Catheter directed interventions for acute deep vein thrombosis
Introduction
Venous thromboembolism (VTE) is the third leading vascular disease after acute myocardial infarction and stroke (1). VTE, which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), contributes to a yearly economic burden of $7–10 billion in the USA (2).
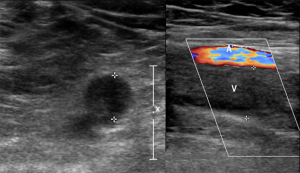
Clinical presentations of DVT of the legs include swelling or pitting edema, erythema, pain, and presence of collateral veins (3). Duplex ultrasound is the recommended modality for the diagnosis of DVT (4).
A thrombus is typically classified as acute if it has formed within 2–4 weeks of diagnosis. Duplex ultrasound characteristic of acute DVT include a smooth homogenous thrombus appearance, soft or spongy texture, hypoechogenicity, poor wall attachment or free floating, surrounding dilated vessel size and absence of collaterals with no flow noted in the vein on spectral Doppler (4).
Approximately 25–50% of patients with lower extremity DVT develop post-thrombotic syndrome (PTS), despite anticoagulation therapy. Symptoms of PTS include swelling, pain, heaviness, and venous claudication which can worsen with dependency of the leg. The primary goal of catheter directed interventions for acute DVT is to prevent or reduce the likelihood of developing PTS (5).
A number of different treatment strategies are available for patients diagnosed with DVT. This review paper will discuss the different catheter directed interventions for acute DVT.
Anticoagulation therapy
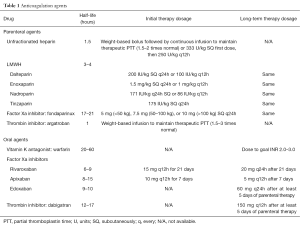
Anticoagulation is the mainstay for the treatment of patients with VTE (3). In addition, such agents also play an important adjuvant role in catheter directed interventions for acute DVT. Table 1 demonstrates the currently available pharmacologic agents and Table 2 illustrates the recommend duration of anticoagulation therapy for patients diagnosed with DVT (6).
Full table
Catheter-directed pharmacologic thrombolysis (CDPT)
Indications and contraindications
CDPT may be performed emergently, urgently, or electively. CDPT should be performed emergently in the setting of phlegmasia cerulea dolens, where severe edema from venous obstruction threatens limb viability (5). Urgent CDPT should be considered for inferior vena cava (IVC) thrombosis, especially when patients are at risk for renal failure and Budd-Chiari syndrome due to renal or hepatic vein involvement, respectively. Patients at high risk for bleeding, however, may be more appropriately managed with mechanical or aspiration thrombectomy techniques without the use of thrombolytic agents.
Elective CDPT may be pursued for acute iliofemoral (iliac and/or common femoral vein involvement) DVT in symptomatic patients in order to prevent PTS (5). Since many symptomatic patients with acute femoropopliteal DVT improve with anticoagulation and compression stockings, CDPT for prevention of PTS should be reserved for femoropopliteal DVT patients with severe symptoms or worsening thrombosis despite anticoagulation. Absolute contraindications to catheter-directed pharmacologic thrombolysis include active internal bleeding, cerebral infraction, neurological and eye procedures or head trauma within 3 months, and known intracranial tumor, aneurysm, or vascular malformation. Relative contraindications include major trauma, surgery or obstetrical delivery within 10 days, uncontrolled hypertension (systolic >180 mmHg or diastolic >110 mmHg), gastrointestinal bleeding within 3 months, pregnancy, infected venous thrombus, severe renal or liver disease, hemorrhagic diabetic retinopathy, and bleeding diathesis. Known severe allergy to iodinated contrast agent, anticoagulant, or thrombolytic agent may also be considered a relative contraindication, unless the patient can be premeditated.
Procedural technique
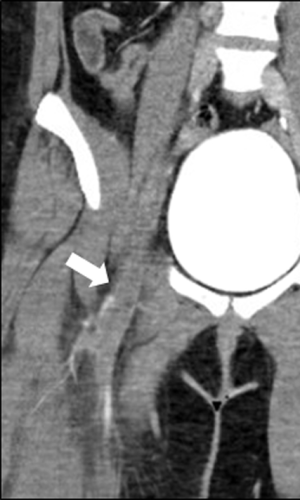
Pre-procedure imaging is reviewed to determine the appropriate access site, in order to maximize the infusion of thrombolytic agents throughout the extent of the thrombus (Figures 1,2). Access options include the common femoral, femoral, internal jugular, popliteal or posterior tibial veins. Moderate sedation or general anesthesia is typically employed throughout the procedure.
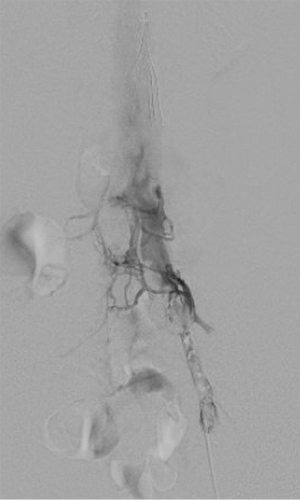
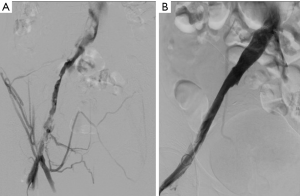
Following access, an angiographic catheter (5 Fr Kumpe or similar) and hydrophilic wire are used to traverse the thrombus. If venograms of the thrombosed segments are obtained, low-volume hand injections are preferred to avoid disrupting the thrombus (Figure 3). A venogram is performed through the catheter to confirm patency of the outflow vein, which is typically the IVC or an iliac vein. The angiographic catheter is then exchanged for a multi-side hole infusion catheter, the side holes of which should traverse the entire extent of the thrombosed venous segment. A catheter tip-occluding wire is then placed, and the infusion catheter and sheath are connected to their appropriate infusion pumps to initiate thrombolysis (7).
One mg per hour of recombinant tissue plasminogen activator (tPA) is infused through the catheter overnight (12–24 hours). If two catheters are used, 0.5 mg/hr is administered through each catheter. In addition, 500 units of unfractionated heparin are administered through the vascular sheath per hour; this is divided accordingly, as above, if multiple sheaths are used. Patients are monitored in an ICU setting during CDPT to allow for frequent vital signs, neurological and limb assessments. Aggressive blood pressure control is advised, maintaining systolic pressures <180 mmHg (ideally <160 mmHg) and diastolic pressures <110 mmHg. New arterial or central venous punctures should be avoided. CBC, PT, and partial thromboplastin time (PTT) are checked every 6 hours during CDPT. The PTT should remain sub-therapeutic (PTT <50 sec) as tPA is administered. Fibrinogen levels may be followed every 6 hours, although high-level evidence supporting the use of this parameter is currently lacking. The infusion dose may be reduced by one half if fibrinogen falls below 150 mg/dL and the infusion may be discontinued if the fibrinogen level is below 100 mg/dL, in order to reduce risk of bleeding.
A recent addition to the infusion catheter armamentarium is the ultrasound-accelerated thrombolysis (UAT) catheter (EKOS Corporation, Bothell, WA, USA). After placing the 5.4 Fr EKOS catheter of appropriate treatment length, the guidewire is removed and replaced with a core wire containing multiple, evenly-spaced transducer elements (Figure 4). High frequency ultrasound energy is delivered to the thrombus during thrombolytic infusion. Of note, the EKOS catheter also requires a normal saline infusion (serving as a catheter coolant) through a second port, usually run at 35 mL/hr.
After overnight thrombolytic therapy, the patient returns to the angiography suite the following day. The infusion catheter is removed and venography is performed to assess the results (Figure 5). In some cases, incomplete clot lysis will be evident, and balloon maceration (Figure 6) or other adjunctive therapy such as mechanical or aspiration thrombectomy may be necessary to achieve the desired result (Figure 7). Upsizing the vascular sheath may be necessary in this setting. Evaluation for venous obstruction should be made during venography, as inadequate outflow even after complete clot lysis may predispose to re-thrombosis. Balloon angioplasty or venous stenting may be required. Occasionally, CDPT may be repeated for an additional night if desired results are not achieved.
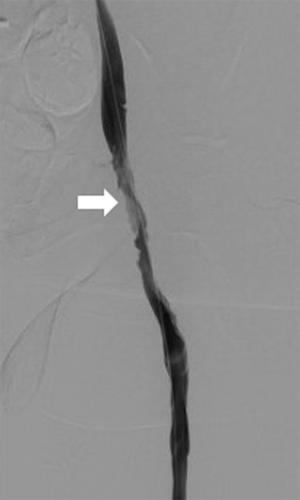
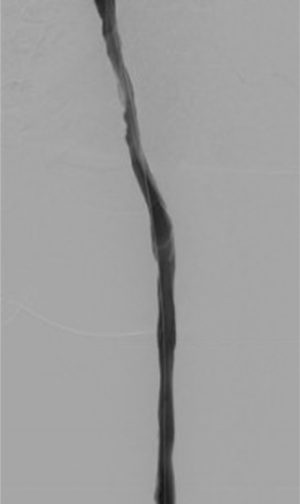
After removing the vascular access sheath(s), gentle compression is used for hemostasis. Bed rest is continued for an additional 2–4 hours, but full anticoagulation is re-initiated immediately. Early ambulation and use of compression stockings is recommended, as both have been shown to be effective adjunctive measures in preventing PTS (8-10).
Outcomes
Vedantham et al. (5) demonstrated venographic success, typically defined as a greater than 50% reduction in thrombus burden, in greater than 90% of patients. In the same study, resolution of symptoms and venographic success occurred in approximately 85% of patients undergoing CDPT. Twenty percent of patients suffered re-thrombosis within 30 days of CDPT, underscoring the importance of adjunctive treatments for associated venous obstructive lesions. With regard to long-term results, increasing volumes of residual thrombus are associated with higher rates of PTS, and complete clearance of thrombus may prevent PTS entirely in patients with iliofemoral DVT (11). In a recent randomized controlled trial, CDPT was associated with an absolute risk reduction of 14% for PTS at 24 months when compared with anticoagulation alone (12).
UAT has been shown to be both safe and efficacious for the treatment of DVT (13,14). Its benefit over standard CDPT through a multi-side hole infusion catheter remains controversial, however. A retrospective analysis of 83 patients failed to demonstrate a clinical benefit of UAT over standard CDPT (15). More recently, a randomized controlled trial of 48 patients concluded that UAT did not result in improved clot resolution in patients with DVT (16). Further trials are necessary to elucidate whether any short or long-term benefit can be derived from UAT.
Complications
Major complications from CDPT occur in approximately 9% of cases (5). The most relevant major complications identified in a pooled analysis of more than 1,000 patients included major hemorrhage (8.3%), symptomatic PE (0.9%), death (0.3%), and intracranial hemorrhage (0.2%) (5).
Mechanical and pharmacomechanical thrombectomy
Indications and contraindications
Percutaneous mechanical thrombectomy refers to clot removal using one of several endovascular mechanical devices and is commonly used as part of a pharmacomechanical thrombectomy. In this process mechanical thrombectomy is combined with direct infusion of lytic agents into the thrombus in an attempt to rapidly clear the thrombus in a single session. It can also be used as an adjunct following CDPT if residual thrombus persists. If a patient has a contraindication to fibrinolysis, mechanical thrombectomy can be used as a standalone therapy without the administration of lytic medications.
Since there is mechanical manipulation of thrombus during mechanical thrombectomy, there is an accepted risk for PE. As such, poor cardiopulmonary reserve and inability to tolerate PE represents a potential relative contraindication to mechanical thrombectomy. Temporary IVC filtration can be considered in such patients (17).
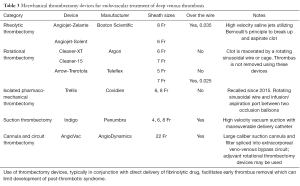
Available devices
Several devices with various mechanisms of clot maceration with or without clot removal have been developed over the last two decades. Currently available and historically important devices are list in Table 3.
Full table
Procedural technique
Following venous access, the thrombosed segment is infused with 5–25 mg of tPA, depending on thrombus burden. This can be performed through a side-hole infusion catheter that is lodged within the clot, through an end-hole catheter that is pulled back through the clot, or through an AngioJet (Boston Scientific, Marlborough, MA, USA) thrombectomy catheter set to power pulse mode (Figure 8). The tPA is allowed to dwell within the thrombosed venous segment for 20–45 minutes. A mechanical thrombectomy device is then used to clear the thrombus. If working within a large caliber venous segment such as the iliac veins or IVC, accessing eccentric thrombus adherent to the vein wall can be challenging. Inserting the thrombectomy device through an angled sheath or guiding catheter can help achieve wall apposition. Adjuvant angioplasty and/or stenting are necessary if thrombus removal unmasks an anatomical lesion (such as May-Thurner syndrome) that incited the DVT.
Outcomes
There is a paucity of prospective randomized data from which to draw conclusions about using thrombectomy or pharmacomechanical thrombectomy over other acute DVT treatment options. The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial is a recently completed prospective randomized trial, which aims to determine if adjunctive pharmacomechanical catheter directed thrombolysis can prevent PTS in patients with symptomatic DVT compared with standard DVT therapy alone (18).
The Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths (PEARL) registry was a prospective multicenter study performed in the US and Europe and included 329 patients with lower extremity DVT. Thirty-nine percent of patients had 100% clot resolution with either AngioJet alone or with AngioJet and power pulse lytic therapy such that CDPT was not needed—this resulted in procedure times of less than 6 hours. Even when patients did require CDPT after AngioJet, the tPA infusion times were significantly decreased for the AngioJet group (17 vs. 48–58 hours). There was also significant improvement in quality of life at 3, 6 and 12 months (19).
More recent reports have shown that the Cleaner thrombectomy device (Argon Medical Devices, Plano, Texas, USA) (20) and the Arrow-Trerotola device (Arrow, Reading, PA, USA) (21) are effective in treating acute DVT. Similarly, small series and retrospective studies comparing pharmacomechanical thrombectomy to CDPT suggest that similar efficacy can be achieved by pharmacomechanical thrombectomy without the costs of ICU monitoring and decreased hospital stays (22) and that PTS may be decreased with pharmacomechanical thrombectomy at 1 year (23).
Recommendations of an expert panel from the Society for Vascular Surgery and the American Venous Forum include early thrombus removal for acute DVT, particularly if the limb is threatened. Pharmacomechanical treatment was preferred over CDPT alone if the resources were available (24).
Complications
Depending on the mechanism of the thrombectomy device, clot may either be macerated and removed or simply macerated. For devices that only macerate thrombus there is an increased risk for embolization of thrombus that can result in PE (17). The AngioJet system both macerates and removes clot, but also results in hemolysis of red blood cells, which can lead to hemoglobinuria (which can manifests as hematuria), elevated serum potassium level, and acute kidney injury (25). Bradyarrhythmias are also a specific complication to AngioJet, although the mechanism is poorly understood (26). This complication occurs with prolonged thrombectomy times when used near the heart and usually resolves on its own with cessation of device use. While early clot removal has been shown to help limit damage to venous valvular function, aggressive mechanical thrombectomy may however result in valvular damage (27).
Aspiration thrombectomy
Indications and contraindications
Aspiration thrombectomy refers to the mechanical removal of thrombus from the body by the application of suction through a catheter or other device. Manual aspiration thrombectomy is the simplest form of aspiration thrombectomy. A guiding catheter is inserted through a vascular sheath to the site of thrombus. Negative pressure is then applied via a syringe attached to the guiding catheter as the thrombus is engaged. Aspiration of thrombus is aided by imparting a gentle to-and-fro and rotational motion on the guiding catheter (28) or by continuously withdrawing the catheter under suction (29,30). This process is repeated until thrombus has been cleared. Overall initial technical success rates of 96–100% (28-30) have been reported. Zhu et al. (28) and Oğuzkurt et al. (30) have reported primary patency rates of 77% and 96% and secondary patency rates of 93% and 100%, respectively. Complications of symptomatic PE and contralateral DVT within 3 months of intervention were also described (28,30).
Vacuum-assisted thrombectomy is very similar to manual aspiration thrombectomy. The key difference is that suction is applied to the catheter via an external vacuum pump attached to the proprietary catheter via the side port of a Tuohy-Borst adapter. The Penumbra Indigo (Penumbra Inc., Alameda, CA, USA) series of aspiration thrombectomy catheters is an example of this device, available in 3.4–8 Fr sizes (Figure 9). The end port of the Tuohy-Borst adapter accommodates a “separator” device. The “separator” is used to clear the catheter when thrombus obstructs the lumen. Until recently, the use of the Penumbra Indigo system was limited to the cerebral and coronary circulation. Consequently, there is no available data demonstrating the device’s safety and efficacy in the treatment of acute DVT.
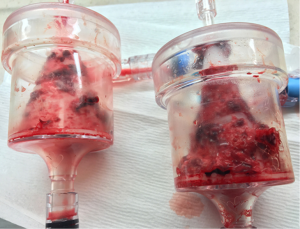
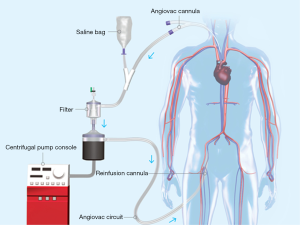
Vacuum-assisted aspiration thrombectomy utilizes an extracorporeal veno-venous circuit which places a filter in series with an aspiration cannula and a reinfusion cannula. This technique allows for prolonged aspiration thrombectomy to proceed without the risk of blood loss. The AngioVac Cannula and Circuit (AngioDynamics Inc., Latham, NY, USA) is an example of this device. Suction is applied to a large bore (22 Fr) cannula (Figure 10) with a balloon-expandable tip that has been inserted through a 26 French sheath (Gore DrySeal Sheath, W. L. Gore & Associates, Flagstaff, AZ, USA) and advanced near to the site of thrombus. Blood and any thrombus removed is drawn through a filtered extracorporeal circuit which only returns the blood to the patient via a 16–19 Fr reinfusion cannula placed at a separate venous access site. The thrombus is trapped in the filter (Figure 11). A centrifugal pump capable of creating flows up to 5 liters/minute drives the veno-venous circuit and is operated by a perfusion team. Due to the large caliber of the suction and reinfusion cannulas, access sites are limited to the internal jugular and common femoral veins (Figure 12).

The AngioVac cannula typically cannot be advanced caudal to the common femoral vein, making clearance of thrombus in the lower extremities difficult without adjunctive techniques to dislodge thrombus into the cannula. As such, this technique is best applied to thrombus in the superior or IVC and iliac veins. The published experience of the use of the AngioVac cannula for the treatment of DVT is limited to case series and reports comprising in total <40 patients. While there is heterogeneity in the method of outcome reporting in these reports (31-35), the various series report very high technical success rates. For example Moriarty et al. (32) reported a 100% technical success rate (n=10) for the treatment of caval thrombi, where technical success was defined as the restoration of antegrade flow in caval lesions.
Given the novel nature of these aspiration thrombectomy devices, further trials are needed to demonstrate their safety, efficacy and comparative effectiveness in the treatment of patients with acute DVT.
May-Thurner syndrome
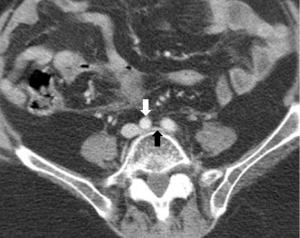
May-Thurner syndrome, also known as iliac vein compression syndrome, is a common cause of iliac vein obstruction of nonthrombotic etiology. First described in 1957, it is due to compression of the left common iliac vein as it traverses behind the right common iliac artery and in front of the vertebral bodies of the lumbar spine (36), (Figure 13). The role of endovascular intervention for patients with iliac venous outflow obstruction leading to thrombotic complications is well described (37), and more recent work suggests benefits in treating symptomatic patients without thrombosis as well (38,39). Typically, this involves stent placement to ameliorate mechanical obstruction produced by compression and fibrous spur formation.
Stenting for May-Thurner syndrome is typically performed within the proximal left common iliac vein near its confluence with the right common iliac vein to form the IVC, following venography to elucidate the site of compression and/or stenosis (Figure 14). This may follow thrombolysis if clot is present. Self-expanding stents are most commonly used, 12–16 mm in size, which may be made of nitinol (SMART Stent, Cordis, Miami Lakes, FL, USA; Luminexx, Bard, Temple, AZ, USA; Zilver, Cook, Bloomington, IN, USA) or stainless steel (e.g., Wallstent, Boston Scientific, Marlborough, MA, USA). Advantages of these stents include their large deployed diameter for a relatively low-profile delivery size and their ability to be recaptured and repositioned during the early stage of deployment. However, radial hoop strength of these stents is lower than that of balloon-expandable stents (40). To determine whether self-expanding stents have fully relieved the compression after deployment, the use of both intravascular ultrasound and cone-beam CT has been proposed (39,41). If these reveal the stent to be persistently “pancaked”, which may be challenging to see in the frontal projection, then deployment of an appropriately sized balloon-expandable stent within the self-expanding stent can increase the radial force and hopefully remove any residual compression.
Following endovascular intervention for iliac vein compression syndrome, optimal post-procedure care and the use of anticoagulation depends primarily on the presence or absence of thrombotic disease at the time of intervention, and on patient risk factors for re-thrombosis. Rates of stent occlusion are reasonably low in the venous system provided that stent placement and extent is adequate to completely treat the precipitating lesion(s), however primary, assisted primary and secondary patency rates are higher in patients with non-thrombotic disease compared to patients with DVT (42,43). This is a complex topic, and although understanding of the pathophysiology of venous disease has increased in recent years, many of the guidelines for post-procedure pharmacologic therapy are adapted from work on arterial interventions. In general, however, following endovascular treatment for thrombotic venous disease, anticoagulation will most often be recommended for a period of at least 3–6 months with warfarin, low molecular weight heparin or a newer thrombin or factor Xa inhibitor (44). Factors such as the completeness of thrombus removal and restoration of flow, presence of ongoing pro-thrombotic states and risk to the patient of remaining on anticoagulation must all be considered. In the case of non-thrombotic venous disease such as symptomatic May-Thurner syndrome without DVT, traditional anticoagulation may be pursued for only a short time (39), however the longer-term use of anti-platelet agents is considered reasonable in these instances based on extrapolation from arterial studies. Additionally, for both non- and post-thrombotic disease, long-term use of aspirin can be considered, given its low risk of complications (45).
Conclusions
Several catheter-directed interventions are available for the treatment of patients with acute DVT. The procedure and the device used will depend on the clinical nature of the patient with a strong discussion of risks, benefits, and alternatives. With the advent of more novel devices, it is likely that interventional radiology will reinforce its important role in the treatment of patients with acute DVT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34:2363-71. [Crossref] [PubMed]
- Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb Res 2016;137:3-10. [Crossref] [PubMed]
- Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2017;388:3060-73. [Crossref] [PubMed]
- Barleben A, Bandyk DF. Interpretation of peripheral venous duplex testing. Semin Vasc Surg 2013;26:111-9. [Crossref] [PubMed]
- Vedantham S, Thorpe PE, Cardella JF, et al. Quality improvement guidelines for the treatment of lower extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol 2006;17:435-47. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Protack CD, Bakken AM, Patel N, et al. Long-term outcomes of catheter directed thrombolysis for lower extremity deep venous thrombosis without prophylactic inferior vena cava filter placement. J Vasc Surg 2007;45:992-7; discussion 997. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med 2004;141:249-56. [Crossref] [PubMed]
- Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997;349:759-62. [Crossref] [PubMed]
- Aissaoui N, Martins E, Mouly S, et al. A meta-analysis of bed rest versus early ambulation in the management of pulmonary embolism, deep vein thrombosis, or both. Int J Cardiol 2009;137:37-41. [Crossref] [PubMed]
- Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg 2012;55:768-73. [Crossref] [PubMed]
- Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet 2012;379:31-8. [Crossref] [PubMed]
- Parikh S, Motarjeme A, McNamara T, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol 2008;19:521-8. [Crossref] [PubMed]
- Grommes J, Strijkers R, Greiner A, et al. Safety and feasibility of ultrasound-accelerated catheter-directed thrombolysis in deep vein thrombosis. Eur J Vasc Endovasc Surg 2011;41:526-32. [Crossref] [PubMed]
- Baker R, Samuels S, Benenati JF, et al. Ultrasound-accelerated vs standard catheter-directed thrombolysis--a comparative study in patients with iliofemoral deep vein thrombosis. J Vasc Interv Radiol 2012;23:1460-6. [Crossref] [PubMed]
- Engelberger RP, Spirk D, Willenberg T, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv 2015;8:e002027. [Crossref] [PubMed]
- Bozkurt A, Kırbaş İ, Kösehan D, et al. Pharmacomechanical thrombectomy in the management of deep vein thrombosis using the cleaner device: an initial single-center experience. Ann Vasc Surg 2015;29:670-4. [Crossref] [PubMed]
- Vedantham S, Goldhaber SZ, Kahn SR, et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J 2013;165:523-530.e3. [Crossref] [PubMed]
- Garcia MJ, Lookstein R, Malhotra R, et al. Endovascular Management of Deep Vein Thrombosis with Rheolytic Thrombectomy: Final Report of the Prospective Multicenter PEARL (Peripheral Use of AngioJet Rheolytic Thrombectomy with a Variety of Catheter Lengths) Registry. J Vasc Interv Radiol 2015;26:777-85. [Crossref] [PubMed]
- Köksoy C, Yilmaz MF, Başbuğ HS, et al. Pharmacomechanical thrombolysis of symptomatic acute and subacute deep vein thrombosis with a rotational thrombectomy device. J Vasc Interv Radiol 2014;25:1895-900. [Crossref] [PubMed]
- Park KM, Moon IS, Kim JI, et al. Mechanical thrombectomy with Trerotola compared with catheter-directed thrombolysis for treatment of acute iliofemoral deep vein thrombosis. Ann Vasc Surg 2014;28:1853-61. [Crossref] [PubMed]
- Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg 2006;192:782-8. [Crossref] [PubMed]
- Huang CY, Hsu HL, Kuo TT, et al. Percutaneous pharmacomechanical thrombectomy offers lower risk of post-thrombotic syndrome than catheter-directed thrombolysis in patients with acute deep vein thrombosis of the lower limb. Ann Vasc Surg 2015;29:995-1002. [Crossref] [PubMed]
- Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 2012;55:1449-62. [Crossref] [PubMed]
- Dukkipati R, Yang EH, Adler S, et al. Acute kidney injury caused by intravascular hemolysis after mechanical thrombectomy. Nat Clin Pract Nephrol 2009;5:112-6. [Crossref] [PubMed]
- Jeyabalan G, Saba S, Baril DT, et al. Bradyarrhythmias during rheolytic pharmacomechanical thrombectomy for deep vein thrombosis. J Endovasc Ther 2010;17:416-22. [Crossref] [PubMed]
- Sharafuddin MJ, Gu X, Han YM, et al. Injury potential to venous valves from the Amplatz thrombectomy device. J Vasc Interv Radiol 1999;10:64-9. [Crossref] [PubMed]
- Zhu QH, Zhou CY, Chen Y, et al. Percutaneous manual aspiration thrombectomy followed by stenting for iliac vein compression syndrome with secondary acute isolated iliofemoral deep vein thrombosis: a prospective study of single-session endovascular protocol. Eur J Vasc Endovasc Surg 2014;47:68-74. [Crossref] [PubMed]
- Kwon SH, Oh JH, Seo TS, et al. Percutaneous aspiration thrombectomy for the treatment of acute lower extremity deep vein thrombosis: is thrombolysis needed? Clin Radiol 2009;64:484-90. [Crossref] [PubMed]
- Oğuzkurt L, Ozkan U, Gülcan O, et al. Endovascular treatment of acute and subacute iliofemoral deep venous thrombosis by using manual aspiration thrombectomy: long-term results of 139 patients in a single center. Diagn Interv Radiol 2012;18:410-6. [PubMed]
- Donaldson CW, Baker JN, Narayan RL, et al. Thrombectomy using suction filtration and veno-venous bypass: single center experience with a novel device. Catheter Cardiovasc Interv 2015;86:E81-7. [Crossref] [PubMed]
- Moriarty JM, Al-Hakim R, Bansal A, et al. Removal of Caval and Right Atrial Thrombi and Masses Using the AngioVac Device: Initial Operative Experience. J Vasc Interv Radiol 2016;27:1584-91. [Crossref] [PubMed]
- Resnick SA, O'Brien D, Strain D, et al. Single-Center Experience Using AngioVac with Extracorporeal Bypass for Mechanical Thrombectomy of Atrial and Central Vein Thrombi. J Vasc Interv Radiol 2016;27:723-729.e1. [Crossref] [PubMed]
- Salsamendi J, Doshi M, Bhatia S, et al. Single Center Experience with the AngioVac Aspiration System. Cardiovasc Intervent Radiol 2015;38:998-1004. [Crossref] [PubMed]
- Smith SJ, Behrens G, Sewall LE, et al. Vacuum-assisted thrombectomy device (AngioVac) in the management of symptomatic iliocaval thrombosis. J Vasc Interv Radiol 2014;25:425-30. [Crossref] [PubMed]
- May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology 1957;8:419-27. [Crossref] [PubMed]
- Neglén P, Raju S. Proximal lower extremity chronic venous outflow obstruction: recognition and treatment. Semin Vasc Surg 2002;15:57-64. [Crossref] [PubMed]
- Lou WS, Gu JP, He X, et al. Endovascular treatment for iliac vein compression syndrome: a comparison between the presence and absence of secondary thrombosis. Korean J Radiol 2009;10:135-43. [Crossref] [PubMed]
- Ahmed O, Ng J, Patel M, et al. Endovascular Stent Placement for May-Thurner Syndrome in the Absence of Acute Deep Vein Thrombosis. J Vasc Interv Radiol 2016;27:167-73. [Crossref] [PubMed]
- Duda SH, Wiskirchen J, Tepe G, et al. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol 2000;11:645-54. [Crossref] [PubMed]
- Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg 2006;44:136-43; discussion 144. [Crossref] [PubMed]
- Raju S, Neglén P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg 2009;50:360-8. [Crossref] [PubMed]
- Neglén P, Raju S. In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg 2004;39:181-7. [Crossref] [PubMed]
- Sista AK. Postprocedural management of patients undergoing endovascular therapy for acute and chronic lower-extremity deep venous disease. Tech Vasc Interv Radiol 2014;17:127-31. [Crossref] [PubMed]
- Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology 2013;28 Suppl 1:91-8. [Crossref] [PubMed]