
Safety, effectiveness, and complications of the first-in-human minimally invasive transthoracic ventricular septal defect closure using a bioabsorbable occluder: a cohort study with 12-month follow-up
Highlight box
Key findings
• Transesophageal echocardiography (TEE)-guided minimally invasive ventricular septal defect (VSD) occlusion using fully biodegradable occluders has the advantages of minimal trauma, high safety, and few complications, with satisfactory recent efficacy, and good prospects for clinical safety applications.
What is known and what is new?
• Percutaneous intervention or transthoracic minimally invasive occlusion has been considered a safe and effective method for treating VSDs.
• The closure devices used in both medical and surgical transcatheter interventions are commonly made of nickel-titanium alloy, which are non-degradable.
What is the implication, and what should change now?
• Minimally invasive occlusion treatment of perimembranous VSD with the application of a fully biodegradable occluder guided by TEE is a treatment method worthy of further clinical investigation.
Introduction
Ventricular septal defect (VSD) is one of the most common congenital heart diseases (1,2), accounting for about 20–30% of all congenital heart diseases. Percutaneous intervention or transthoracic minimally invasive occlusion has been considered a safe and effective method for treating VSDs (3-7), which not only effectively reduces the difficulty and duration of surgical operation and trauma, but also the need for circulation However, the closure devices used in both medical and surgical transcatheter interventions are commonly made of nickel-titanium alloy (8), which are non-degradable. Six months after implantation, the occluder surface is endothelialized and loses its value (9). An ideal occluder should gradually degrade after endothelialization until it disappears, avoiding the permanent presence of a foreign body in the body.
Almost all types of VSD occluders used in clinical practice at home and abroad are made or modified according to the principle of the Amplatzer occluders (10,11), although the structure and performance of domestic occluders have been further optimized and their performance is better than that of similar imported products. However, these occluders are made of nickel-titanium alloy wire as the main stent and lined with flow blocking membrane, and the metal foreign body will remain in the heart for life after implantation. An increasing number of clinical follow-up data shows that some children have intermediate and long-term complications related to the implanted metal foreign body (12-15), especially delayed fatal complete atrioventricular block (16-18). The safety of this technology has been questioned, and the U.S. Food and Drug Administration has not approved the Amplatzer nickel-titanium VSD occluder for clinical use. Various types of biodegradable VSD occluders are still in the animal experimental stage (9,19-22), and there are still many problems to be solved regarding whether they can be used in humans.
In Children’s Hospital of Nanjing Medical University, minimally invasive VSD closure using a completely biodegradable occluder was successfully performed between June 2019 and June 2022 under the guidance and monitoring of transesophageal echocardiography (TEE). This study aimed to investigate the clinical value and application of a fully degradable occluder, as well as the importance of using TEE for guidance and monitoring in a minimally invasive setup. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-361/rc).
Methods
Ethical consideration
This study adheres to the principles of the Declaration of Helsinki (as revised in 2013). This study obtained approval from the Ethics Committee of the Children’s Hospital of Nanjing Medical University (No. 2019-02-001-F01). Informed consent has been obtained from all patients’ parents or legal guardians.
Participants
This is a retrospective cohort study. We included 24 children with VSDs from Children’s Hospital of Nanjing Medical University, consisting of 10 males and 14 females, aged from 1 year and 6 months to 10 years and 3 months, with weights ranging from 10 to 58 kg. Prior to surgery, all patients underwent transthoracic echocardiography (TTE) examinations. Thirteen of them were diagnosed with perimembranous inflow tract VSDs, and eleven had VSDs accompanied by membranous tumor formation. Apart from five cases that presented with patent foramen ovale (PFO), the remaining 19 cases were identified as having simple VSDs. None of the children exhibited significant valve regurgitation. The treatment plan was approved after obtaining informed consent from the patients’ families.
The inclusion criteria for children in this clinical trial were as follows: (I) age ≥18 months, weight ≥10 kg; (II) a simple VSD with hemodynamic abnormalities and no other congenital malformations of the heart; (III) a perimembranous type of VSD; (IV) an effective shunt diameter of ≥3 and ≤10 mm; (V) defect distance ≥2 mm from the aortic valve without aortic right coronary valve prolapse into the VSD and aortic regurgitation; (VI) a VSD with membranous aneurysm formation and a normal right ventricular outflow tract; and (VII) no infective endocarditis, intracardiac bulge, or other infectious diseases.
Equipment
Philips IE 33 and Epic 7C color Doppler ultrasound diagnostic instruments (Philips, USA) were used. The TTE probes were S8-3 and S5-1 probes with frequencies from 1 to 8 MHz, and the TEE probes were S7-3t pediatric multiplanar probes with a diameter of 6 mm and frequencies from 4 to 7 MHz.
Occluder
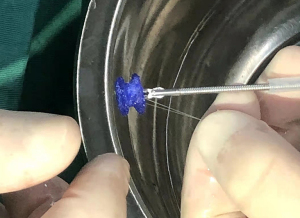
The fully biodegradable occluders (Figure 1) are manufactured by Shanghai Shape Memory Alloy Materials Co. Ltd. The occluder structure is made of a polydioxanone (PDO) filament woven and shaped, with a polylactic acid membrane, a PDO filament suture, and a flow-blocking membrane sewn inside the skeleton. All components are completely biodegradable. After implantation, the occluder serves as a temporary bridge to guide epithelialization along the occluder until the defect is covered with tissue. Then, the occluder gradually degrades until it completely disappears.
Surgical technique on TEE guidance
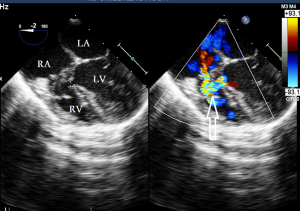
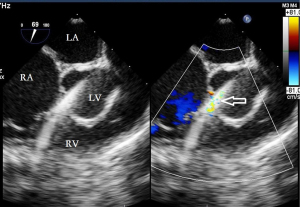
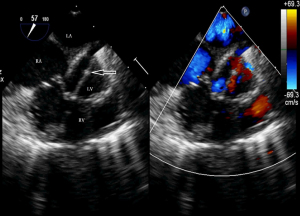
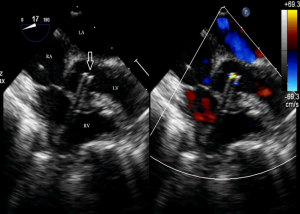
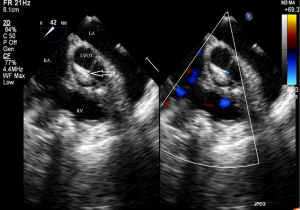
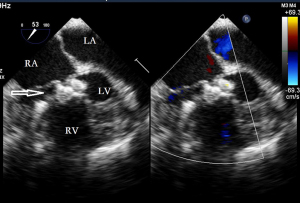
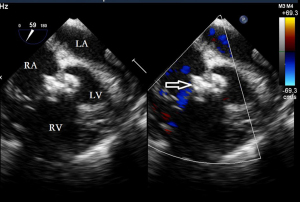
Before the surgery, the TEE probe is examined in 0°–180° sections to observe the VSD site and its relationship with the surrounding anatomy. The size of the VSD is measured, and a suitable occluder model for backup is selected. The patient is placed in a supine position, and a 1–2 cm incision is made on the lower part of the sternum. The lower part of the pericardium is cut longitudinally and suspended. The surface of the anterior wall of the right ventricle is lightly pressed with the index finger to avoid the coronary vessels. The point with the smallest shunt angle and the closest distance to the VSD is selected as the puncture point under TEE guidance. The position of the VSD is continuously monitored via TEE (Figure 2). A suture is made at the point directly opposite to the VSD so that the guiding wire can pass as vertically through the ventricular septum as possible. A 20G puncture needle is chosen, and the straight end of the guiding wire is inserted into the right ventricular cavity (≤1 cm) after suspension. The guiding wire enters the left ventricle through the shunt bundle of the VSD (Figure 3). The outer sheath of the puncture needle is withdrawn, and a 9F or 10F delivery sheath tube is introduced through the guiding wire in the VSD into the left ventricular cavity (Figure 4). This is confirmed on TEE as a sheath-like echo in the left ventricular cavity, the ‘double line sign’ (Figure 5). The inner core and guidewire are then removed. The loaded fully biodegradable VSD occluder is inserted into the delivery sheath, and the left disc of the occluder is delivered (Figure 6). The left ventricular disc is attached to the ventricular septum by pulling back the push rod, carefully avoiding injury to the aortic and mitral valves. If the VSD base with the combined membranous aneurysm is closer to the aortic valve, it is necessary to gently pull the left ventricular disc of the occluder into the aneurysm, and then release the occluder waist and right disc surface, bringing the right disc surface close to the right ventricular surface of the septum. Repeated push-pull experiments are performed under TEE guidance to confirm the correct occluder position without a residual shunt in color flow visualization (Figure 7). The occluder is then released (Figure 8). The safety cord is cut and withdrawn along one end. Monitoring continues for 15 minutes after occluder release to prevent occluder dislodgement and other complications.
Statistical analysis
SPSS version 24.0 (IBM Corp., Armonk, NY, USA) statistical software was used for the statistical analysis. Continuous variables were tested for normality and data conforming to a normal distribution were expressed as mean ± standard deviation. Two-sided one-way repeated measures ANOVA was used for comparison between pre- and post-treatment time points. A P value of <0.05 indicates a statistical difference.
Results
Results of the pre-operative TTE examination
In the 13 cases of VSD with perimembranous inflow tract (Figure 9), the effective shunt size was 3–5.1 mm, and the edge of the left ventricular surface defect was 2–5 mm from the aortic valve. In eleven cases of VSD with membranous aneurysm formation (Figure 10), the size of the base of the aneurysm was 5–10 mm, including three cases with one breach, five cases with double breaches, and three cases with multiple breaches (≥3 breaches), and the largest breach of the defect was 2.6–3.4 mm. The base of the membranous aneurysm was close to the aortic valve and less than 1 mm from the aortic valve in the remaining ten cases, except for one case in which the base of VSD was 3 mm from the aortic valve. All membranous aneurysm ruptures were >2 mm from the aortic valve. Five cases in this group had a combined PFO at the same time.
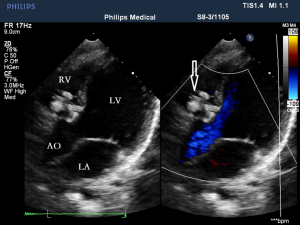
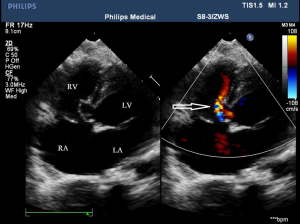
Intraoperative TEE measurements, occluder size selection, and evaluation of immediate occlusion effect
Intraoperative TEE measurements revealed that the effective shunt size of the VSDs ranged from 2.8–4.9 mm, with an average of 3.31±0.45 mm. This finding showed no statistically significant difference when compared with the TTE measurement method (P>0.05). The defects were located 2–6 mm from the aortic valve. Occluders used had diameters between 6–8 mm, averaging 6.54±0.66 mm. In 17 cases, occluders with a height of 2.8 mm were chosen, while in 7 cases, occluders with a height of 5 mm were selected. For simple perimembranous inflow tract VSDs, the chosen occluder model (waist diameter) was 2–3 mm larger than the actual measured diameter of the VSD. In cases of VSDs associated with membranous aneurysm formation, the selected occluder model (waist diameter) was 2–4 mm larger than the measured VSD diameter. All 24 minimally invasive occlusion surgeries were successful. TEE examinations showed that, except for one case with a small left-to-right shunt at the edge of the occluder (shunt bundle <1.5 mm), the occluders closely conformed to the edges of the VSDs in all other cases. The occluders did not interfere with the opening activity of the aortic valve and did not cause any aggravation of the mitral and tricuspid valves. There were no instances of occluder displacement, detachment, or thrombosis formation.
Postoperative TTE follow-up results
All 24 children with successful VSD closure were followed up during hospitalization (within 3 days after surgery), and the longest follow-up period was 18 months and the shortest was 12 months due to the sequence of surgery. Their baseline conditions are listed in Table 1. A 100% (24/24) follow-up rate was achieved within 12 months post-surgery.
Table 1
| No. | Gender | Age | Weight (kg) | Clinical symptoms | Defect type | Defect size (mm) | Occluder size (mm) |
|---|---|---|---|---|---|---|---|
| 1 | Female | 3 y 8 m | 13.5 | Cardiac murmur | PIT | 3.8 | 6 |
| 2 | Female | 5 y 1 m | 18.5 | Cardiac murmur | VMA | 3.2 | 6 |
| 3 | Female | 5 y 0 m | 13 | Cardiac murmur | PIT | 3.6 | 6 |
| 4 | Male | 3 y 4 m | 14 | Cardiac murmur | VMA | 2.8 | 6 |
| 5 | Female | 2 y 8 m | 13 | Cardiac murmur | VMA | 3 | 7 |
| 6 | Female | 3 y 1 m | 13 | Cardiac murmur | PIT | 4.9 | 7 |
| 7 | Male | 1 y 6 m | 10.5 | Cardiac murmur, growth retardation | PIT | 3 | 6 |
| 8 | Female | 2 y 5 m | 12.5 | Cardiac murmur | VMA | 2.6 | 6 |
| 9 | Female | 2 y 3 m | 11 | Cardiac murmur | VMA | 4.5 | 8 |
| 10 | Female | 3 y 4 m | 15 | Cardiac murmur | PIT | 4 | 7 |
| 11 | Male | 5 y 8 m | 18.5 | Cardiac murmur | PIT | 3.6 | 6 |
| 12 | Female | 3 y 2 m | 13 | Cardiac murmur | PIT | 3.7 | 6 |
| 13 | Male | 2 y 0 m | 10 | Cardiac murmur | PIT | 4.1 | 8 |
| 14 | Male | 6 y 8 m | 12.6 | Cardiac murmur | VMA | 4.2 | 7 |
| 15 | Male | 10 y 3 m | 33 | Cardiac murmur, chest tightness, palpitations | PIT | 4 | 6 |
| 16 | Male | 3 y 1 m | 13 | Cardiac murmur | VMA | 3.4 | 6 |
| 17 | Female | 6 y 4 m | 22 | Cardiac murmur | PIT | 4 | 7 |
| 18 | Female | 9 y 9 m | 58 | Cardiac murmur, chest tightness, palpitations | VMA | 2.8 | 6 |
| 19 | Male | 3 y 9 m | 17 | Cardiac murmur | PIT | 3.4 | 6 |
| 20 | Female | 4 y 10 m | 19.5 | Cardiac murmur | VMA | 4.2 | 6 |
| 21 | Male | 3 y 10 m | 16.5 | Cardiac murmur | PIT | 3.5 | 6 |
| 22 | Female | 1 y 11 m | 12 | Cardiac murmur, growth retardation | PIT | 4.7 | 6 |
| 23 | Male | 3 y 7 m | 21 | Cardiac murmur | VMA | 4.6 | 6 |
| 24 | Female | 1 y 8 m | 11 | Cardiac murmur, excessive sweating | VMA | 3.1 | 6 |
m, month; PIT, perimembranous inflow tract; VMA, ventricular septal defect with membranous aneurysm; y, year; VSD, ventricular septal defect.
In 23 cases, the position of the occluder was normal without shunt or other complications (Figure 11). Only in one case, there was still a small residual shunt of 1 mm (Figure 12), which was considered a small shunt, and no complications appeared during the follow-up period. The position and morphology of the occluders were observed using multiple views on echocardiogram (short-axis view of the aorta, four-chamber cardiac view, and five-chamber cardiac view). The size of the left and right discs was accurately measured to assess the degradation of the occluders. There was no difference in the sizes of the left and right disc surfaces of the occluders measured in each section at postoperative month 1 and 3 (Figure 13) compared with postoperative day 3 (P>0.05). The size of the left and right disc surfaces of the occluder at postoperative month 6 and 12 (Figure 14) was significantly smaller than that at postoperative day 3, with statistically significant differences (P<0.05) (Table 2).
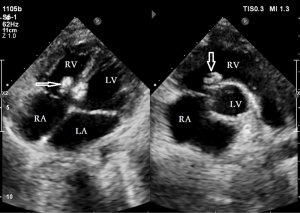
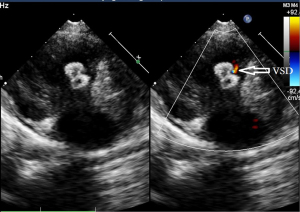
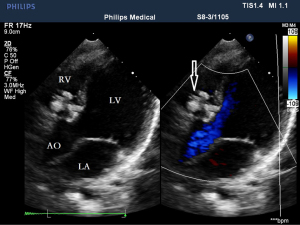
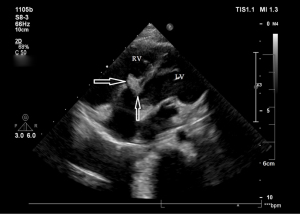
Table 2
| Group | Total population | Short-axis view of the aorta (mm) | Four-chamber cardiac view (mm) | Five-chamber cardiac view (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Left disc | Right disc | Left disc | Right disc | Left disc | Right disc | ||||
| Postoperative day 3 | 24 | 10.4±1.3 | 10.2±1.2 | 10.5±1.4 | 10.4±1.4 | 10.5±1.4 | 10.4±1.4 | ||
| Postoperative month 1 | 24 | 10.2±1.2 | 10.1±1.2 | 10.4±1.4 | 10.3±1.4 | 10.3±1.4 | 10.3±1.5 | ||
| Postoperative month 3 | 24 | 10.0±1.4 | 10.0±1.5 | 10.0±1.4 | 10.1±1.4 | 9.9±1.5* | 10.1±1.4 | ||
| Postoperative month 6 | 24 | 8.2±1.0** | 8.7±1.0** | 8.1±1.0** | 8.5±1.0** | 8.1±0.9** | 8.5±1.1** | ||
| Postoperative month 12 | 24 | 6.5±1.0** | 7.0±1.1** | 6.4±1.0** | 7.0±1.1** | 6.3±0.9** | 6.8±1.2** | ||
Data are presented as mean ± standard deviation. Data at postoperative month 1, 3, 6, and 12 were compared with postoperative day 3, *, P<0.05; **, P<0.01.
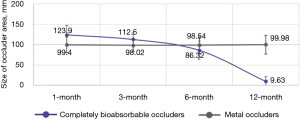
We conducted a comparative study with a control group of 30 cases where traditional nickel-titanium alloy occluders were used in cardiovascular interventional procedures. The occluder area data were analyzed using echocardiographic imaging. In the control group, there were no significant differences in the size of the occluder area from 1 to 12 months post-surgery (P=0.14). In the experimental group, there were no significant differences in the occluder area size between 1 and 3 months post-surgery (P=0.30) (Figure 15). However, a significant reduction in the occluder area was observed between 3 and 6 months post-surgery (P=0.003), and a marked decrease in the occluder area was noted from 6 to 12 months post-surgery (P=0.02) (Figure 15).
Preoperatively, the left atrial, ventricular, and pulmonary artery pathways of all children were larger than in the age-matched general population. The pathways measured in the postoperative period were slightly smaller than those before surgery (P>0.05), In the postoperative periods of 3, 6, and 12 months, the pathways decreased significantly compared with those before surgery and returned to normal levels (Table 3).
Table 3
| Group | Total population | LASd (mm) | LVDd (mm) | MPA (mm) |
|---|---|---|---|---|
| Pre-operation | 24 | 38.2±3.8 | 24.4±2.5 | 17.3±2.5 |
| Postoperative month 1 | 24 | 37.3±3.6 | 23.6±2.5 | 16.9±2.4 |
| Postoperative month 3 | 24 | 34.9±2.1* | 21.2±2.2* | 15.9±2.1* |
| Postoperative month 6 | 24 | 34.4±2.9* | 20.8±2.1* | 15.6±2.0* |
| Postoperative month 12 | 24 | 34.8±2.6* | 21.2±1.7* | 15.6±1.8* |
Data are presented as mean ± standard deviation. Data at month 1, 3, 6 and 12 were compared with pre-operative, *, P<0.05. LVDd, left ventricular diastolic diameter; LASd, left atrial systolic diameter; MPA, main pulmonary artery.
Discussion
The fully biodegradable VSD occluder used in this study was produced by Shanghai Shape Memory Alloy Materials Co. Ltd., and was the first to be approved by the relevant authorities and used in human clinical trials by Children’s Hospital of Nanjing Medical University.
Since the fully biodegradable VSD occluder could not be visualized under digital subtraction angiography (DSA), all cases in this group were completed with minimally invasive transthoracic blocking under TEE guidance and monitoring. Therefore, TEE plays a pivotal role in the preoperative measurement of VSD size, understanding the VSD site and its relationship with the surrounding anatomy, the intraoperative localization of the puncture site for the operator, selection of occluder type, the guidance of occluder release, and the evaluation of immediate postoperative efficacy, which are all directly related to the success or failure of the operation.
TEE before the implementation of blocking
Multiple views are required for further screening of children before the start of surgery, particularly the four-chamber cardiac view and the five-chamber cardiac view at 0° in the middle esophagus, the short-axis view of the aorta at 30°–60°, and the long-axis view of the left ventricle at 110°–135°. Previous studies have demonstrated that TEE was safe and effective in guiding surgery for children with VSD (23,24), but whether TEE shows better performance than TTE in minimally invasive transthoracic VSD closure surgery remains unclear. In the present study, the measurements made by TEE and TTE were similar in terms of defect type and size in 24 children. However, TEE was significantly better than TTE in observing the relationship between defects and surrounding structures, especially in measuring the distance between the defect and the aortic valve. In this group, there was originally one case in which VSD measured by TTE was 2.2 mm away from the aortic valve, but the distance measured by TEE was only 1 mm away from the aortic valve during surgery. We had to change to surgical repair under extracorporeal circulation. During the surgical exploration, the defect was found to be close to the right aortic valve with a distance <1 mm. In addition, preoperative TEE is most important to determine the size of the VSD accurately and select the appropriate occluder and delivery sheath. Although the biodegradable occluder was used in our case series, it is still possible for an oversized occluder to result in atrioventricular block. Therefore, it is advisable to select an occluder model (waist diameter) that is 2–3 mm larger than the measured VSD diameter. The TEE-guided transthoracic minimally invasive blocking technique is easy to repeat, and if a residual shunt exists, the diameter should be increased by only 1 mm each time until the shunt disappears, which can reduce the possibility of using a larger occluder.
TEE intraoperative application
In selecting the puncture point for VSD cases, especially those combined with a membranous tumor, accurate positioning is crucial, using views like the four-chamber heart and mid-esophagus short-axis sections for the smallest angle and proximity to the VSD. In our study, initial misalignments in two cases necessitated repositioning for successful guidewire entry into the left ventricle. Post-entry, it is vital to direct the guidewire towards the left ventricular cavity, avoiding the ascending aorta to prevent potential valve or aortic damage. When releasing the biodegradable occluder’s left disc surface, positioning is key to prevent damage to valve structures, with careful monitoring of valve function post-deployment. Our experience showed that in cases where the occluder’s morphology appeared abnormal or inadequately opened, retraction, and subsequent re-release were necessary. In one particular case with multiple distant shunts, a small residual shunt was deemed hemodynamically insignificant, leading to the final release of the occluder. In one case of VSD combined with a membranous aneurysm in this group, there were multiple shunts in the defect, and the shunts were far apart from each other. A small shunt of 1.2 mm still existed after two consecutive models of occluders were replaced, but the flow velocity of the shunt was less than 3 m/s. The occluder was released, considering that there would be no significant hemodynamic effect. After it was established that there was no shunt at the ventricular level, each valve was intact, and that the occluder opened adequately, the operator was prompted to withdraw the sheath, cut the safety cord, and gently withdraw it along one end. Continued monitoring was required for more than 15 min after release to prevent dislodgement of the occluder and other complications.
As the first clinical study of using biodegradable VSD occluders in human subjects, the application of intraoperative TEE in children with VSD requires further accumulation of experience and research, although small sample size is the limitation of this study. To the best of our knowledge, no ultrasound equipment manufacturer produces probes for pediatric TEE, resulting in the lack of richer spatial information from multiple planes and the increase in complexity of intraoperative positioning. Furthermore, fully biodegradable VSD occluders are symmetrical, imposing stringent selection criteria for patient enrolment. VSDs with defect edges less than 2 mm from the aortic valve are ineligible for occlusion treatment, limiting widespread implementation. Moreover, most patients in our group were followed for about 1 year. Although the short-term degradation effects were significant, the long-term efficacy of this treatment still requires more extensive clinical research and longer follow-up and observations.
Conclusions
In conclusion, minimally invasive occlusion treatment of perimembranous VSD with the application of a fully biodegradable occluder guided by TEE is a treatment method worthy of further clinical investigation.
Acknowledgments
We thank Dr. Kang Liu for the statistics and collation of data. We also thank Ultrasound Department Children’s Hospital affiliated to Nanjing Medical University for these cases.
Funding: This study was supported by grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-361/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-361/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-361/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-361/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study adheres to the principles of the Declaration of Helsinki (as revised in 2013). This study obtained approval from the Ethics Committee of the Children’s Hospital of Nanjing Medical University (No. 2019-02-001-F01). Informed consent has been obtained from all patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bendriss L, Sedrati M, Haddour L, et al. Ventricular septal defects: anatomic, clinical, therapeutic and prognostic aspects. 44 cases. Presse Med 2006;35:593-7. [Crossref] [PubMed]
- Minette MS, Sahn DJ. Ventricular septal defects. Circulation 2006;114:2190-7. [Crossref] [PubMed]
- Aluri M, Alfares F, Sandhu SK. The role of transesophageal echocardiography in device closure of perimembranous ventricular septal defects with the hybrid approach. J Card Surg 2022;37:1180-1. [Crossref] [PubMed]
- Bai W, An Q, Tang H. Application of transesophageal echocardiography in minimally invasive surgical closure of ventricular septal defects. Tex Heart Inst J 2012;39:211-4. [PubMed]
- Chen TY, Ju YT, Wei YJ, et al. Clinical Experience of Transcatheter Closure for Ventricular Septal Defects in Children Weighing under 15 kg. Acta Cardiol Sin 2021;37:618-24. [PubMed]
- Jiang-Shan H, Kai-Peng S, Ning X, et al. Transthoracic closure of ventricular septal defects guided by transesophageal echocardiography. Turk Gogus Kalp Damar Cerrahisi Derg 2020;28:250-6. [Crossref] [PubMed]
- Zhang GC, Chen Q, Chen LW, et al. Transthoracic echocardiographic guidance of minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: initial experience. Eur Heart J Cardiovasc Imaging 2012;13:739-44. [Crossref] [PubMed]
- Yılmazer MM, Meşe T, Güven B, et al. Recent experience with transcatheter closure of perimembranous ventricular septal defects using duct occluders. Cardiol Young 2016;26:1452-3. [Crossref] [PubMed]
- Bu H, Yang Y, Hu S, et al. A novel biodegradable occluder for the closure of ventricular septal defects: immediate and medium-term results in a canine model. Interact Cardiovasc Thorac Surg 2019;29:783-92. [Crossref] [PubMed]
- Marinakis A, Vydt T, Dens J, et al. Percutaneous transcatheter ventricular septal defect closure in adults with Amplatzer septal occluders. Acta Cardiol 2007;62:391-5. [Crossref] [PubMed]
- Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defect with Amplatzer septal occluders. Am J Cardiol 2005;96:52L-8L. [Crossref] [PubMed]
- Butera G, Carminati M, Chessa M, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol 2007;50:1189-95. [Crossref] [PubMed]
- Ghaderian M, Merajie M, Mortezaeian H, et al. Mid-term Follow-up of the Transcatheter Closure of Perimembranous Ventricular Septal Defects in Children Using the Amplatzer. J Tehran Heart Cent 2015;10:182-7. [PubMed]
- Mandal KD, Su D, Pang Y. Long-Term Outcome of Transcatheter Device Closure of Perimembranous Ventricular Septal Defects. Front Pediatr 2018;6:128. [Crossref] [PubMed]
- Chessa M, Butera G, Negura D, et al. Transcatheter closure of congenital ventricular septal defects in adult: mid-term results and complications. Int J Cardiol 2009;133:70-3. [Crossref] [PubMed]
- Zhou T, Shen XQ, Zhou SH, et al. Atrioventricular block: a serious complication in and after transcatheter closure of perimembranous ventricular septal defects. Clin Cardiol 2008;31:368-71. [Crossref] [PubMed]
- Xie L, Zhang H, Zhang R, et al. Management of Late-Onset Complete Atrioventricular Block Post Transcatheter Closure of Perimembranous Ventricular Septal Defects. Front Pediatr 2020;7:545. [Crossref] [PubMed]
- Zhu YF, Huang XM, Cao J, et al. Animal experimental study of the fully biodegradable atrial septal defect (ASD) occluder. J Biomed Biotechnol 2012;2012:735989. [Crossref] [PubMed]
- Huang XM, Zhu YF, Cao J, et al. Development and preclinical evaluation of a biodegradable ventricular septal defect occluder. Catheter Cardiovasc Interv 2013;81:324-30. [Crossref] [PubMed]
- Wu W, Yip J, Tang YD, et al. A novel biodegradable septal defect occluder: the “Chinese Lantern” design, proof of concept. Innovations (Phila) 2011;6:221-30. [Crossref] [PubMed]
- Duong-Hong D, Tang YD, Wu W, et al. Fully biodegradable septal defect occluder-a double umbrella design. Catheter Cardiovasc Interv 2010;76:711-8. [Crossref] [PubMed]
- Jux C, Bertram H, Wohlsein P, et al. Interventional atrial septal defect closure using a totally bioresorbable occluder matrix: development and preclinical evaluation of the BioSTAR device. J Am Coll Cardiol 2006;48:161-9. [Crossref] [PubMed]
- Ye JJ, Jiang GP, Zhang ZW, et al. Transesophageal echocardiography-guided hybrid therapy for ventricular septal defects in children. Zhejiang Da Xue Xue Bao Yi Xue Ban 2009;38:311-4. [PubMed]
- Bu H, Yang Y, Wu Q, et al. Results of two different echocardiography-guided approaches to closure of perimembranous ventricular septal defects. Eur J Cardiothorac Surg 2021;59:1304-11. [Crossref] [PubMed]


