Minimally invasive treatments for perforator vein insufficiency
Introduction
Approximately 25% of women and 15% of men in the United States suffer from lower extremity venous insufficiency, and accordingly lower extremity venous insufficiency is responsible for significant health care expenditures in USA and worldwide (1,2). Cosmetic causes are the main reason patients seek medical treatment for varicose veins, but presentation with lower extremity venous insufficiency symptoms including aching, pain, night cramps, fatigue, heaviness, or restlessness are also common (3). Untreated significant superficial venous insufficiency may eventually progress to advanced chronic venous insufficiency, including lower extremity swelling, eczema, pigmentation, hemorrhage, and ulceration (4).
A common cause of recurrence after treatment of incompetent superficial veins is perforator vein insufficiency. Recent advances in the treatment of superficial venous insufficiency have significantly changed management in this subset of patients. Physicians specialized in treatment of venous diseases can now choose from a variety of minimally invasive techniques and treatment modalities including ultrasound guided sclerotherapy (USGS) and endovascular thermal ablation (EVTA), with either laser or radiofrequency energy sources. These techniques can be performed in an office setting with local anesthesia or with conscious sedation, unlike their surgical counterpart, subfascial endoscopic perforator surgery (SEPS) which requires general anesthesia and a hospital setting (2,5-7). Data and experience regarding minimally invasive techniques in insufficient perforator veins are increasing. These newly introduced techniques have their own advantages, disadvantages and efficiency rates, both in superficial and perforator vein use. Lack of experience and awareness of newly introduced equipment designed for perforator vein treatments may be limiting factor in widespread use.
Perforator vein anatomy and pathogenesis of incompetency
Perforator veins connect the deep and superficial venous systems, allowing passage of blood in between them. Lower extremity perforators are named depending on their topographical location. Thigh level perforators are named Hunter veins; perforators located just above and below the knee are named Dodd and Boyd veins respectively; and calf level perforators are named Cockett veins (8,9). Perforator veins run in close proximity to the arteries, but their anatomy is variable. This variability is more evident after significant dilatation and tortuosity due to insufficiency, and it may render perforator veins difficult to identify with US and difficult to access for EVTA.
Re-entry points are where superficial lower extremity veins and perforator veins join. Volume overload at reentry points may lead to weakening of the perforator vein walls, dilatation, and eventually reflux (Figure 1). Reflux within incompetent superficial veins triggers perforator veins to enlarge and become incompetent (10,11). High flow from the deep venous system during muscular contraction rendering perforating veins incompetent was a previously suggested theory, that is now widely abandoned (12,13). Perforator vein incompetence generally follows reflux within the superficial veins in a temporal fashion, supporting the former theory (9,14). Pathologic perforator veins are described as having reversed flow from deep system to superficial vein for more than 500 ms, and with diameter more than 3.5 mm. Risk factors for incompetent perforator veins are the same as for all chronic superficial venous disease, including history of deep venous thrombosis, multiple pregnancies, advanced age and genetic factors (15).
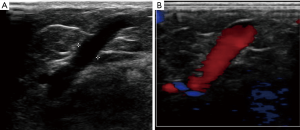
Incompetent perforator veins have been linked to chronic venous insufficiency including recurrence of superficial venous reflux after treatment, varicose veins and ulcer development. Despite the fact that compression stockings therapy is the primary treatment for chronic superficial venous disease, non-compliance amongst patients is common. Interventions to relieve venous hypertension have been shown to improve wound healing and decrease risk of recurrence (16-18). Treatment and closure of incompetent perforators minimizes long-term sequelae of chronic venous insufficiency, and reduces the rate of venous stasis ulceration. Current guidelines recommend perforator treatment in cases of clinical severity, etiology, anatomy, pathophysiology score (CEAP) 5 and 6, with treatment of the perforator at the level of previous or active venous ulceration (5,19,20). Several authors also suggest treating incompetent perforator veins in cases of focal pain, focal swelling, associated varicose veins, focal skin irritation and/or discoloration in the area of the incompetent perforator vein (21,22).
USGS
USGS uses chemical agents to treat venous perforators and is the most commonly utilized and oldest minimally invasive ablation method used (23). It offers several advantages in that the technique is relatively easy technically and less complicated than other methods. US-guided access to the perforator vein is established; with confirmation with aspiration of blood ensure endoluminal position before ablation. Sodium morrhuate, sodium tetradecyl sulfate (STS), and aethoxysclerol are reported sclerosants in the literature (24-26). When in contact with the venous walls, these sclerosants cause denaturation of proteins, denude the endothelium, and cause direct tissue damage just beyond the vessel wall. The response is a result of this cell damage with fibroblast proliferation that leads to sclerosis and fibrosis. In addition to fibrosis, agents may produce other effects such as thrombosis, extraction of proteins from lipids, denaturation of proteins, cell dehydration by osmosis, and physical obstruction by polymerization.
Sclerotheraphy with a chemical foam, where the agents are mixed with air, has been reported to be more efficacious than injection of liquid (27), as it increases the time the ablation agents are in contact with the venous walls. This technique has benefits in that it can be injected into a tortuous perforator vein or its tributary, and with proper technique, sclerose the perforator vein up to its connection with the deep system. It can also treat all varicosities in relation to an incompetent perforator with a single injection (Figure 2). USGS can be performed quickly and without expensive equipment and catheters; although, the side effect profile may be wider when compared to other minimal invasive techniques. An early closure rate of 98% and a 20-month follow-up closure rate of 78% have been reported for incompetent perforator treatment. Patients with successfully treated incompetent perforator veins also had a significant improvement in their clinical scores and symptoms, proving the clinical success of this technique (28). Several small perforators frequently accompany the dominant perforator, and Doppler US can be effective in showing these perforators in close proximity to main dilated perforator vein (29,30). These small adjacent perforators can become insufficient after an initial treatment (29), and new or recurrent perforator disease is a well-described entity. USGS can easily be repeated in these situations.
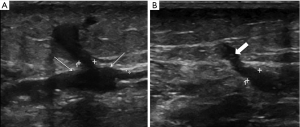
Local side effects include allergic reactions and phlebitis. Major complications include deep vein thrombosis (DVT) in the communicating deep venous system and pulmonary emboli. Case reports related to systemic embolization, including transient loss of vision and stroke have been reported (25,27,30). Close proximity of perforating veins to arteries has reported inadvertent embolization of the artery and subsequent extensive skin necrosis. This should be preventable with proper technique and confirmation of needle tip position within the vein and target vessel prior to injection or treatment. Hyperpigmentation may occur from heme trapped with the sclerosed superficial veins. Phlebectomy of the affected vein can be used to minimize this side effect (31).
EVTA
EVTA uses catheters equipped with radiofrequency or laser energy to thermally damage the endothelial lining of venous structures. EVTA has been used in superficial vein insufficiency treatment with satisfactory short and long-term results. EVTA is technically more complex than USGS. Specially designed 16- and 18-gauge cannulas are used for accessing the diseased perforator vein, often over a guidewire for endoluminal perforator access. A laser or radiofrequency fiber is then advanced coaxially through the introductory sheath. The fiber is usually placed at or just below the fascia to minimize deep vessel and nerve injury. Perivenous tumescent anesthesia is usually applied to minimize discomfort, protect surrounding tissues, and enhance device-wall apposition. Tumescent local anesthetic, pressure applied by the US probe, and Trendelenberg position can all be used to drain the perforator vein and provide better energy transmission through direct contact between the probe and perforator vein wall. The catheter is then withdrawn 1 to 2 mm between treatments, and consecutive levels of the target vein are treated depending on the length. Perforators can be focally treated at two or three different levels depending on the length of perforator. However, the deepest level of treatment should be 1–1.5 cm away from the deep venous system to minimize DVT risk. After energy delivery, pressure is applied to compress the walls of the treated perforator. Local side effects include ecchymosis, induration or paraesthesia. Rare systemic complications include DVT and pulmonary emboli (6,32,33).
For laser energy, commercially available 940-nm diode, 1,320 nm Nd:YAG and 1,470-nm microfibers can be used for perforator vein ablation (34-37). Commercially available radiofrequency probes are also available for perforator ablation treatment (Covidien, Mansfield, MA, USA). These probes have the capability of measuring impedance in the tissues as an additive security measure to real-time US visualization of the probe. An appropriate impedance value indicates endoluminal position and thermal ablative energy directed to the endothelial lining. Some treatment failures are attributed to perivascular positioning, resulting in insufficient contact with endothelial cells. Impedance value between 150 and 350 ohms indicates intraluminal placement while soft tissue placement registers higher values (18). Incompetent perforator veins can be treated in the same session as insufficient superficial vein ablation with the same equipment.
EVTA is technically more challenging than USGS; however, several studies have shown highly effective and durable closure rates, with closure rates ranging between 61% and 95% (37-40). A recent comparison between minimally invasive techniques for perforator ablation showed EVTA had better early closure rates when compared to USGS; however, this rate narrowly missed significance (40). Also, closure rates of laser and radiofrequency EVTA after failed USGS were 85% and 89% respectively. Closure rate of a second USGS after failure was reported at 50%, with thermal ablative techniques significantly better for repeat closure attempt than USGS, further indicating thermal ablation may be preferred for repeat procedures for incompetent perforators (40). BMI over 50 was reported as non-closure risk factor for all minimally invasive perforator treatments (18,40,41). Perforator size and the presence of deep vein reflux were not risk factors for non-closure (18,40).
Open surgery techniques for perforator vein disease have been mostly abandoned due to the invasiveness, possible complications, and these minimally invasive surgical substitutes. SEPS has been shown to offer the same success rate with significant decreased hospital stay and complications when compared with open surgery (42,43). However, the percutaneous treatments described here are gaining momentum as a less invasive alternative when compared to SEPS. These treatments offer several advantages in that they can be applied with local anesthesia or oral/IV sedation, and distal perforators around the malleolus are easily treatable unlike SEPS (24,32,44). These treatments are performed without incisions, and are easily repeatable if necessary. Older patients and patients with lower extremity edema and obesity can easily be treated, a usual contraindication to open surgery or SEPS (2,6,43). Both short-term and long-term closure rates at 10 years have been shown to be high and it is accepted as effective treatment for insufficient superficial veins (34,35,45). Short-term closure rates of perforator veins with minimally invasive treatment options is effective and similar to superficial vein closure rates (18).
Conclusions
Chemical and thermal ablative technology for perforator treatment will continue to improve. USGS can be recommended as a first-line treatment before thermal ablation because it is fast, minimally painful, and less expensive when compared to other modalities. USGS is also preferable in multiple communicating perforator veins. EVTA with laser or radiofrequency energy may be recommended in patients with initial failures by USGS and for patients with possible risk factors for failure, such as obesity. However, treatment modality for insufficient perforators is dependent on individual expertise, treatment modalities, clinical setting and patient preference.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Evans CJ, Fowkes FG, Ruckley CV, et al. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health 1999;53:149-53. [Crossref] [PubMed]
- Miller GV, Lewis WG, Sainsbury JR, et al. Morbidity of varicose vein surgery: auditing the benefit of changing clinical practice. Ann R Coll Surg Engl 1996;78:345-9. [PubMed]
- Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. Int Angiol 1999;18:83-102. [PubMed]
- Nicholls SC. Sequelae of Untreated Venous Insufficiency. Semin Intervent Radiol 2005;22:162-8. [Crossref] [PubMed]
- Pierik EG, Wittens CH, van Urk H. Subfascial endoscopic ligation in the treatment of incompetent perforating veins. Eur J Vasc Endovasc Surg 1995;9:38-41. [Crossref] [PubMed]
- Subramonia S, Lees T. Radiofrequency ablation vs conventional surgery for varicose veins - a comparison of treatment costs in a randomised trial. Eur J Vasc Endovasc Surg 2010;39:104-11. [Crossref] [PubMed]
- Gloviczki P, Gloviczki ML. Guidelines for the management of varicose veins. Phlebology 2012;27 Suppl 1:2-9. [Crossref] [PubMed]
- Caggiati A, Bergan JJ, Gloviczki P, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg 2002;36:416-22. [Crossref] [PubMed]
- Delis KT. Leg perforator vein incompetence: functional anatomy. Radiology 2005;235:327-34. [Crossref] [PubMed]
- Masuda EM, Kistner RL, Eklof B, et al. Practical application of the CEAP classification. In: Ballard JL, Bergan JJ. editors. Practical Application of the CEAP Classification. London: Springer London, 2000:41-9.
- Labropoulos N, Tassiopoulos AK, Bhatti AF, et al. Development of reflux in the perforator veins in limbs with primary venous disease. J Vasc Surg 2006;43:558-62. [Crossref] [PubMed]
- Cockett FB, Jones DE. The ankle blow-out syndrome; a new approach to the varicose ulcer problem. Lancet 1953;1:17-23. [Crossref] [PubMed]
- Labropoulos N, Mansour MA, Kang SS, et al. New insights into perforator vein incompetence. Eur J Vasc Endovasc Surg 1999;18:228-34. [Crossref] [PubMed]
- Kistner RL, Eklof B, Masuda EM. Diagnosis of chronic venous disease of the lower extremities: the "CEAP" classification. Mayo Clin Proc 1996;71:338-45. [Crossref] [PubMed]
- Fischer H. Socio-epidemiological study on distribution of venous disorders among a residential population. Int angiol 1984;3:89.
- Black CM, Smilanich RP, Worth ER. Endovascular Perforator Ablation. Endovascular Today 2007;63-70.
- Nael R, Rathbun S. Treatment of varicose veins. Curr Treat Options Cardiovasc Med 2009;11:91-103. [Crossref] [PubMed]
- Dillavou ED, Harlander-Locke M, Labropoulos N, et al. Current state of the treatment of perforating veins. J Vasc Surg Venous Lymphat Disord 2016;4:131-5. [Crossref] [PubMed]
- Petrie D, Chopra A, Chochinov A, et al. CAEP 2015 Academic Symposium: Recommendations for University Governance and Administration for Emergency Medicine. CJEM 2016:1-8. [Epub ahead of print].
- Hanrahan LM, Araki CT, Rodriguez AA, et al. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg 1991;13:805-11; discussion 811-2. [Crossref] [PubMed]
- Lawrence PF, Alktaifi A, Rigberg D, et al. Endovenous ablation of incompetent perforating veins is effective treatment for recalcitrant venous ulcers. J Vasc Surg 2011;54:737-42. [Crossref] [PubMed]
- Kiguchi MM, Hager ES, Winger DG, et al. Factors that influence perforator thrombosis and predict healing with perforator sclerotherapy for venous ulceration without axial reflux. J Vasc Surg 2014;59:1368-76. [Crossref] [PubMed]
- Isobe J, Onyeachom U, Taylor R, et al. Sclerotherapy Use for Chronic Venous Insufficiency Across the United States: A Report From the Venous Patient Outcome Registry. J Vasc Surg Venous Lymphat Disord 2016;4:144-5. [Crossref]
- Alden PB, Lips EM, Zimmerman KP, et al. Chronic venous ulcer: minimally invasive treatment of superficial axial and perforator vein reflux speeds healing and reduces recurrence. Ann Vasc Surg 2013;27:75-83. [Crossref] [PubMed]
- Bush R, Bush P. Percutaneous foam sclerotherapy for venous leg ulcers. J Wound Care 2013;22:S20-2. [PubMed]
- Bush RG, Bush P, Flanagan J, et al. Factors associated with recurrence of varicose veins after thermal ablation: results of the recurrent veins after thermal ablation study. ScientificWorldJournal 2014;2014:505843.
- Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg 2007;94:925-36. [Crossref] [PubMed]
- Masuda EM, Kessler DM, Lurie F, et al. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg 2006;43:551-6; discussion 556-7. [Crossref] [PubMed]
- Pierik EG, Toonder IM, van Urk H, et al. Validation of duplex ultrasonography in detecting competent and incompetent perforating veins in patients with venous ulceration of the lower leg. J Vasc Surg 1997;26:49-52. [Crossref] [PubMed]
- de Waard MM, der Kinderen DJ. Duplex ultrasonography-guided foam sclerotherapy of incompetent perforator veins in a patient with bilateral venous leg ulcers. Dermatol Surg 2005;31:580-3. [Crossref] [PubMed]
- Scultetus AH, Villavicencio JL, Kao TC, et al. Microthrombectomy reduces postsclerotherapy pigmentation: multicenter randomized trial. J Vasc Surg 2003;38:896-903. [Crossref] [PubMed]
- Beale RJ, Mavor AI, Gough MJ. Minimally invasive treatment for varicose veins: a review of endovenous laser treatment and radiofrequency ablation. Int J Low Extrem Wounds 2004;3:188-97. [Crossref] [PubMed]
- Proebstle TM, Alm BJ, Göckeritz O, et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. Br J Surg 2015;102:212-8. [Crossref] [PubMed]
- Min RJ, Zimmet SE, Isaacs MN, et al. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol 2001;12:1167-71. [Crossref] [PubMed]
- Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol 2003;14:991-6. [Crossref] [PubMed]
- Shepherd AC, Gohel MS, Lim CS, et al. Pain following 980-nm endovenous laser ablation and segmental radiofrequency ablation for varicose veins: a prospective observational study. Vasc Endovascular Surg 2010;44:212-6. [Crossref] [PubMed]
- Dumantepe M, Tarhan A, Yurdakul I, et al. Endovenous laser ablation of incompetent perforating veins with 1470 nm, 400 µm radial fiber. Photomed Laser Surg 2012;30:672-7. [Crossref] [PubMed]
- Zerweck C, von Hodenberg E, Knittel M, et al. Endovenous laser ablation of varicose perforating veins with the 1470-nm diode laser using the radial fibre slim. Phlebology 2014;29:30-6. [PubMed]
- Bozoglan O, Mese B, Eroglu E, et al. Comparison of Endovenous Laser and Radiofrequency Ablation in Treating Varicose Veins in the Same Patient. Vasc Endovascular Surg 2016;50:47-51. [Crossref] [PubMed]
- Hager ES, Washington C, Steinmetz A, et al. Factors that influence perforator vein closure rates using radiofrequency ablation, laser ablation, or foam sclerotherapy. J Vasc Surg Venous Lymphat Disord 2016;4:51-6. [Crossref] [PubMed]
- Hingorani AP, Ascher E, Marks N, et al. Predictive factors of success following radio-frequency stylet (RFS) ablation of incompetent perforating veins (IPV). J Vasc Surg 2009;50:844-8. [Crossref] [PubMed]
- Scriven JM, Bianchi V, Hartshorne T, et al. A clinical and haemodynamic investigation into the role of calf perforating vein surgery in patients with venous ulceration and deep venous incompetence. Eur J Vasc Endovasc Surg 1998;16:148-52. [Crossref] [PubMed]
- Roka F, Binder M, Bohler-Sommeregger K. Mid-term recurrence rate of incompetent perforating veins after combined superficial vein surgery and subfascial endoscopic perforating vein surgery. J Vasc Surg 2006;44:359-63. [Crossref] [PubMed]
- Laufer MD, Montgomery HD, inventor; Vnus Medical Technologies, Inc., assignee. Method and apparatus for minimally invasive treatment of chronic venous insufficiency. United States patent PCT/US1995/014803. 1996.
- Min RJ, Roizental M, Fernandez CF. EVLT of the SSV and Other Truncal Veins. Endovascular Today (Supplement) 2004:11-14.


