 116.0±5.16 | 122.50±6.77 | 117.50±3.54 | 120.00±6.67 | 129.5±4.38 | 126.00±5.68 | 122.00±6.32 | <0.001* |
| Diastolic blood pressure (mmHg) | 80.00±0.00 | 80.00±2.36 | 80.00±0.00 | 79.50±2.84 | 82.00±2.58 | 81.00±2.11 | 80.50±1.58 | 0.11 |
| IMT (mm) | 0.67±0.08 | 0.81±0.11 | 0.78±0.08 | 0.86±0.05 | 1.00±0.11 | 1.03±0.09 | 1.03±0.09 | <0.001* |
Data are presented as number or mean ± standard deviation. *, a statistically significant difference assessed by the analysis of variance or Kruskal-Wallis test. BSA, body surface area; IMT, intima-media thickness.
WSS differences among age groups
The WSSmax appeared in the systolic phase of each cardiac cycle, and the WSSmin appeared in the diastolic phase of each cardiac cycle in all the participants. The WSSmax, WSSmean, and WSSmin decreased with age (P≤0.001), see Table 2.
Table 2
| WSS parameter (Pa) | 20s group | 30s group | 40s group | 50s group | 60s group | 70s group | 80s group | P |
|---|---|---|---|---|---|---|---|---|
| WSSmax | 2.47±0.27 | 2.25±0.17 | 1.99±0.30 | 1.98±0.25 | 1.8±0.19 | 1.66±0.28 | 1.47±0.32 | <0.001* |
| WSSmean | 1.64±0.41 | 1.51±0.20 | 1.47±0.22 | 1.4±0.25 | 1.37±0.16 | 1.10±0.30 | 0.92±0.29 | <0.001* |
| WSSmin | 1.04±0.46 | 0.92±0.27 | 0.95±0.31 | 1.01±0.34 | 0.96±0.18 | 0.58±0.27 | 0.51±0.33 | 0.001* |
The data were presented as mean ± standard deviation. *, a statistically significant difference assessed by the analysis of variance or Kruskal-Wallis test. WSS, wall shear stress; max, maximum; min, minimum.
Correlation between age and the WSS
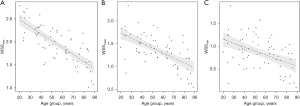
The Pearson correlation coefficient of the WSSmax and age was −0.556 (P<0.001; Figure 2), that of the WSSmean and age was −0.461 (P<0.001; Figure 3), and that of the WSSmin and age was −0.308 (P<0.001; Figure 4). The WSSmax, WSSmean, and WSSmin differ among the age groups, a negative correlation trend was observed (Figure 5). The correlation between WSSmax, WSSmean, and age is more pronounced.
Correlation between IMT and WSS
The Pearson correlation coefficient of the WSSmax and IMT was −0.511 (P<0.001), that of the WSSmean and IMT was −0.410 (P<0.001), and that of the WSSmin and IMT was −0.343 (P<0.001) (Figure 6).

Discussion
In the present study, we used a novel vascular VFM technique to visualize and quantitatively measure the WSSmax, WSSmean, and WSSmin in healthy Chinese adult carotid arteries in different age groups. The WSS parameters differed among the age groups, while the WSSmax, WSSmean, and WSS min gradually decreased with age. The correlation analysis between the WSSmax, WSSmean, and WSSmin and age revealed a negative correlation trend.
Vascular VFM technology was the primary carotid ultrasound method used in this study. Due to the complexity of vascular fluid dynamics, the accurate, objective, real-time, and rapid measurement of WSS is important. Previous studies had been conducted to explore and establish more accurate and objective methods of WSS examination (20-22). For example, Mazzi et al. (23) applied the WSS topological skeleton approach to computational hemodynamic modeling of carotid bifurcations and intracranial aneurysms based on MRI. In this study, we aim to explore the use of vascular ultrasound VFM technique to obtain the WSSmax, WSSmin, and WSSmean. The principle of the vascular VFM is to combine two-dimensional speckle tracking technology with the continuity equation to directly measure and calculate the velocity component of blood flow perpendicular to the beam direction, thereby obtaining the true flow velocity vector of the carotid artery. As a non-invasive hemodynamic imaging method, VFM enables rapid quantitative analysis of carotid artery WSS. Saito et al. (24) detected significantly higher WSS on the upstream side of plaques than on the downstream side of plaques using the ProSound F75 ultrasound blood flow vector device (Hitachi Aloka, Japan) (25).
In this preliminary study we evaluated the quantitative WSS parameters using vascular VFM technology in healthy adults and found that the WSS parameters gradually decreased with age in healthy participants. The healthy participants (aged from 20–89 years) were grouped by age based on 10-year increments. At 20–29 years, the participants had a WSSmax of 2.47±0.27 Pa, a WSSmean of 1.64±0.41 Pa, and a WSSmin of 1.04±0.46 Pa, while at 80–89 years, the participants had a WSSmax of 1.47±0.32 Pa, a WSSmean of 0.92±0.29 Pa, and a WSSmin of 0.51±0.33 Pa. There was a significant difference in these values between these groups, and the WSS gradually decreased with age. Age can affect vascular EC function and elasticity by influencing hemodynamics (26,27). The present study showed that WSS decreased with age. Similarly, other studies that used MRI have also shown that carotid WSS and ascending aortic WSS decreased with age in healthy adults (28,29), which confirms the accuracy and reliability of the results obtained in the present study using the vascular VFM technology.
Human aging begins with vascular aging, which occurs through oxidative stress damage, abnormal glucose and lipid metabolism, the chronic inflammatory response, autophagy, apoptosis, and other mechanisms that induce abnormal vascular EC function (30,31), which in turn lead to reduced vascular blood flow velocity and reduced elasticity of the arterial wall, which leads to the stiffening of the wall and the widening of the lumen inner diameter (32,33).
In addition, in healthy individuals, VSMCs also regulate arterial stiffness by producing various ECM components, such as collagen and elastin, to maintain vascular homeostasis; however, matrix proteases, such as calpain-1, matrix metalloproteinase 1 (MMP1), and matrix metalloproteinase 2 (MMP2), have been found in the aortic walls of healthy older people (34). The increased expression of MMP1 and MMP2 promotes VSMCs osteogenic trans-differentiation, elastin degradation, alkaline phosphatase activation, and collagen production, and reduces the expression of calcification inhibitors, such as osteopontin (35), which indirectly affect vascular EC function through ECM remodeling, resulting in decreased arterial elasticity, reduced vessel wall compliance. It is evident that age affects vascular structure and function in multiple ways, this also explains the negative correlation between WSS parameters and age found in this study. At the same time, it provides preliminary insights into the distribution and trends of WSS in different age groups in China. These findings have clinical significance in the early detection and prevention of age-related atherosclerotic lesions.
WSS represents the tangential force exerted by flowing blood on the vessel wall. Utilizing the measurement method and formula employed in this study, WSS can quantitatively determine the distribution of frictional forces on the vessel wall (both parallel and perpendicular to the wall), and supply insights into the blood flow adjacent to the vessel wall, thus reflecting alterations in vascular structure and function. Table 2 within the research findings illustrates the distribution of WSSmax, WSSmean, and WSSmin across various age groups, as measured using vascular VFM technology. We observed significant statistical differences between WSSmax and WSSmean in different age groups. It is possible that WSSmax and WSSmean exert a greater influence on the overall WSS of the carotid artery. In future investigations, it may be beneficial to focus on these indicators, WSSmax and WSSmean for assessing early functional abnormalities of the carotid artery. The vascular VFM technique used in this study visualized the magnitude of WSS at different ages; a red line on the vessel wall indicated high WSS, and a green line indicated low WSS. As age increased, more green lines were observed (Figure 7). Intima-media thickening is an important manifestation of in early-stage atherosclerosis (36) and has been shown to increase significantly with age (37). Zhang et al. (38) quantified WSS using two-dimensional color Doppler flow imaging in carotid arteries and found that the WSS was lower in the group with intima-media thickening than the healthy control group. The authors suggested that this may be due to the dysfunction of vascular EC, accumulation of foam cells under the intima, and subintimal fat deposition into fatty streaks, resulting in intima-media thickening (39). When early carotid intimal medial thickening occurs, vascular VFM technology offers superiority in early, objective, and quantitative assessment of carotid hemodynamic abnormalities compared to conventional ultrasound methods.
The present study had several limitations. First, the present study largely focused on carotid WSS parameters at different ages, and differences in bilateral carotid WSS were not compared in the same subjects. Second, this was a single-center study with a small sample size; however, this should not have affected the value of this study that sought to provide healthy reference values for WSS. We calculated the further power values based on a statistical significance level of 0.05, which resulted in a minimum statistical efficacy value of 0.942 for the different groups in terms of age differences, and which provided sufficient power for the analysis. Measurements of left ventricular fluid dynamics parameters in healthy Chinese adults based on echocardiographic VFM have been reported (40). However, future multicenter studies on VFM of the carotid vasculature in healthy adults with increased sample sizes need to be conducted. In addition, changes in WSS at different ages in the same study subjects also need to be further studied in the carotid bifurcation and carotid bulb.
Conclusions
In conclusion, this study utilized vascular VFM technology through a visual approach to obtain results in the quantitative assessment of carotid WSS parameters among healthy Chinese adults. In the common carotid artery, as age increased, the carotid WSS-related parameters decreased, the elderly are significantly lower than the young. The descriptions of the carotid WSS parameters (WSSmax, WSSmean) of healthy adults in different ages may be of clinical and scientific value for future studies.
Acknowledgments
We would like to thank the reviewers for their time and effort in reviewing and providing constructive suggestions for the manuscript. We thank the Shanghai Eighth People’s Hospital for supporting this study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-134/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-134/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-134/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-134/coif). F.C. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2023 to August 2025. All authors report that this work was supported by Shanghai Municipal Health Commission Health Industry Clinical Research Project (No. 202340181) and Shanghai Xuhui District-Academia Collaborative Project (Life and Health Sciences) (No. 23XHYD-22). Y.F. is employed by FUJIFILM Healthcare. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Shanghai Eighth People’s Hospital (No. 2022-018-12-02). The requirement of informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Libby P. The changing landscape of atherosclerosis. Nature 2021;592:524-33. [Crossref] [PubMed]
- Gijsen F, Katagiri Y, Barlis P, et al. Expert recommendations on the assessment of wall shear stress in human coronary arteries: existing methodologies, technical considerations, and clinical applications. Eur Heart J 2019;40:3421-33. [Crossref] [PubMed]
- Frösen J, Cebral J, Robertson AM, et al. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus 2019;47:E21. [Crossref] [PubMed]
- Kang H, Duran CL, Abbey CA, et al. Fluid shear stress promotes proprotein convertase-dependent activation of MT1-MMP. Biochem Biophys Res Commun 2015;460:596-602. [Crossref] [PubMed]
- Ben-Saadon S, Gavriel M, Zaretsky U, et al. Tissue-engineered arterial intima model exposed to steady wall shear stresses. J Biomech 2021;117:110236. [Crossref] [PubMed]
- Wang L, Rice M, Swist S, et al. BMP9 and BMP10 Act Directly on Vascular Smooth Muscle Cells for Generation and Maintenance of the Contractile State. Circulation 2021;143:1394-410. [Crossref] [PubMed]
- Costopoulos C, Huang Y, Brown AJ, et al. Plaque Rupture in Coronary Atherosclerosis Is Associated With Increased Plaque Structural Stress. JACC Cardiovasc Imaging 2017;10:1472-83. [Crossref] [PubMed]
- Samady H, Eshtehardi P, McDaniel MC, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 2011;124:779-88. [Crossref] [PubMed]
- Nemoto T, Minami Y, Yamaoka-Tojo M, et al. Endothelial glycocalyx and severity and vulnerability of coronary plaque in patients with coronary artery disease. Atherosclerosis 2020;302:1-7. [Crossref] [PubMed]
- Miranda CH, de Carvalho Borges M, Schmidt A, et al. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis 2016;247:184-8. [Crossref] [PubMed]
- Chen Y, Canton G, Kerwin WS, et al. Modeling hemodynamic forces in carotid artery based on local geometric features. Med Biol Eng Comput 2016;54:1437-52. [Crossref] [PubMed]
- Zhuang B, Sirajuddin A, Zhao S, et al. The role of 4D flow MRI for clinical applications in cardiovascular disease: current status and future perspectives. Quant Imaging Med Surg 2021;11:4193-210. [Crossref] [PubMed]
- Shokina N, Teschner G, Bauer A, et al. Parametric Sequential Method for MRI-Based Wall Shear Stress Quantification. IEEE Trans Med Imaging 2021;40:1105-12. [Crossref] [PubMed]
- Dilba K, van Dam-Nolen DHK, Korteland SA, et al. The Association Between Time-Varying Wall Shear Stress and the Development of Plaque Ulcerations in Carotid Arteries From the Plaque at Risk Study. Front Cardiovasc Med 2021;8:732646. [Crossref] [PubMed]
- Asami R, Tanaka T, Shimizu M, et al. Ultrasonic Vascular Vector Flow Mapping for 2-D Flow Estimation. Ultrasound Med Biol 2019;45:1663-74. [Crossref] [PubMed]
- Goudot G, Poree J, Pedreira O, et al. Wall Shear Stress Measurement by Ultrafast Vector Flow Imaging for Atherosclerotic Carotid Stenosis. Ultraschall Med 2021;42:297-305. [Crossref] [PubMed]
- Goudot G, Mirault T, Khider L, et al. Carotid Stiffness Assessment With Ultrafast Ultrasound Imaging in Case of Bicuspid Aortic Valve. Front Physiol 2019;10:1330. [Crossref] [PubMed]
- Urschel K, Tauchi M, Achenbach S, et al. Investigation of Wall Shear Stress in Cardiovascular Research and in Clinical Practice-From Bench to Bedside. Int J Mol Sci 2021;22:5635. [Crossref] [PubMed]
- He L, Cai Y, Feng Y, et al. Utility of vector flow mapping technology in quantitative assessment of carotid wall shear stress in hypertensive patients: A preliminary study. Front Cardiovasc Med 2022;9:967763. [Crossref] [PubMed]
- Samady H, Molony DS, Coskun AU, et al. Risk stratification of coronary plaques using physiologic characteristics by CCTA: Focus on shear stress. J Cardiovasc Comput Tomogr 2020;14:386-93. [Crossref] [PubMed]
- Ko S, Yang B, Cho JH, et al. Novel and facile criterion to assess the accuracy of WSS estimation by 4D flow MRI. Med Image Anal 2019;53:95-103. [Crossref] [PubMed]
- Zhang B, Gu J, Qian M, et al. Study of correlation between wall shear stress and elasticity in atherosclerotic carotid arteries. Biomed Eng Online 2018;17:5. [Crossref] [PubMed]
- Mazzi V, Gallo D, Calò K, et al. A Eulerian method to analyze wall shear stress fixed points and manifolds in cardiovascular flows. Biomech Model Mechanobiol 2020;19:1403-23. [Crossref] [PubMed]
- Saito K, Abe S, Kumamoto M, et al. Blood Flow Visualization and Wall Shear Stress Measurement of Carotid Arteries Using Vascular Vector Flow Mapping. Ultrasound Med Biol 2020;46:2692-9. [Crossref] [PubMed]
- Kamimura T, Aoki S, Nezu T, et al. Association between Carotid Wall Shear Stress-Based Vascular Vector Flow Mapping and Cerebral Small Vessel Disease. J Atheroscler Thromb 2023;30:1165-75. [Crossref] [PubMed]
- Heiss C, Rodriguez-Mateos A, Bapir M, et al. Flow-mediated dilation reference values for evaluation of endothelial function and cardiovascular health. Cardiovasc Res 2023;119:283-93. [Crossref] [PubMed]
- López-Otín C, Kroemer G. Hallmarks of Health. Cell 2021;184:33-63. [Crossref] [PubMed]
- Zhang G, Wang Z, Zhang S, et al. Age and anatomical location related hemodynamic changes assessed by 4D flow MRI in the carotid arteries of healthy adults. Eur J Radiol 2020;128:109035. [Crossref] [PubMed]
- Ebel S, Kühn A, Aggarwal A, et al. Quantitative normal values of helical flow, flow jets and wall shear stress of healthy volunteers in the ascending aorta. Eur Radiol 2022;32:8597-607. [Crossref] [PubMed]
- Krüger-Genge A, Blocki A, Franke RP, et al. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci 2019;20:4411. [Crossref] [PubMed]
- Scott MB, Huh H, van Ooij P, et al. Impact of age, sex, and global function on normal aortic hemodynamics. Magn Reson Med 2020;84:2088-102. [Crossref] [PubMed]
- Spencer EB, Sheafor DH, Hertzberg BS, et al. Nonstenotic internal carotid arteries: effects of age and blood pressure at the time of scanning on Doppler US velocity measurements. Radiology 2001;220:174-8. [Crossref] [PubMed]
- Jeon SJ, Kwak HS, Chung GH. Widening and Rotation of Carotid Artery with Age: Geometric Approach. J Stroke Cerebrovasc Dis 2018;27:865-70. [Crossref] [PubMed]
- Wen J, Liu C, Deng C. Research progress on the mechanism of aging of vascular endothelial cells and the intervention of traditional Chinese medicine: A review. Medicine (Baltimore) 2022;101:e32248. [Crossref] [PubMed]
- Méndez-Barbero N, Gutiérrez-Muñoz C, Blanco-Colio LM. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int J Mol Sci 2021;22:7284. [Crossref] [PubMed]
- Nezu T, Hosomi N, Aoki S, et al. Carotid Intima-Media Thickness for Atherosclerosis. J Atheroscler Thromb 2016;23:18-31. [Crossref] [PubMed]
- Koç AS, Sümbül HE. Age should be considered in cut-off values for increased carotid intima-media thickness. Turk Kardiyol Dern Ars 2019;47:301-11. [PubMed]
- Zhang B, Ma Y, Ding F. Evaluation of spatial distribution and characterization of wall shear stress in carotid sinus based on two-dimensional color Doppler imaging. Biomed Eng Online 2018;17:141. [Crossref] [PubMed]
- Zhang B, Gu J, Qian M, et al. Correlation between quantitative analysis of wall shear stress and intima-media thickness in atherosclerosis development in carotid arteries. Biomed Eng Online 2017;16:137. [Crossref] [PubMed]
- Wang S, Yin L, Luo A, et al. Measurement of left ventricular fluid dynamic parameters in healthy Chinese adults based on echocardiographic vector flow mapping. Chin Med J (Engl) 2023;136:616-8. [Crossref] [PubMed]






