
Number of attempts and interventions to obtain a valid pulmonary artery wedge pressure
Highlight box
Key findings
• We found that a valid pulmonary artery wedge pressure (PAWP) was obtained in the first or second attempt in 71% and 91% of the patients, respectively. A valid PAWP was obtained in the right pulmonary circulation in 86% of the patients with a pulmonary artery catheter balloon inflation of around 1 mL and at a distance from the introducer hub of about 53 cm. The main reason for repeating the PAWP was incomplete wedge, and the main intervention was to advance the pulmonary artery catheter.
What is known and what is new?
• Errors in the determination of the PAWP are common and may lead to hemodynamic misclassification and inadequate management. There is limited information regarding the methodology used for measuring the PAWP.
• The present manuscript carefully evaluates the process of acquiring a valid PAWP, describing the methodology, including number of attempts required and interventions performed to obtain a valid PAWP.
What is the implication, and what should change now?
• This is the first study that systematically evaluated the process of obtaining a valid PAWP in a relatively large cohort of patients who underwent right heart catheterization predominantly for pulmonary hypertension. The data presented provide guidance on what to expect and do when obtaining a valid PAWP determination.
Introduction
Right heart catheterization (RHC) with flow-guided balloon-tipped pulmonary artery catheter (PAC) was introduced by Swan and Ganz in 1970 (1). Currently, over 2 million RHC are performed annually in the US alone. RHC is essential for the diagnosis and hemodynamic classification of pulmonary hypertension (PH). Key hemodynamic determinations include cardiac output (CO), mean pulmonary artery pressure (mPAP) and pulmonary artery wedge pressure (PAWP). A PAWP >15 mmHg in the presence of PH (mPAP >20 mmHg) indicates either isolated post-capillary PH [when pulmonary vascular resistance (PVR) is <2 Wood units (WU)] or combined pre- and post-capillary PH (when PVR is ≥2 WU) (2).
The most challenging hemodynamic determination during RHC is the PAWP, given potential errors in the measurement that lead to hemodynamic misclassification and inadequate management (3,4). Five criteria are used to define an adequate PAWP measurement: (I) PAWP is less than the diastolic pulmonary artery pressure (PAP); (II) tracing is compatible with the atrial pressure waveform; (III) fluoroscopic image demonstrates a stationary PAC; (IV) free flow is present within the PAC (flush test) and (V) highly oxygenated blood is obtained from the distal port of the PAC (5).
Although the acquisition of valid PAWP is of utmost importance, there is limited information regarding the methodology for measuring this pressure (5). Therefore, the main objective of the present study is to carefully evaluate the process of acquiring a valid PAWP, describing the methodology, including number of attempts required and interventions performed to obtain a valid PAWP. In addition, we recorded prespecified clinical, hemodynamic, and radiographic factors to assess their role with difficulties in obtaining a valid PAWP in the first attempt. We present this article in accordance with STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-189/rc).
Methods
Study participants and design
In this prospective observational cohort study, we included consecutive patients who underwent PAC at the Cleveland Clinic Pulmonary Vascular Disease program, between February 2023 and May 2023. Hemodynamic data during RHC was prospectively collected as part of a quality improvement project, with the goal of identifying factors affecting a valid PAWP acquisition. All patients during the study period were included as we were able to obtain a valid PAWP in all of them. Standard of care was followed in all patients, and no specific interventions were performed as part of the present study. Additional clinical, echocardiographic, and radiographic data were retrospectively collected as part of a Cleveland Clinic Insitutional Review Board (IRB) approved project (IRB No. 23-517). Informed consent was waived. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The RHC was ordered by the patient’s treating physician for a variety of reasons including suspected PH, re-evaluation of previously known PH, or as a component of an invasive cardiopulmonary test performed for dyspnea of unknown origin, exercise intolerance and/or chronic thromboembolic disease.
RHC including PAWP measurement:
RHC was performed in the outpatient setting by three experienced operators using local anesthesia (6,7). In all cases we accessed the right internal jugular vein with ultrasound guidance. An 8.5 Arrow (Arrow International LLC, Morrisville, NC, USA) introducer was placed. We used 3 types of C-tip, thermodilution PAC manufactured by Edwards (Edwards Lifesciences LLC, Irvine, CA, USA), including (I) a standard 4 lumen 7 Fr (131F7) for regular RHC, (II) a Paceport 5 lumen 7.5 Fr (931F75) for patients also undergoing exercise in whom we additionally measure the right ventricular pressure, and (III) a synthetic ControlCath 4 Lumen 7 Fr (C146F7) for subjects with latex allergy.
After advancing the PAC to the mid-right atrium, the mean right atrial pressure (RAP) was recorded. Subsequently, the PAC with the balloon inflated was advanced using fluoroscopy until the PAC achieve a stationary position in one of the pulmonary artery (PA) branches. During PAC insertion, no specific interventions were performed to select the right or left pulmonary arteries or a particular third (upper, middle or lower) of the lung for initial hemodynamic measurements.
After the PAC achieved a stationary position by fluoroscopy, the balloon was deflated, and PAP determined. After recording the PAP, the balloon of the PAC was progressively inflated until obtaining an adequate PAWP waveform or reaching the maximum balloon inflation of 1.5 mL. The minimal volume of air required to obtain an adequate PAWP waveform was recorded. We recorded whether the PAC wedged in the right or left pulmonary circulation and in upper, middle, or lower third of the lung. If the PAWP waveform was considered adequate, then other criteria for a valid PAWP determination were checked (5). In the event of over wedge in the waveform tracing, the PAC was withdrawn (about 1 cm) and if incomplete PAWP was suspected, the PAC was advanced (about 1 cm) (Figure 1).
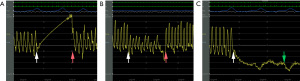
After the PAC was repositioned, the PAWP was remeasured. If the PAWP waveform was considered adequate, then the other criteria for a valid PAWP determination were checked (5). Similarly, in the presence of over wedge or incomplete PAWP by waveform analysis, the catheter was again withdrawn or advanced, respectively, and PAWP remeasured. If the PAWP waveform was still inadequate, the PAC was relocated in the contralateral pulmonary circulation using fluoroscopy and a J tip 0.025” wire. The PAC was then advanced until stationary on fluoroscopy, following the steps previously described. Both the mPAP and the valid PAWP were measured at end-expiration, using electronic calipers and waveform tracings including three respiratory cycles. CO was measured by thermodilution and PVR was calculated as (mPAP − PAWP)/CO.
Once a valid PAWP was obtained, we recorded the PA location, number of attempts, the reason for repeating the determination, interventions performed, the depth of the PAC from the introducer hub, and the need to access the contralateral pulmonary circulation. In all patients we tried to obtain PAW blood for gasometry using co-oximetry and recorded if this was successful. If PAW blood could not be obtained or wedge blood oxygen saturation (SwO2) was <88%, we still consider a PAWP valid if all other criteria for a valid PAWP were met (8). The depth of the PAC from the introducer hub to wedge position was added later in the protocol and therefore only recorded in 1/3 of patients.
Additional data collected
We retrospectively collected data on demographics, type of PH based on the 2022 ERS/ESC guidelines, need for oxygen supplementation, World Health Organization (WHO) functional class, risk assessment based on the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) light 2 and 3-strata Noninvasive French criteria, and right heart function by echocardiography (2,8,9). In addition, we measured the PA diameter and the PA/aorta (Ao) diameter ratio in patients with available contrast and non-contrast computed tomography of the chest.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median [interquartile range (IQR)], as appropriate. Normal distribution of the data was assessed with Q-Q plots and Shapiro-Wilk test. Categorical data are summarized as discrete values and percentages [n (%)]. We chose a convenience sample size that included all patients that underwent RHC during the 4-month period of our quality improvement project. We specifically chose to use a 4-month window based on the prior year number of cases and the goal of including a representative sample size of >150 patients in our project. Comparison of continuous variables among three subgroups (1, 2 or ≥3 PAWP measuring attempts) was performed with analysis of variance (ANOVA) or Kruskal-Wallis based on normality. Categorical data among three groups was compared with Chi-square test. Groups of independent continuous variables were contrasted with t-test. We tested the association between number of PAWP measuring attempts and a series of continuous prespecified variables using Pearson or Spearman rank correlation. A P value of <0.05 was considered significant. The statistical analyses were performed using the statistical package IBM SPSS, version 22 (IBM; Armonk, NY, USA).
Results
Patient characteristics
We included 195 patients, age: 57.5±15.7 years, 111 (57%) women. A total of 39 (20%) patients had no PH, while the rest had PH due to a variety of conditions (Table 1). Only 41 (21%) patients were on PAH-specific medications at the time of RHC, including 12 who were treated with parenteral prostacyclin therapy. The PA diameter on computed tomography (n=163) was 32.0±7.1 mm with a PA/Ao ratio of 1.03±0.22 (Table 1).
Table 1
| Variables | Overall (N=195) | 1 PAWP attempt (N=139) | 2 PAWP attempts (N=39) | ≥3 PAWP attempts (N=17) | P (ANOVA, Kruskal-Wallis, Chi-square test) |
|---|---|---|---|---|---|
| Age (years) | 57.5±15.7 | 57.3±15.7 | 56.7±17.0 | 61.3±12.8 | 0.58 |
| Female | 111 [57] | 78 [56] | 23 [59] | 10 [59] | 0.94 |
| BMI (kg/m2) | 32.5±9.8 | 31.7±8.7 | 34.3±12.6 | 34.9±10.6 | 0.19 |
| BSA (m2) | 2.00±0.28 | 1.98±0.27 | 2.04±0.34 | 2.04±0.28 | 0.31 |
| O2 supplementation (yes) | 54 [28] | 36 [26] | 12 [31] | 6 [35] | 0.64 |
| FiO2 (%) | 27.4±15.6 | 27.6±16.6 | 27.6±14.9 | 25.8±7.5 | 0.91 |
| COPD (yes) | 46 [24] | 37 [27] | 8 [21] | 1 [6] | 0.14 |
| Asthma (yes) | 55 [28] | 39 [28] | 13 [33] | 3 [18] | 0.49 |
| Obstructive sleep apnea (yes) | 98 [50] | 74 [53] | 17 [44] | 7 [41] | 0.42 |
| Hx of pulmonary embolism (yes) | 36 [19] | 22 [16] | 9 [23] | 5 [29] | 0.28 |
| Diabetes mellitus (yes) | 59 [30] | 39 [28] | 11 [28] | 9 [53] | 0.10 |
| Coronary artery disease (yes) | 65 [33] | 42 [30] | 16 [41] | 7 [41] | 0.15 |
| Connective tissue disease (yes) | 34 [17] | 22 [16] | 7 [18] | 5 [29] | 0.38 |
| PH groups^ | 0.25 | ||||
| No PH | 39 [20] | 34 [24] | 4 [10] | 1 [6] | |
| Group 1 | 39 [20] | 25 [18] | 10 [26] | 4 [24] | |
| Group 2 | 51 [26] | 34 [24] | 13 [33] | 4 [24] | |
| Group 3 | 35 [18] | 26 [19] | 7 [18] | 2 [12] | |
| Group 4 | 27 [14] | 17 [12] | 5 [13] | 5 [29] | |
| Group 5 | 4 [2] | 3 [2] | 0 [0] | 0 [0] | |
| Severity of PAH (N=34) | |||||
| REVEAL lite 2* | 7.2±3.0 | 7.1±3.0 | 8.2±5.3 | 5.3±2.5 | 0.34 |
| French risk score* | 2.2±0.8 | 2.2±0.9 | 2.3±0.9 | 2±0.0 | 0.84 |
| Echocardiogram | |||||
| RVSP (N=131)* | 52±25 | 52±24 | 53±25 | 52±28 | 0.98 |
| TAPSE (N=139)* | 2.0±0.5 | 2.0±0.5 | 2.1±0.6 | 1.9±0.5 | 0.72 |
| RV function (N=177)* | 0.93 | ||||
| Normal | 112 [63] | 80 [64] | 22 [61] | 10 [67] | |
| Reduced | 65 [37] | 46 [37] | 14 [39] | 5 [33] | |
| NT-pro BNP (N=154)* | 250 [68–1,638] | 212 [63–1,332] | 266 [106–1,835] | 299 [125–1,478] | 0.59 |
| PAH-specific medications at RHC (yes) | 41 [21] | 28 [20] | 7 [18] | 6 [35] | 0.31 |
| Number of PAH medications | 0.45 | ||||
| 1 | 17 [42] | 12 [43] | 1 [14] | 4 [67] | |
| 2 | 12 [29] | 8 [29] | 3 [43] | 1 [17] | |
| 3 | 12 [29] | 8 [29] | 3 [43] | 1 [17] | |
| Parenteral prostacyclin at RHC (yes) | 12 [6] | 8 [6] | 3 [8] | 1 [6] | 0.91 |
| PA diameter (mm) (N=163) | 32.0±7.1 | 31.4±6.5 | 32.1±7.3 | 35.7±9.5 | 0.08 |
| PA/Ao diameter (N=163) | 1.03±0.22 | 1.02±0.23 | 1.02±0.21 | 1.09±0.25 | 0.53 |
Data are presented as mean ± SD, median [IQR] or n [%]. *, only variables with n<195; ^, PH groups based on the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. BMI, body mass index; BSA, body surface area; FiO2, fraction of inspired oxygen; COPD, chronic obstructive pulmonary disease; Hx, history; PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; NT-pro BNP, N-terminal pro-brain natriuretic peptide; RHC, right heart catheterization; PA, pulmonary artery; Ao, aorta; PAWP, pulmonary artery wedge pressure; SD, standard deviation; IQR, interquartile range; ESC, European Society of Cardiology; ERS, European Respiratory Society.
Hemodynamic determinations
The PAWP was 16.4±5.9 mmHg and the PVR was 2.7 (1.5–4.6) WU. Pre-capillary, isolated post-capillary and combined pre- and post-capillary PH were observed in 55 (28%), 24 (12%) and 64 (33%) patients, respectively (Table 2).
Table 2
| Variables | Overall (N=195) | 1 PAWP attempt (N=139) | 2 PAWP attempts (N=39) | ≥3 PAWP attempts (N=17) | P (ANOVA, Kruskal-Wallis, Chi-square test) |
|---|---|---|---|---|---|
| Physician performing the procedure | 0.53 | ||||
| #1 | 143 [73] | 105 [73] | 28 [20] | 10 [7] | |
| #2 | 30 [15] | 19 [63] | 6 [20] | 5 [17] | |
| #3 | 22 [11] | 15 [68] | 5 [23] | 2 [9] | |
| PAC used | 0.10 | ||||
| Edwards 7F | 124 [64] | 84 [60] | 27 [69] | 13 [76] | |
| Edwards Paceport 7.5F | 59 [30] | 49 [35] | 8 [21] | 2 [12] | |
| Edwards synthetic ControlCath 7F | 12 [6] | 6 [4] | 4 [10] | 2 [12] | |
| Initial PAC distance from hub (cm) (N=65*) | 52.3±4.6 | 51.9±4.4 | 54.8±4.8 | 52.5±6.4 | 0.27 |
| Final PAC distance from hub (cm) (N=65*) | 52.6±5.2 | 51.9±4.4 | 56.9±4.9 | 52.8±10.0 | 0.04 |
| Initial location of the PAC | 0.82 | ||||
| Right PA | 184 [94] | 132 [95] | 36 [92] | 16 [94] | |
| Left PA | 11 [6] | 7 [5] | 3 [8] | 1 [6] | |
| Final location of the PAC | <0.001 | ||||
| Right PA | 168 [86] | 132 [95] | 31 [80] | 5 [29] | |
| Left PA | 27 [14] | 7 [5] | 8 [21] | 12 [71] | |
| Initial location of the PAC | <0.001 | ||||
| Lower third of the lung | 136 [70] | 93 [67] | 27 [69] | 16 [94] | |
| Middle third of the lung | 52 [27] | 45 [32] | 7 [18] | 0 [0] | |
| Upper third of the lung | 7 [4] | 1 [1] | 5 [13] | 1 [6] | |
| Final location of the PAC | 0.30 | ||||
| Lower third of the lung | 134 [69] | 93 [57] | 30 [77] | 11 [65] | |
| Middle third of the lung | 58 [30] | 45 [32] | 8 [21] | 5 [29] | |
| Upper third of the lung | 3 [2] | 1 [1] | 1 [3] | 1 [6] | |
| Initial PAC balloon volume (mL) | 1.1±0.3 | 1.0±0.3 | 1.1±0.4 | 1.2±0.3 | 0.02 |
| Final PAC balloon volume (mL) | 1.0±0.3 | 1.0±0.3 | 1.0±0.3 | 1.2±0.4 | 0.14 |
| Atrial fibrillation at RHC (yes) | 8 [4] | 6 [4] | 2 [5] | 0 [0] | 0.66 |
| RAP (mmHg) | 11.2±5.5 | 10.6±4.8 | 12.6±7.5 | 12.7±4.9 | 0.07 |
| mPAP (mmHg) | 34.4±15.9 | 32.9±15.2 | 36.2±17.1 | 43.0±16.9 | 0.03 |
| PAWP (mmHg) | 16.4±5.9 | 15.7±5.2 | 17.1±7.1 | 20.4±6.2 | 0.005 |
| CI (L/min/m2) | 3.1±0.8 | 3.0±0.8 | 3.2±1.0 | 3.0±1.1 | 0.68 |
| PVR (WU) | 2.7 [1.5–4.6] | 2.6 [1.4–4.4] | 2.7 [1.7–6.1] | 3.6 [2.3–6.0] | 0.33 |
| Pulmonary hemodynamic classification | 0.08 | ||||
| No PH | 39 [20] | 34 [25] | 4 [10] | 1 [6] | |
| Pre-capillary PH | 55 [28] | 39 [28] | 13 [33] | 3 [18] | |
| Isolated post-capillary PH | 24 [12] | 16 [12] | 6 [15] | 2 [12] | |
| Combined pre- and post-capillary PH | 64 [33] | 41 [30] | 12 [31] | 11 [65] | |
| Unclassified PH | 13 [7] | 9 [7] | 4 [10] | 0 [0] | |
| Wedge blood obtained (yes) | 141 [72] | 100 [72] | 29 [74] | 12 [71] | 0.94 |
| SwO2 (N=141)* | 97 [95–99] | 97 [95–98] | 98 [96–99] | 97 [93–99] | 0.40 |
Data are presented as mean ± SD, median [IQR] or n [%]. *, only variables with n <195. PAC, pulmonary artery catheter; PA, pulmonary artery; RAP, right atrial pressure; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; CI, cardiac index; PVR, pulmonary vascular resistance; WU, Wood units; PH, pulmonary hypertension, SwO2, oxygen saturation in wedge blood; SD, standard deviation; IQR, interquartile range.
Number of attempts to obtain a valid PAWP
The number of attempts to get a valid PAWP was 1, 2 and ≥3 in 139 (71%), 39 (20%) and 17 (9%) patients, respectively (Tables 1,2). In the group with ≥3 PAWP attempts, 10 patients required 3, 5 patients required 5, while the remaining 2 patients required 5 and 9 attempts, respectively.
Methodology to obtain a valid PAWP
In 124 (64%) patients we used a conventional triple lumen Edwards 7F PAC. The distance from the introducer hub to the tip of the PAC, at first PAWP attempt was 52.3±4.6 cm (n=64) with a PAC balloon inflation of 1.1±0.3 mL. The PAC preferentially went to the right pulmonary circulation in 184 (94%) patients, predominantly to the lower and middle third of the lung in 188 (96%) patients (Table 2).
Factors associated with valid PAWP at first attempt
A valid PAWP at first, versus more than one attempt, required a lower air volume of the PAC balloon (1.0±0.3 vs. 1.2±0.4 mL, P=0.006) and a shorter distance from the hub [51.9±4.4 (n=52) vs. 54.0±5.2 cm (n=12), P=0.16]. PAWP was valid in the first attempt in 132/184 (72%) of the patients in whom the PAC initially went to the right pulmonary circulation and in 7/11 (64%) patients in whom the PAC initially went to the left side (P=0.56). Interestingly, the PAWP was valid in 93/136 (68%) patients in whom the initial PAWP was attempted in the lower, 45/52 (87%), in the middle and 1/7 (14%) in the upper third of the lung (P<0.001).
PAWP remeasurement
Reasons for repeating the initial PAWP measurement (n=56) were unusual waveform: 7 (13%), incomplete wedge: 38 (68%) and over wedge: 11 (20%). Initial PAC manipulation consisted in advancing and withdrawing the PAC in 36 (64%) and 12 (21%) patients, respectively; or due to the vascular anatomy directing the PAC to the contralateral PA in 8 (14%) patients (Table 3). Pertinent data on subsequent PAWP remeasurements are provided in Table 3.
Table 3
| Variables | First PAWP remeasurement (N=56) | Second PAWP remeasurement (N=17) | Third PAWP remeasurement (N=8) | Forth PAWP remeasurement (N=2) |
|---|---|---|---|---|
| Reason for a non-valid PAWP | ||||
| Incomplete | 38 [68] | 10 [59] | 4 [50] | 1 [50] |
| Over wedge | 11 [20] | 3 [18] | 3 [38] | 1 [50] |
| Unusual waveform | 7 [13] | 4 [24] | 1 [13] | 0 [0] |
| PAC manipulation | ||||
| Advance | 36 [64] | 10 [59] | 4 [50] | 1 [50] |
| Pullback | 12 [21] | 2 [12] | 1 [13] | 1 [50] |
| Reposition to the contralateral pulmonary circulation | 8 [14] | 5 [29] | 3 [38] | 0 [0] |
Data are presented as n [%]. PAWP, pulmonary artery wedge pressure; PAC, pulmonary artery catheter.
In 2 patients after the third unsuccessful attempt to obtain a valid PAWP, including PAC repositioning to the contralateral circulation, the standard Edwards PAC was changed to a polymer blend Control Catheter (C144F7). In both cases, the PAC was wedged in the lower third of the left pulmonary circulation with balloon inflation of 0.7 and 1.1 mL, at 60 and 61 cm from the introducer hub, respectively.
Characteristics of a valid PAWP
Overall, a valid PAWP was obtained in the right pulmonary circulation in 168 (86%) patients. In the rest [n=27 (14%)], the PAWP was obtained in the left pulmonary circulation (spontaneously in 11 patients and as a result of PAC repositioning in 16 patients) (Table 2). The PAC balloon inflation at valid PAWP was 1.0±0.3 mL (range, 0.3–1.5 mL), at a distance from the hub of 52.6±5.2 cm (range, 41–70 cm, n=65). The final lung location of the valid PAWP was the lower in 134 (69%), middle in 58 (30%), and upper third in 3 (2%) patients (Table 2). The PAC distance from the hub to a valid PAWP was similar in the right and left lung [52.4±4.6 (n=56) vs. 53.8±8.2 cm (n=9), P=0.47], as it was the degree of balloon inflation (1.0±0.3 vs. 1.1±0.3 mL, respectively, P=0.66).
PAW blood gasometry
Blood from the PAW position was obtained in 141 (72%) patients, with SwO2 of 97% (95–99%). Of the patients in whom PAW blood was obtained, only 5 had a SwO2 <88%. Even though wedge blood showed a lower saturation than expected, all other criteria for a valid PAWP were met and no further attempts to remeasure the PAWP were made in these subjects.
Factors associated with the number of measuring attempts to get a valid PAWP
The number of PAWP measurements to obtain a valid PAWP was directly associated with RAP (r=0.16, P=0.03), mPAP (r=0.18, P=0.01), PAWP (r=0.22, P=0.002), PA diameter (r=0.16, P=0.04) and initial PAC balloon inflation (r=0.18, P=0.007); but not PVR or severity of PH by REVEAL light 2 or French noninvasive criteria risk scores.
Discussion
In the present study we found that a valid PAWP was obtained in the first or second measuring attempt in 71% and 91% of the patients, respectively. A valid PAWP was obtained in the right pulmonary circulation in 86% of the patients with a PAC balloon inflation of around 1 mL and at a distance from the introducer hub of about 53 cm. PAW blood could only be obtained in 72% of the patients, even when all other criteria for a valid PAWP were met. The main reason for repeating the PAWP was incomplete wedge, and the main intervention was to advance the PAC catheter. In 8% of the patients the PAC had to be repositioned to the contralateral pulmonary circulation using wire support, and in two patients the standard PAC had to be replaced for a more rigid PAC. Factors weakly associated with the number of attempts to obtain a valid PAWP included a higher RAP, mPAP, PAWP, PA diameter and initial wedging of the PAC in the upper third of the lung.
The PAWP is an essential determination for the hemodynamic classification of the patients with PH; however, errors in the measurement are common (3,4). We have previously demonstrated that in order to obtain a valid PAWP, both repositioning of the PAC and a lower PAC balloon inflation may be needed to facilitate the complete occlusion of the PA branch that is wedged (5,10). For the first time, we have prospectively recorded the number of measuring attempts needed to obtain a valid PAWP, the reasons for repeating it and corrective maneuvers applied. It is critical to mention that the PAWP determination was done under fluoroscopy, in a large volume academic center, by three experienced operators, and following a strict protocol (11). Therefore, it is likely that the number of measuring attempts to obtain a valid PAWP will increase when performing this procedure without fluoroscopy guidance and/or by less experienced operators.
A valid PAWP was obtained at a PAC distance from the introducer hub of slightly more than 50 cm and at a PAC balloon inflation of 1 mL, values that can serve as guide in the absence of fluoroscopy (5,12). Advancing or withdrawing the PAC based on the PAWP waveform analysis is certainly possible at bedside. However, more complicated interventions (noted in 9% of our cohort) such as repositioning the PAC in the contralateral PA and/or a different portion of the lung and switching the PAC for a more rigid one are best performed under fluoroscopy guidance (12).
We were not able to identify factors strongly associated with the difficulty in obtaining a valid PAWP. In fact, neither the PH clinical groups or the PH hemodynamic groups were associated with the number of attempts to obtain a valid PAWP. Similarly, in our cohort, the operator, type of PAC, and the initial location of the PAC in the right vs left PA had not significant impact in the process of acquiring valid PAWP. However, the initial PAC position in the upper third of the lung frequently led to an inadequate PAWP measurement and hence the need to reposition the PAC. Importantly, both mid and lower lung zones yield similar percentages of a valid PAWP. Ideally, PAWP is obtained in zone 3 of the lung, which is in the lung bases in the upright position (13). However, since all hemodynamic measurements obtained in our study are in supine position, traditional lung zones differ, since the dominant effect of gravity is in the anterior to posterior axis instead of apical to basal. In fact, in supine position zone 3 is the most posterior. The methodology of advancing the PAC with balloon inflated until stationary tends to guide the balloon to a vessel with adequate flow, likely in zone 3 (14). No patients had conditions in which the zone 3 was reduced in supine position such as the use of positive end-expiratory pressure, mechanical ventilation or hypovolemia.
Factors that were weakly associated with the number of measuring attempts before a valid PAWP determination were a higher RAP, mPAP and PAWP. The explanation for these associations, could be that a more tortuous pulmonary circulation due to vascular remodeling secondary to higher pulmonary pressures, interfere with an adequate pulmonary vascular seal (15). The need to confirm a higher PAWP may trigger a remeasurement; although this factor did not impact our numbers as we followed a strict protocol to determine a valid PAWP. In the rare event an operator wanted to confirm a valid PAWP determination with a second measurement (even when all criteria for a valid PAWP were present), this was not considered a remeasurement. Importantly, when all criteria were met for a valid PAWP, the rare remeasurement (<10 occasions) always confirmed the initial determination (11).
A larger PA was weakly associated (r=0.16) with a higher number of measuring attempts for a valid PAWP. This was noted when the PAWP attempts were incorporated as continuous variable. We noted a trend towards a significant association between a larger PA and measuring attempts when we clustered them into three groups (1, 2 and ≥3). It is plausible that larger proximal PA vessels with rapid tapering may complicate the seal of the PAC balloon, likely causing an incomplete wedge and requiring the advancement of the PAC to a more distal vascular area.
Our study has limitations such as the use of fluoroscopy and a strict protocol that limits the extrapolation of our findings to less experienced centers, bedside PAC placement, or RHC for conditions other than PH. Our findings are only applicable when performing the RHC through the right internal jugular vein, using 7 or 7.5 F PAC. The variables reported were available in all patients, except for the PAC distance from the vascular introducer hub to wedge position that was not part of our initial data collection protocol, and a number of missing echocardiograms, NT-pro BNP, and PA diameter determinations. Despite these limitations, this is the first study that systematically evaluated the process of obtaining a valid PAWP in a relatively large cohort of patients who underwent RHC predominantly for PH.
Conclusions
A valid PAWP was obtained at the first or second attempt in about 90% of the patients that undergo a RHC, with a PAC balloon inflation volume of about 1 mL. Incomplete PAWP or over wedge are relatively common and can generally be corrected by advancing or withdrawing the PAC, respectively. PAC that wedge in the upper third of the lung usually provide inadequate PAWP determinations and need repositioning. In about 10% of patients more advanced interventions requiring fluoroscopy for reposition or switching to a stiffer PAC are needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-189/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-189/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-189/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-189/coif). A.R.T. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from September 2023 to August 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swan HJ, Ganz W, Forrester J, et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med 1970;283:447-51. [Crossref] [PubMed]
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618-31. Erratum in: Eur Heart J 2023;44:1312. [Crossref] [PubMed]
- Naeije R, Tonelli AR. Pulmonary Artery Wedge Pressure in the Diagnosis of Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2024;209:242-4. [Crossref] [PubMed]
- Sahay S, Lane J, Sharpe MG, et al. Impact on Pulmonary Hypertension Hemodynamic Classification Based on the Methodology Used to Measure Pulmonary Artery Wedge Pressure and Cardiac Output. Ann Am Thorac Soc 2023;20:1752-9. [Crossref] [PubMed]
- Tonelli AR, Mubarak KK, Li N, et al. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest 2011;139:115-21. [Crossref] [PubMed]
- Ryan JJ, Rich JD, Thiruvoipati T, et al. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in the classification of patients with pulmonary hypertension. Am Heart J 2012;163:589-94. [Crossref] [PubMed]
- Oudiz RJ, Langleben D. Cardiac Catheterization in Pulmonary Arterial Hypertension: An Updated Guide to Proper Use. Advances in Pulmonary Hypertension 2005;4:15-25. [Crossref]
- Benza RL, Kanwar MK, Raina A, et al. Development and Validation of an Abridged Version of the REVEAL 2.0 Risk Score Calculator, REVEAL Lite 2, for Use in Patients With Pulmonary Arterial Hypertension. Chest 2021;159:337-46. [Crossref] [PubMed]
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017;50:1700889. [Crossref] [PubMed]
- Ennala S, Melillo CA, Lane JE, et al. Effect of pulmonary artery catheter balloon inflation on pulmonary hemodynamics. Cardiovasc Diagn Ther 2022;12:37-41. [Crossref] [PubMed]
- Tang WHW, Wilcox JD, Jacob MS, et al. Comprehensive Diagnostic Evaluation of Cardiovascular Physiology in Patients With Pulmonary Vascular Disease: Insights From the PVDOMICS Program. Circ Heart Fail 2020;13:e006363. [Crossref] [PubMed]
- Grinstein J, Houston BA, Nguyen AB, et al. Standardization of the Right Heart Catheterization and the Emerging Role of Advanced Hemodynamics in Heart Failure. J Card Fail 2023;29:1543-55. [Crossref] [PubMed]
- Del Rio-Pertuz G, Nugent K, Argueta-Sosa E. Right heart catheterization in clinical practice: a review of basic physiology and important issues relevant to interpretation. Am J Cardiovasc Dis 2023;13:122-37. [PubMed]
- Ragosta M Ragosta M, Kennedy JLW. Normal waveforms, artifacts, and pitfalls. In: Ragosta M. editor. Textbook of Clinical Hemodynamics. 2nd Edn. Philadelphia: Elsevier, 2018:17-55.
- Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 2013;91:297-309. [Crossref] [PubMed]


