
Ultra-fast-track cardiac anesthesia in minimally invasive cardiac surgery: a retrospective observational study
Highlight box
Key findings
• Ultra-fast-track cardiac anesthesia (UFTCA) can promote rapid postoperative recovery in patients undergoing minimally invasive cardiac surgery (MICS). The potential benefits of UFTCA were demonstrated in shortening intensive care unit and postoperative hospital stay, a reduction in postoperative chest drainage volume and decreasing the occurrence of postoperative delirium in patients undergoing MICS.
What is known and what is new?
• Although patients with UFTCA are prone to transient hypoxia in the early stage after extubation and require high-flow oxygen therapy, it does not increase the occurrence of postoperative pulmonary complications, such as atelectasis and pulmonary edema. And the subsequent oxygenation seems to be restored better.
• However, patients with UFTCA are susceptible to postoperative nausea and vomiting (PONV), and the prevention of PONV needs to be improved.
What is the implication, and what should change now?
• UFTCA can promote enhanced recovery after cardiac surgery for patients undergoing MICS.
• Transient hypoxia in the early stage after extubation does not increase the incidence of postoperative pulmonary complications in patients undergoing UFTCA, and high-flow oxygen therapy can effectively improve this transient hypoxia.
• Patients with UFTCA are susceptible to PONV, and the prevention of PONV needs to be improved.
Introduction
In recent years, minimally invasive cardiac surgery (MICS) has witnessed rapid improvements, attributable to its associated benefits such as reduced trauma, expedited recovery, and aesthetically acceptable incisions. Since 2018, our cardiac surgery team has been proficiently executing thoracoscopic-assisted right-side axillary incision approach minimally invasive cardiac surgeries—a technique identified as scarless MICS (1). To date, approximately 83% of cardiac surgeries in Zhejiang Provincial People’s Hospital have been completed using minimally invasive methodologies.
Expanding upon the foundations of fast-track cardiac anesthesia (FTCA) and Ultra-fast-track cardiac anesthesia (UFTCA), the concept of Enhanced Recovery After Cardiac Surgery (ERACS) has been introduced (2,3). This necessitates collaborative efforts from cardiac surgery, anesthesia, extracorporeal circulation, and intensive care teams. This comprehensive approach incorporates a series of preoperative, intraoperative, and postoperative measures designed to facilitate prompt recovery for patients undergoing significant cardiac and vascular surgeries (4-6). FTCA refers to the removal of the tracheal tube within 6 hours postoperatively, while UFTCA involves extubation either immediately or within 1 hour postoperatively, and may even encompass immediate awakening and extubation on the operating table (7-9). Since April 2021, more than 700 patients undergoing MICS have been successfully performed with UFTCA in Zhejiang Provincial People’s Hospital (10-12).
This retrospective observational study focuses on patients who underwent MICS in Zhejiang Provincial People’s Hospital between January 2022 and July 2023. The aim is to compare the impact of UFTCA and conventional anesthesia on postoperative recovery, oxygenation, and complications in patients undergoing MICS. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-175/rc).
Methods
General information
Ethical approval for this study was obtained from the Ethics Committee of Zhejiang Provincial People’s Hospital (ethics No. QT2021KY045). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. Patients who underwent thoracoscopic-assisted right-side axillary incision approach MICS at Zhejiang Provincial People’s Hospital between January 2022 and July 2023 were included. They were retrospectively segregated into two groups: UFTCA group and conventional general anesthesia (CGA group).
Inclusion criteria were as follows: (I) American Society of Anesthesiologists (ASA) classification levels I–III; (II) New York Heart Association (NYHA) functional classification III or below; (III) left ventricular diastolic function grade II or below; (IV) age 18–79 years; (V) no contraindications to neuraxial blockade. Exclusion criteria were: (I) severe communication barriers; (II) NYHA functional classification IV; (III) severe pulmonary hypertension (pulmonary artery systolic pressure ≥70 mmHg); (IV) intraoperative cerebrovascular incidents; (V) re-entry thoracotomy for postoperative hemostasis; (VI) emergency cardiac surgery; (VII) patients with incomplete or lost data.
A total of 107 patients were excluded, of whom 62 had NYHA class IV, 17 had severe pulmonary hypertension, 11 were age ineligible for inclusion, 16 were ASA grade IV, and 1 case was intraoperative cerebrovascular accident. A total of 543 patients were finally included, comprising 42 cases of left ventricular outflow tract obstruction surgery, 40 atrial septal defect repairs, 20 ascending aorta replacements, 8 cardiac tumor excisions, 116 aortic valve replacements (AVR), 145 mitral valve replacements (MVR), 43 mitral valve repairs (MVP), 17 tricuspid valvuloplasty (TVP), 21 combined AVR and MVR, 20 combined AVR and MVP, 22 combined MVP and TVP, and 49 combined MVR and TVP surgeries. Out of these 543 patients, 327 underwent UFTCA, while 216 received CGA. The research flowchart was shown in Figure 1.

Anesthesia, extracorporeal circulation, and surgical methods
Upon admission to the surgical theater, patients underwent electrocardiogram (ECG) assessment, measurement of blood oxygen saturation (SpO2), and the establishment of invasive arterial blood pressure monitoring through radial artery cannulation. Anesthesia depth was assessed using the bispectral index (BIS), and external defibrillation electrode pads were connected. Cannulation of peripheral veins was conducted subsequent to anesthesia induction. Following induction, internal jugular vein cannulation and placement of the transesophageal echocardiography (TEE) probe were carried out. Intraoperative monitoring included the observation of end-tidal carbon dioxide partial pressure (PETCO2), central venous pressure (CVP), urine output, as well as nasopharyngeal and rectal temperatures.
CGA is used because some patients and some cardiac anesthesiologists have concerns about the safety of UFTCA. Therefore, we conducted this retrospective analysis to verify the security of UFTCA.
UFTCA group
The following medications were administered to induce anesthesia: propofol (0.5–1.0 mg/kg), etomidate (0.2 mg/kg), cisatracurium besylate (0.15 mg/kg), and sufentanil (0.4–0.5 µg/kg), followed by the initiation of single-lumen tracheal intubation. Subsequent to induction, a composite solution of 0.375% ropivacaine with 1 µg/kg dexmedetomidine, constituting a total volume of 40 mL, was used for thoracic fascial blocks: (I) deep anterior serratus plane block: 20 mL of the mixture was instilled between the serratus anterior muscle and the rib surface along the midaxillary line at the 4th rib; (II) pectoral fascial I (PECS I) block: 10 mL was injected between the pectoralis major and pectoralis minor muscles along the anterior axillary line below the clavicle; (III) pectoral fascial II (PECS II) block: 10 mL was injected between the serratus anterior and pectoralis minor muscles along the anterior axillary line below the clavicle.
The maintenance of anesthesia involved the intravenous infusion of propofol (100–200 µg/kg/min), remifentanil (0.1–0.5 µg/kg/min), and cisatracurium besylate (1.0–2.0 µg/kg/min). Intraoperatively, contingent upon hemodynamics and surgical timing, sufentanil was administered as a single intravenous dose of 0.1–0.15 µg/kg as required, with the total sufentanil dosage ranging from 1–1.5 µg/kg. The depth of anesthesia was sustained within a BIS value range of 40–60.
Anesthesia recovery: approximately 30 minutes before the conclusion of the surgical procedure, adjustments were made to the maintenance doses of propofol and remifentanil based on the degree of surgical stimulation. Additionally, the infusion of cisatracurium besylate was ceased. All intravenous anesthetic drugs were terminated 20 minutes prior to the conclusion of the surgery. Propofol (20–30 mg) was administered as necessary to sustain sedation until the conclusion of the surgical intervention. Patients who promptly regained consciousness and fulfilled extubation criteria at the conclusion of the procedure underwent tracheal tube removal at the operating table. Patients anticipated to be incapable of tracheal tube removal within the first 30 minutes post-surgery were transitioned to the post-anesthesia care unit (PACU). In cases where a patient could not be roused for tracheal tube removal within 1 hour in the PACU, the patient was expeditiously transferred to the intensive care unit (ICU) with the tracheal tube remaining in situ for further resuscitation and extubation. The flowchart of UFTCA was shown in Figure 2. The above was the protocols for UFTCA in Zhejiang Provincial People’s Hospital. However, only one of the patients included in this study who planned to undergo UFTCA gave up UFTCA due to intraoperative cerebrovascular complications and was excluded. All the rest were successfully implemented with UFTCA.

Criteria for tracheal tube removal: (I) hemodynamic stability characterized by the absence or minimal requirement for vasopressors, dopamine not exceeding ≤5.0 µg/kg/min, norepinephrine not surpassing ≤0.05 µg/kg/min, hemoglobin levels equal to or exceeding ≥80 g/L, and the absence of uncorrectable arrhythmias. (II) Arterial blood gas pH value falling within the range of 7.35 to 7.45, indicative of an uncompromised internal environment. (III) Lack of bleeding and absence of coagulation dysfunction. (IV) Consciousness, as evidenced by the ability to comply with commands such as opening the eyes, lifting the head, sticking out the tongue, and forming a fist. (V) Uninterrupted spontaneous breathing accompanied by restored airway protective reflexes. SpO2 of ≥95% during mask oxygenation, arterial oxygen partial pressure (PaO2) equal to or exceeding ≥60 mmHg, and arterial carbon dioxide partial pressure (PaCO2) within the range of 35–45 mmHg.
CGA group
The following medications were administered to induce anesthesia: propofol (0.5–1 mg/kg), etomidate (0.2 mg/kg), cisatracurium besylate (0.2 mg/kg), and sufentanil (0.4–0.5 µg/kg), followed by the implementation of single-lumen tracheal intubation. Anesthesia maintenance entailed the intravenous infusion of propofol (100–200 µg/kg/min), remifentanil (0.1–0.5 µg/kg/min), and cisatracurium besylate (1.0–2.0 µg/kg/min). Sufentanil was incrementally introduced, culminating in a total intraoperative dose of approximately 3 µg/kg. The doses of remifentanil, propofol, and sufentanil were adjusted in response to the intensity of surgical stimulation, maintaining anesthetic depth within a targeted entropy index (EI) range of 40–60. Intravenous anesthetic drugs were sustained until the conclusion of the surgical procedure, after which patients were promptly transferred to the ICU for recovery and extubation. The criteria for tracheal extubation in the CGA group were the same as those in the UFTCA group.
Postoperative pain management for all patients encompassed the use of an intravenous analgesia pump. In cases where the Visual Analogue Scale (VAS) score was ≥3, tramadol was administered for rescue analgesia.
Surgical method: extracorporeal circulation was established through the femoral artery and vein, with a 4-cm longitudinal incision in the right midaxillary line at the fourth intercostal space for thoracic access. Throughout the procedure, continuous insufflation of the thoracic field with 3–5 L/min of CO2 was maintained until the conclusion of intrathoracic manipulations. Following cardiac resumption, TEE was used to assess surgical outcomes, cardiac function, valve conditions, guide venting from the heart chambers, and detect any residual intracardiac shunts. Upon confirming adequate hemostasis, a closed chest drainage tube was inserted through a small axillary incision prior to thoracic closure and suturing of the incision.
All patients followed the same intraoperative lung protection strategy: (I) glucocorticoids were administered intravenously, with a dose of 1.5–2.0 mg/kg of methylprednisolone administered before the initiation of extracorporeal circulation. (II) Throughout full-flow extracorporeal circulation, minimal tidal volume ventilation was maintained to the extent possible without compromising surgical manipulations, using a 30% oxygen concentration, tidal volume set at 60–80 mL, respiratory rate maintained at 5–8 breaths per minute, and positive end-expiratory pressure (PEEP) set at 3–5 cmH2O. (III) Preloading fluids for extracorporeal circulation encompassed the administration of 500 mL of dextran, 100 mL of 20% albumin, and 300,000 U of ulinastatin. (IV) Ultrafiltration of extracorporeal fluids was conducted as deemed necessary during extracorporeal circulation, guided by overall volume status and hematocrit levels, sustaining a hematocrit level of ≥24%.
For patients in the UFTCA group who were transferred to the ICU post-extubation, or for the CGA group patients who underwent extubation in the ICU, high-flow nasal cannula oxygen therapy was administered in cases where, despite nasal cannula or mask oxygenation, their SpO2 remained below 95%, PaO2 fell below 80 mmHg, or the oxygenation index dropped below 200 mmHg.
Observation indicators
Primary indicators: duration of postoperative tracheal tube intubation, ICU stay, and hospital stay.
Secondary indicators: the parameters assessed included the in-hospital mortality rate and the survival rate within 3 months post-discharge. Additionally, intraoperative opioid dosage and the occurrence of postoperative rescue analgesia were analyzed. Measurements of the oxygenation index [PaO2/fraction of inspired oxygen (FiO2)], alveolar-arterial oxygen tension difference [P(A-a)O2], and respiratory index [P(A-a)O2/PaO2] were recorded before surgery (T0), immediately after extubation (T1), 6 hours (T2), and 12 hours (T3) after extubation. We also investigated the number of cases requiring high-flow humidified oxygen therapy in the ICU, the prevalence of postoperative atelectasis and pulmonary edema, and preoperative and 1-hour postoperative neutrophil counts. Other considerations encompassed chest drainage volume within the initial 24 hours postoperatively, total chest drainage volume, and the occurrence of postoperative nausea and vomiting (PONV) and delirium.
Statistical analysis
Data analysis was conducted using SPSS software version 26.0. Quantitative data displaying a normal distribution are expressed as mean ± standard deviation, and group comparisons were performed using grouped t-tests and repeated measures analysis of variance (ANOVA). Skewed data are presented as median [interquartile range] and analyzed using rank-sum tests. Count data were compared using the Chi-squared test or Fisher’s exact test. With two-sided test, P value <0.05 was deemed statistically significant.
Results
No statistically significant differences were observed between the two groups in terms of general patient characteristics, types of surgery, extracorporeal circulation time, arterial clamping time, and total operation time (P>0.05) (refer to Table 1).
Table 1
| Indicators | UFTCA group (n=327) | CGA group (n=216) | t/Z/χ2 value | P value |
|---|---|---|---|---|
| Age (years) | 55.5 [22] | 57 [17] | 1.446 | 0.14 |
| Male | 166 (50.8) | 111 (51.4) | 0.020 | 0.88 |
| Height (cm) | 165 [10] | 165 [10] | 0.470 | 0.63 |
| Weight (kg) | 62 [16] | 62 [16] | 0.463 | 0.64 |
| BMI (kg/m2) | 23.43 [4.44] | 23.04 [4.42] | 0.796 | 0.42 |
| ASA class | 1.353 | 0.24 | ||
| Class II | 5 (1.5) | 1 (0.5) | ||
| Class III | 322 (98.5) | 215 (99.5) | ||
| NYHA class | 1.329 | 0.24 | ||
| Class II | 186 (56.9) | 112 (51.9) | ||
| Class III | 141 (43.1) | 104 (48.1) | ||
| Comorbidities | ||||
| Hypertension | 85 (26.0) | 70 (32.4) | 2.623 | 0.10 |
| Diabetes | 17 (5.2) | 16 (7.4) | 1.112 | 0.29 |
| Atrial fibrillation | 83 (25.4) | 62 (28.7) | 0.733 | 0.39 |
| Coronary heart disease | 29 (8.9) | 19 (8.8) | 0.001 | 0.97 |
| History of cerebral infarction | 14 (4.3) | 11 (5.1) | 0.195 | 0.65 |
| Types of surgery | ||||
| Left ventricular outflow tract obstruction | 27 (8.3) | 15 (6.9) | ||
| Atrial septal defect repair | 24 (7.3) | 16 (7.4) | ||
| Ascending aorta replacement | 12 (3.7) | 8 (3.7) | ||
| Cardiac tumor excision | 5 (1.5) | 3 (1.4) | ||
| AVR | 70 (21.4) | 46 (21.3) | ||
| AVR and MVP | 12 (3.7) | 8 (3.7) | ||
| AVR and MVR | 12 (3.7) | 9 (4.2) | ||
| MVP | 26 (8) | 17 (7.9) | ||
| MVP and TVP | 15 (4.6) | 8 (3.7) | ||
| MVR | 86 (26.3) | 59 (27.3) | ||
| MVR and TVP | 29 (8.9) | 20 (9.3) | ||
| TVP | 9 (2.8) | 7 (3.2) | ||
| Extracorporeal circulation time (min) | 140 [53] | 143 [57] | 1.487 | 0.13 |
| Aortic clamping time (min) | 91 [47] | 91 [44] | 0.275 | 0.78 |
| Surgery duration (min) | 270 [70] | 285 [70] | 1.885 | 0.059 |
Skewed data are presented as median [interquartile range] and categorical data as percentage (%). Statistically significant difference (P<0.05). BMI, body mass index; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; AVR, aortic valve replacement; MVP, mitral valve repair; MVR, mitral valve replacement; TVP, tricuspid valve repair; UFTCA, ultra-fast-track cardiac anesthesia; CGA, conventional general anesthesia.
Intraoperative and postoperative analgesia
The intraoperative sufentanil dosage in the UFTCA group was 60 [20] µg, demonstrating a significant reduction compared to the 80 [40] µg in the CGA group (P=0.001). There were no noteworthy differences between the two groups in remifentanil usage and the number of patients requiring rescue analgesia (P>0.05). See Table 2.
Table 2
| Indicators | UFTCA group (n=327) | CGA group (n=216) | t/Z/χ2 value | P value |
|---|---|---|---|---|
| Intraoperative sufentanil (μg) | 60 [20] | 80 [40] | 8.073 | 0.001 |
| Intraoperative remifentanil (mg) | 3 [1.3] | 2.9 [1.2] | 1.770 | 0.07 |
| Rescue analgesia | 151 (46.2) | 82 (38.0) | 3.583 | 0.058 |
Skewed data are presented as median [interquartile range] and categorical data as percentage (%). Statistically significant difference (P<0.05). UFTCA, ultra-fast-track cardiac anesthesia; CGA, conventional general anesthesia.
Postoperative recovery and complications
The postoperative recovery details and complications are outlined in Table 3.
Table 3
| Indicators | UFTCA group (n=327) | CGA group (n=216) | t/Z/χ2 value | P value |
|---|---|---|---|---|
| Time to tracheal tube removal postoperatively (minutes) | 10 [15] | 920 [644] | 19.774 | 0.001 |
| ICU stay duration (hours) | 23 [20] | 43 [45] | 9.019 | 0.001 |
| Hospital stay duration (days) | 8 [3] | 10 [5] | 6.074 | 0.001 |
| In-hospital mortality | 0 | 1 (0.5) | 1.519 | 0.21 |
| 3-month post-discharge survival rate | 327 (100.0) | 215 (99.5) | – | – |
| 3-month post-discharge NYHA class | 0.846 | 0.65 | ||
| Class I | 281 (86.0) | 190 (88.0) | ||
| Class II | 43 (13.1) | 22 (10.2) | ||
| Class III | 3 (0.9) | 4 (1.8) | ||
| 24-hour chest drainage volume (mL) | 180 [163] | 180 [200] | 0.881 | 0.37 |
| Total chest drainage volume (mL) | 465 [470] | 610 [665] | 4.070 | 0.001 |
| Preoperative blood neutrophil count (109/L) | 3.4 [1.56] | 3.34 [1.85] | 0.144 | 0.88 |
| Postoperative blood neutrophil count (109/L) | 12.93 [4.97] | 10.49 [5.82] | 6.094 | 0.001 |
| Atelectasis | 85 (26.0) | 68 (31.5) | 1.936 | 0.16 |
| Pulmonary edema | 14 (4.3) | 12 (5.6) | 0.463 | 0.49 |
| High-flow oxygen therapy in the ICU | 172 (52.6) | 67 (31.0) | 25.322 | 0.001 |
| Nausea/vomiting | 55 (16.8) | 20 (9.3) | 6.246 | 0.01 |
| Delirium | 3 (0.9) | 14 (6.5) | 13.361 | 0.001 |
Skewed data are presented as median [interquartile range] and categorical data as percentage (%). Statistically significant difference (P<0.05). ICU, intensive care unit; NYHA, New York Heart Association; UFTCA, ultra-fast-track cardiac anesthesia; CGA, conventional general anesthesia.
In the UFTCA group, the postoperative tracheal tube removal time was 10 [15] minutes, significantly shorter compared to the CGA group, which had a median time of 920 [644] minutes (P=0.001). The ICU stay for the UFTCA group was 23 [20] hours, markedly less than the 43 [45] hours for the CGA group (P=0.001). The hospital stay for the UFTCA group was 8 [3] days, which was shorter than the 10 [5] days for the CGA group (P=0.001). All patients in the UFTCA group were successfully discharged, while there was one in-hospital death in the CGA group; however, there was no significant difference in the in-hospital mortality rate between the two groups (P>0.05). No deaths occurred in either group within 3 months postoperatively, furthermore, there was no significant difference in cardiac function classification (P>0.05).
The 24-hour postoperative chest drainage volume revealed no significant difference between the two groups (P>0.05), however, the total chest drainage volume in the UFTCA group was 465 [470] mL, which was less than the 610 [665] mL in the CGA group (P=0.001).
No significant differences were observed in the incidence of postoperative atelectasis and pulmonary edema between the two groups (P>0.05). However, 172 patients (52.6%) in the UFTCA group required high-flow oxygen therapy after extubation to maintain oxygenation, a higher proportion than the 67 patients (31.0%) in the CGA group (P=0.001). The postoperative blood neutrophil count was higher in the UFTCA group than in the CGA group (P=0.001).
The prevalence of PONV in the UFTCA group was 55 cases (16.8%), higher than the 20 cases (9.3%) in the CGA group (P=0.01). However, the incidence of delirium was lower in the UFTCA group, with 3 cases (0.9%) compared to 14 cases (6.5%) in the CGA group (P=0.001).
Oxygenation status
The preoperative and postoperative oxygenation statuses are elaborated in Table 4 and Figures 3-5.
Table 4
| Indicators | Time point | UFTCA group (n=327) | CGA group (n=216) | P value |
|---|---|---|---|---|
| PaO2/FiO2 (mmHg) | T0 | 404.48±82.64 | 398.39±92.71 | 0.42 |
| T1 | 245.79±110.46 | 341.85±101.79 | 0.001 | |
| T2 | 317.94±117.94 | 299.17±113.65 | 0.06 | |
| T3 | 338.55±121.23 | 299.84±106.50 | 0.001 | |
| P(A-a)O2 (mmHg) | T0 | 25.75±24.11 | 24.04±18.07 | 0.37 |
| T1 | 201.00±119.56 | 128.85±65.21 | 0.001 | |
| T2 | 152.01±82.41 | 144.18±80.46 | 0.27 | |
| T3 | 128.56±76.43 | 140.21±77.59 | 0.08 | |
| P(A-a)O2/PaO2 | T0 | 0.31±0.27 | 0.29±0.21 | 0.33 |
| T1 | 2.14±1.65 | 1.18±0.86 | 0.001 | |
| T2 | 1.32±1.03 | 1.41±1.06 | 0.30 | |
| T3 | 1.12±0.93 | 1.35±0.93 | 0.006 |
Continuous data were represented as mean ± standard deviation. Statistically significant difference (P<0.05). PaO2/FiO2: oxygenation index; P(A-a)O2/PaO2: respiratory index; T0: before surgery; T1: immediately after extubation; T2: 6 h after extubation; T3: 12 h after extubation. UFTCA, ultra-fast-track cardiac anesthesia; CGA, conventional general anesthesia; PaO2, arterial oxygen partial pressure; FiO2, fraction of inspired oxygen; P(A-a)O2, alveolar-arterial oxygen partial pressure difference.
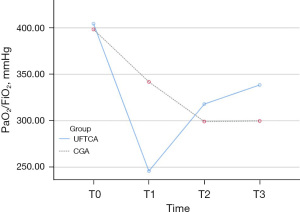


At T1, the PaO2/FiO2 was significantly lower in the UFTCA group (245.79±110.46 mmHg) compared to the CGA group (341.85±101.79 mmHg, P=0.001). However, at T3, the UFTCA group exhibited a higher PaO2/FiO2 (338.55±121.23 mmHg) than the CGA group (299.84±106.50 mmHg, P=0.001). There were no notable differences between the two groups at T0 and T2 (P>0.05). Neither the UFTCA nor CGA group had patients who needed reintubation.
At T1, the P(A-a)O2 in the UFTCA group was 201.00±119.56 mmHg, significantly higher than the CGA group (128.85±65.21 mmHg, P=0.001). No significant differences in P(A-a)O2 were observed between the groups at T0, T2, and T3 (P>0.05).
The P(A-a)O2/PaO2 value at T1 in the UFTCA group was 2.14±1.65, higher than in the CGA group (1.18±0.86, P=0.001). At T3, the P(A-a)O2/PaO2 value in the UFTCA group (1.12±0.93) was lower than in the CGA group (1.35±0.93, P=0.006).
Discussion
MICS has become a predominant trend, constituting nearly 83% of cardiac procedures at Zhejiang Provincial People’s Hospital. This encompasses a diverse array of surgeries, including AVR, MVP or MVR, TVP, multiple valve replacements, surgical treatment of atrial fibrillation such as the maze procedure, radiofrequency ablation, left atrial appendage plication or clipping, various corrections for congenital heart diseases, ascending aorta replacement, and myxoma removal. A distinctive procedure pioneered at Zhejiang Provincial People’s Hospital involves a small axillary incision for hypertrophic cardiomyopathy excision and outflow tract clearance through the aorta (1).
The goal is to advance recovery following cardiac surgery, with a focus on diminishing postoperative complications and expediting the recuperative process through initiatives such as early weaning from mechanical ventilation, prompt extubation, and reduction in ICU stay. Since April 2021, Zhejiang Provincial People’s Hospital has successfully implemented UFTCA in approximately 700 cases for patients undergoing thoracoscopic-assisted right-side axillary incision approach MICS. Employing predefined inclusion and exclusion criteria, we retrospectively compared UFTCA with CGA with respect to postoperative recovery, oxygenation, and complications in patients undergoing MICS, in this study. The primary objective was to affirm the safety, feasibility, and potential complications associated with UFTCA.
In the UFTCA group, comprising of 327 patients, tracheal tubes were expeditiously removed post-surgery, with a median time of 10 [15] minutes. Subsequently, patients were transferred to the ICU and typically relocated to the cardiac surgery ward the next day, following stabilization, with an ICU stay duration of 23 [20] hours. Conversely, in the CGA group of 216 patients, tracheal tubes remained in place post-surgery when transferred to the ICU. The time for tube removal in the CGA group was significantly prolonged at 920 [644] minutes (P=0.001). Upon stabilization, patients were returned to the cardiac surgery ward, but the ICU stay duration was protracted to 43 [45] hours. This elongated duration was primarily attributed to staff shortages in the ICU during night hours at Zhejiang Provincial People’s Hospital, resulting in intentional delays in extubation to avoid night shifts, consequently extending the ICU stay (P=0.001). This circumstance played a pivotal role in the initial proposal of UFTCA by the surgical team of Zhejiang Provincial People’s Hospital. The postoperative hospital stay for the UFTCA group was 8 [3] days, significantly shorter than the 10 [5] days observed in the CGA group (P=0.001). In the CGA group, one patient successfully transitioned out of the ICU postoperatively but later experienced malignant arrhythmia in the ward and passed away, leading to a 0.5% in-hospital mortality rate. No in-hospital deaths occurred in the UFTCA group. However, there was no significant difference in in-hospital mortality rates between the two groups. Three months post-discharge, all 327 patients in the UFTCA group survived, with their heart function classified by the NYHA as grade I/II/III for 281/43/3 patients, respectively. The 215 patients in the CGA group survived 3 months post-discharge, with NYHA grades I/II/III for 190/22/4 patients respectively. There was no significant difference in the 3-month postoperative survival rates and cardiac function recovery between the two groups.
These findings align with the study by Berretta et al., suggesting that implementing UFTCA in MICS is safe (13). In this study, early extubation in the UFTCA group led to significantly shorter ICU and postoperative hospital stay, thereby conserving medical resources. Bianchi et al. revealed that extubation in the operating room for adult congenital heart disease is feasible, and early extubation can reduce postoperative dependence on vasopressors (14). Ahmad et al. also achieved favorable outcomes by combining minimally invasive left ventricular assist device surgery with ultra-fast-track anesthesia (15).
The success of UFTCA at Zhejiang Provincial People’s Hospital can be attributed to the implementation of a balanced anesthesia protocol with precise control over anesthesia depth. In minimally invasive cardiac surgeries, we employ a low-dose opioid balanced anesthesia protocol, maintaining BIS values between 40–60. This approach reduced intraoperative opioid usage while achieving effective analgesia. The intravenous anesthesia in the UFTCA group incorporated a three-point pectoral and serratus plane block, including the deep anterior serratus plane block, PECS I block, and PECS II block. Both groups received continuous intravenous infusion of remifentanil at 0.1–0.5 µg/kg/min. In the UFTCA group, sufentanil dosage is controlled at 1–1.5 µg/kg, totaling 60 [20] µg. In contrast, the CGA group did not use the pectoral muscles serratus plane block and used approximately 3 µg/kg of sufentanil, totaling 80 [40] µg.
Traditional cardiac anesthesia protocols suggest that high-dose opioids can maintain hemodynamic stability, prevent increased myocardial oxygen consumption, and reduce adverse reactions caused by surgical stress. However, high-dose opioids can significantly increase the incidence of postoperative respiratory dysfunction, the need for mechanical ventilation, and the length of ICU stays. This not only increases hospitalization costs but also raises the likelihood of nosocomial infections. Low-dose opioids, whether short-acting or long-acting, are safe and effective in adult patients who have undergone cardiac surgery, regardless of the clinical characteristics of the patients and the types of opioids used (16).
Lung function is crucial for successful tracheal tube removal and patient prognosis, with PaO2/FiO2, P(A-a)O2, and P(A-a)O2/PaO2 being important indicators for assessing lung function (17). The PaO2/FiO2 and P(A-a)O2/PaO2 are not affected by the concentration of inhaled oxygen. A decrease in the PaO2/FiO2 suggests reduced pulmonary ventilation or increased intrapulmonary shunting, while a higher P(A-a)O2/PaO2 indicates poorer lung ventilation and oxygen exchange. In this study, we found that immediately after extubation, the UFTCA group had a lower PaO2/FiO2 compared to the CGA group (P=0.001), indicating reduced oxygenation. Zhejiang Provincial People’s Hospital uses high-flow nasal cannula oxygen therapy for patients whose SpO2 remains below 95%, PaO2 is below 80 mmHg, or the PaO2/FiO2 is below 200 mmHg under routine nasal cannula or mask oxygenation. A significantly higher proportion of patients in the UFTCA group (52.8%, 172 patients) required high-flow nasal cannula oxygen therapy compared to the CGA group (30.9%, 67 patients) (P=0.001). But neither the UFTCA nor CGA group had patients who needed reintubation. However, at 12 hours after extubation, the UFTCA group demonstrated better PaO2/FiO2 and P(A-a)O2/PaO2 values than the CGA group (P=0.001, P=0.006), indicating that the initial decrease in oxygenation in the UFTCA group was transient and improved over time with support from high-flow nasal cannula oxygen therapy. In contrast, due to longer mechanical ventilation times, the CGA group may have experienced ventilator-induced lung injury. These results indicate that although there is a transient decline in lung function immediately after extubation, this does not impair the recovery of postoperative lung function.
The UFTCA group revealed higher neutrophil counts at 1 hour postoperatively compared to the CGA group (P=0.001). Neutrophils are further activated by hypoxia, bleeding, ischemia-reperfusion injury, and endotoxin release, leading to the release of oxygen radicals and proteases. Although this observation revealed higher postoperative blood neutrophils in the UFTCA group, the correlation between this and lung injury or the lower oxygenation index at 1 hour postoperatively in the UFTCA group requires further study.
The incidence of atelectasis in the UFTCA group was 26.0%, compared to 31.5% in the CGA group, but this difference was not statistically significant. It has been reported that 24% of postoperative cardiac surgery patients experience postoperative atelectasis (18). The incidence of pulmonary edema was 4.3% in the UFTCA group and 5.6% in the CGA group, with no significant difference between the two. Studies by Inoue et al. and Khalil et al. have revealed that the incidence of pulmonary edema ranges from 1.6% to 7.8% (19,20).
Within the first 24 hours, the chest drainage volume was 180 [163] mL for the UFTCA group and 180 [200] mL for the CGA group, showing no significant difference. However, the total chest drainage volume was higher in the CGA group, 610 [665] mL, compared to 465 [470] mL in the UFTCA group. The specific mechanisms behind this difference require further investigation.
UFTCA, although effective in achieving rapid recovery through early mechanical ventilation weaning and early extubation, does face challenges with the incidence of PONV, which not only affects patient comfort but also impacts postoperative recovery. At Zhejiang Provincial People’s Hospital, routine prophylactic use of dexamethasone, palonosetron, and haloperidol significantly reduced PONV, yet it still occurred in 16.8% of patients in the UFTCA group, compared to only 9.3% in the CGA group. Although this overall rate is similar to the findings of Weibel et al. (21), Hijazi et al. observed a difference in PONV incidence between FTCA patients (tracheal tube removal within 6–10 hours postoperatively) and those with tube removal after 24–48 hours (22). They found that the earlier removal group had lower rates of postoperative nausea (10.3%) and vomiting (15.3%) compared to the later removal group. There were no differences in factors such as gender, age, and surgery duration related to PONV between the two groups of patients, yet the PONV incidence was higher in the UFTCA group than in the CGA group (P=0.01). Potential reasons include the lower intraoperative sufentanil dosage in the UFTCA group. Although statistically not significantly different, more patients in the UFTCA group required tramadol for rescue analgesia postoperatively, leading to higher PONV incidence. Another possible reason could be the rapid postoperative awakening of patients, with residual anesthetic drugs or their metabolites in the body causing PONV. Therefore, it is necessary to further explore more optimized anesthesia and postoperative analgesic regimens, and comprehensive prevention of PONV.
The incidence of delirium in the UFTCA group was 0.9%, significantly lower than the 6.5% in the CGA group. This difference could be attributed to the prolonged duration of anesthesia and mechanical ventilation in the ICU, which may exacerbate neuroinflammation.
The limitation of this study is that it is a single-center retrospective study, which may introduce bias. A multicenter, prospective, randomized controlled trial is currently being conducted at Zhejiang Provincial People’s Hospital to obtain more objective clinical outcomes on UFTCA.
Conclusions
In conclusion, the implementation of UFTCA in MICS demonstrates safety. Despite a transient decline in lung function and an increased need for high-flow nasal cannula oxygen therapy after extubation, there are no significant differences in the rates of postoperative atelectasis and pulmonary edema, and this does not hinder the recovery of postoperative lung function. UFTCA contributes to a shortened ICU stay and postoperative hospital duration, a reduction in the incidence of postoperative delirium, a decrease in postoperative chest drainage volume, and facilitates rapid postoperative recovery. However, additional measures are necessary to mitigate the incidence of PONV.
Acknowledgments
We would like to thank Jenny Taylor for her help in polishing our paper.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-175/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-175/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-175/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-175/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval for this study was obtained from the Ethics Committee of Zhejiang Provincial People’s Hospital (ethics No. QT2021KY045). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cui Y. Exploration of a minimally invasive approach for cardiac macrovascular surgery-right underarm microincision approach without visible scar. Cardio-cerebrovascular Disease Prevention and Treatment 2022;(1):17-19+33.
- Zhang TZ, Wu X. Review and prospect of accelerated rehabilitation cardiac surgery. Journal of Clinical Anesthesiology 2020;36:944-7.
- Cohen B, Turan A. Enhanced recovery after cardiac surgery - Is evidence still necessary? J Clin Anesth 2019;54:171-2. [Crossref] [PubMed]
- Yang L, Kaye AD, Venakatesh AG, et al. Enhanced Recovery after Cardiac Surgery: An Update on Clinical Implications. Int Anesthesiol Clin 2017;55:148-62. [Crossref] [PubMed]
- Williams JB, McConnell G, Allender JE, et al. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J Thorac Cardiovasc Surg 2019;157:1881-8. [Crossref] [PubMed]
- Grant MC, Isada T, Ruzankin P, et al. Results from an enhanced recovery program for cardiac surgery. J Thorac Cardiovasc Surg 2020;159:1393-1402.e7. [Crossref] [PubMed]
- Noss C, Prusinkiewicz C, Nelson G, et al. Enhanced Recovery for Cardiac Surgery. J Cardiothorac Vasc Anesth 2018;32:2760-70. [Crossref] [PubMed]
- Li M, Zhang J, Gan TJ, et al. Enhanced recovery after surgery pathway for patients undergoing cardiac surgery: a randomized clinical trial. Eur J Cardiothorac Surg 2018;54:491-7. [Crossref] [PubMed]
- Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg 2019;154:755-66. [Crossref] [PubMed]
- Jiang S, Wang L, Teng H, et al. The Clinical Application of Ultra-Fast-Track Cardiac Anesthesia in Right-Thoracoscopic Minimally Invasive Cardiac Surgery: A Retrospective Observational Study. J Cardiothorac Vasc Anesth 2023;37:700-6. [Crossref] [PubMed]
- Jiang SJ, Teng HK, Wei HW, et al. Efficacy of pectoral muscle fascial plane block using dexmedetomidine combined with ropivacaine in minimally invasive cardiac valve surgery via right axillary approach with ultra-fast track cardiac anesthesia. Zhejiang Medical Journal 2022;44:1627-31.
- Yan MJ, Jiang SJ, Luo XP, et al. Ultrafast channel anesthesia management for minimally invasive cardiac surgery. Zhejiang Clinical Medicine Journal 2023;25:134-7.
- Berretta P, De Angelis V, Alfonsi J, et al. Enhanced recovery after minimally invasive heart valve surgery: Early and midterm outcomes. Int J Cardiol 2023;370:98-104. [Crossref] [PubMed]
- Bianchi P, Constantine A, Costola G, et al. Ultra-Fast-Track Extubation in Adult Congenital Heart Surgery. J Am Heart Assoc 2021;10:e020201. [Crossref] [PubMed]
- Ahmad U, Khattab MA, Schaelte G, et al. Combining Minimally Invasive Surgery With Ultra-Fast-Track Anesthesia in HeartMate 3 Patients: A Pilot Study. Circ Heart Fail 2022;15:e008358. [Crossref] [PubMed]
- Rong LQ, Kamel MK, Rahouma M, et al. High-dose versus low-dose opioid anesthesia in adult cardiac surgery: A meta-analysis. J Clin Anesth 2019;57:57-62. [Crossref] [PubMed]
- DesPrez K, McNeil JB, Wang C, et al. Oxygenation Saturation Index Predicts Clinical Outcomes in ARDS. Chest 2017;152:1151-8. [Crossref] [PubMed]
- Foghsgaard S, Gazi D, Bach K, et al. Minimally invasive aortic valve replacement reduces atelectasis in cardiac intensive care. Acute Card Care 2009;11:169-72. [Crossref] [PubMed]
- Khalil NH, Anders R, Forner AF, et al. Radiological Incidence of Unilateral Pulmonary Edema After Minimally Invasive Cardiac Surgery. J Cardiothorac Vasc Anesth 2020;34:151-6. [Crossref] [PubMed]
- Inoue K, Hiraoka A, Chikazawa G, et al. Preventive Strategy for Reexpansion Pulmonary Edema After Minimally Invasive Cardiac Surgery. Ann Thorac Surg 2020;109:e375-7. [Crossref] [PubMed]
- Weibel S, Rücker G, Eberhart LH, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev 2020;10:CD012859. [PubMed]
- Hijazi EM, Edwan H, Al-Zoubi N, et al. Incidence of Nausea and Vomiting After Fast-Track Anaesthesia for Heart Surgery. Braz J Cardiovasc Surg 2018;33:371-5. [Crossref] [PubMed]


