Multidisciplinary pulmonary embolism response teams and systems
Introduction
The incidence of acute pulmonary embolism (PE) is 1–2 out of 1,000 adults per year (1). It is a complex diagnosis with severity ranging from an incidentally identified PE of minimal clinical significance to a massive PE that can be fatal. The treatment paradigms for PE have changed little in the past half century. However in recent years, many novel tools and strategies have emerged to improve the often devastating morbidity and mortality associated with severe PE. Catheter directed thrombolysis (CDT), percutaneous thrombus aspiration and thrombectomy devices, advanced surgical thrombectomy techniques, and improved utilization of extracorporeal membrane oxygenation (ECMO) are showing promise in advancing the treatment and care of high risk PE patients. The clinical safety and efficacy of many of these methods are being evaluated presently. However, even with the rapid growth of this field, the complexity of PE patients with comorbid diagnoses and the diverse clinical impact of their PE burden have left much of the clinical decision making in acute PE to expert consensus rather than to rigorously vetted scientific guidelines.
Many institutions are building pulmonary embolism response teams (PERTs) to bring together specialists to discuss their expert consensus opinions about the intricacies of challenging PE cases (2-4). PERTs develop in many different forms to take advantage of locally available resources and suit local clinical demands. To activate PERT at Massachusetts General Hospital, referring physicians call a 24-hour telephone number. This activation phone call triggers a “rapid response” consultation by the PERT fellow who gathers pertinent clinical information and assesses the severity of the case. If appropriate, an online meeting of the entire multi-disciplinary team convenes to discuss the case, review clinical findings, lab tests and radiographic images and generate diagnostic and treatment recommendations. The team also assembles appropriate resources if advanced interventions are necessary. This system simultaneously gathers multiple experts to discuss cases and generate treatment plans in real-time. A key aspect of their PERT program is the inclusion of many clinicians such as (but not limited to) vascular medicine and intervention specialists, intensive care unit teams, emergency department teams, cardiologists, pulmonologists, hematologists, radiologists, and cardiothoracic surgeons.
One caveat regarding the treatment of PE is that PE is an acute diagnosis and thus any organization and implementation of PE treatment plans must be performed rapidly and efficiently. To meet this need, infrastructure and tools are available at PERT locations to support PERT programs and other relevant specialists to organize and communicate swiftly. The cases that follow demonstrate how the PERT concept and system generate a multi-disciplinary treatment plan that encompasses the goals and concerns of all clinicians involved. It also provides a forum for a coherent strategy to be vetted and communicated both among specialists and primary treatment team members. Finally, these cases illustrate how the PERT infrastructure can easily extend to include non-traditional PERT clinicians to treat particularly complex PE patients.
Case 1
A 33-year-old female at 28 weeks pregnant presented to the Massachusetts General Hospital emergency room with sudden onset of dyspnea and pre-syncope. Her heart rate was 115, blood pressure 107/52, respiratory rate 24 and she was hypoxic with oxygen saturation of 88% on room air. With immediate concern for acute PE, a CT pulmonary angiogram (CTPA) was performed and demonstrated a large volume of bilateral main pulmonary arterial embolism and evidence of right ventricular (RV) dilatation (Figure 1). Cardiac biomarkers assessing for evidence of RV strain were elevated with Troponin T 0.16 and NT-BNP 550. Lower extremity ultrasound demonstrated no evidence of deep vein thrombosis (DVT).
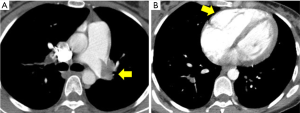
The patient was diagnosed with high risk submassive PE. An urgent PERT conference call was convened including traditional members of the PERT (vascular medicine, cardiology, pulmonary, hematology, cardiothoracic surgery) as well as members of the OBGYN, maternal-fetal medicine and surgical ICU teams. The conversation focused on the complex balance of targeting therapies aimed at the maternal and fetal well-being with primary focus on risks to the mother. With no randomized controlled studies and limited data available on emergent PE therapies in pregnant patients, the importance of “expert consensus” as available in the multi-disciplinary PERT conversation was essential (5). The multi-disciplinary group discussed the available data regarding the use of systemic thrombolytic therapy including the known 1% risk of maternal mortality and 5–10% risk of fetal demise (6).
Though no data exists regarding the use of catheter directed pulmonary arterial thrombolysis in pregnancy, the presumed risks were speculated to be lower than that of systemic thrombolysis. Anticoagulation alone was also considered. The dangers of RV dysfunction in the setting of the hemodynamic challenge of pregnancy and upcoming delivery were weighed carefully (7). After assessing the risks and benefits of the available options for both the mother and fetus, the team reached a consensus to proceed with CDT using an ultrasound-enhanced EKOS catheter (Figure 2). The goal was to minimize risks to the mother and fetus and simultaneously optimize RV recovery (7,8).
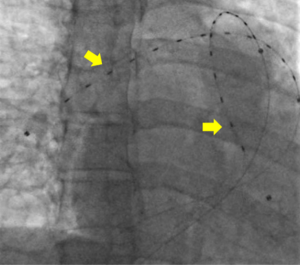
Multi-disciplinary input did not end with the clinical decision for CDT. Little is known about the risks of surgical thrombectomy in pregnancy beyond a presumed high risk of fetal demise secondary to surgical stress and the need for cardiopulmonary bypass. Therefore, the cardiothoracic surgical team organized its resources in advanced preparation for the possible need for an emergent surgical embolectomy if CDT failed (9). Furthermore, the OBGYN and maternal-fetal medicine teams mobilized their resources and planned surgical approaches in case of maternal bleeding due to the thrombolytic therapy or emergent cesarean section to rescue the fetus, if necessary.
This multi-disciplinary organization was orchestrated within 30 minutes of the PERT activation and through a single PERT group conference call. With this planning in place, the patient was brought to the catheterization lab for placement of bilateral EKOS catheters. Via these catheters, she was treated with 12 mg of tPA over 5 hours. Prior to removal of the catheters, she was noted to have a decrease in her PA systolic pressures from 50 mmHg at the time of the procedure to 32 mmHg at 5 hours. No maternal complications or fetal distress occurred during or after the procedure. She was discharged on enoxaparin (1 mg/kg twice daily) on post-procedure day 3. Repeat echocardiogram performed 2 weeks after her hospitalization demonstrated normalization of her right ventricle (Figure 3). The infant was delivered without complications at 39.5 weeks, and the patient and infant continue to do well.
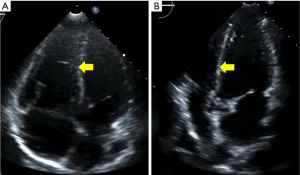
Case 2
A healthy and very active 68-year-old male with no known past medical history presented to an outside facility. He reported that 7 days prior to presentation, he had taken his “weekly 6 mile hike.” Approximately halfway through this exercise, he noted sudden onset of shortness of breath which lasted approximately 30 minutes. When he returned to his baseline breathing, he completed the hike without any additional problems. He went on with his typical weekly activities but noted some progressive mild to moderate dyspnea with chores such as yard and house work. Seven days after the initial episode, while he was “putting on a jacket,” his left arm became suddenly cold and painful. The discomfort worsened over the subsequent 5–10 minutes but then gradually improved. When his arm did not return to normal, he presented to an outside hospital emergency department.
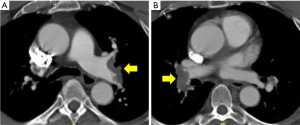
At presentation to the emergency department, he was hemodynamically stable with oxygen saturation of 92% on room air. He had a normal cardiopulmonary examination and normal cardiac biomarkers (Troponin T <0.01 and NT-BNP 284). His left upper extremity was reportedly cool with diminished radial and ulnar pulses. He had full strength and sensation in his left upper extremity and left hand. An arterial phase CT angiogram (CTA) of the chest with runoff of the left upper extremity was performed to evaluate his anatomy and look for the source of this acute arterial insufficiency. The CTA demonstrated abrupt occlusion of the left axillary artery consistent with an embolic event (Figure 4). The same study also demonstrated evidence of bilateral main pulmonary arterial embolism (Figure 5). The PERT was activated given this complex clinical presentation of PE.
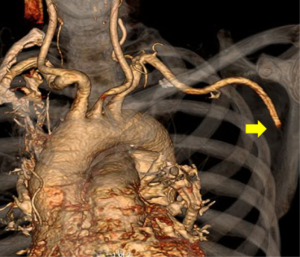
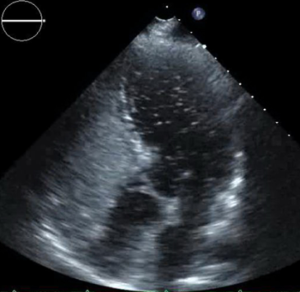
The PERT recommended further work up. Lower extremity venous ultrasound demonstrated evidence of a right popliteal deep venous thrombosis. Suspicion for intracardiac shunt as the source of the axillary arterial embolus prompted an evaluation with transthoracic echocardiogram with agitated saline “bubble study.” The echo revealed a mobile interatrial septum and early passage of “bubbles” from the right to the left atrium consistent with interatrial shunt likely patent foramen ovale (PFO) (Figure 6).
Many simultaneous complex clinical decisions arose with this case. Optimal treatment of the DVT and PE required coordination with complimentary management of the left axillary embolus and PFO. The left upper extremity was a threatened limb secondary to arterial insufficiency and needed urgent intervention prior to onset of any tissue death. Clinical guidelines for the timing and necessity of percutaneous closure of PFO are limited (10). However, in this patient, the PFO was the medium through which a thrombus had already embolized to the systemic arterial circulation and therefore, the decision for urgent closure was entertained. The timing and orchestration of these necessary but independent treatments (PE, axillary thrombus, PFO) was of paramount importance. The PERT organized a conference call including the traditional PERT members as well as the vascular surgery team, the cardiac imaging team and the structural interventional cardiology team.
The PERT conference call concluded that the patient’s hemodynamic stability with evidence of only mild RV strain was consistent with “low risk submassive PE”. With this assessment and the need for urgent vascular surgery and for large bore venous access for PFO closure, conservative treatment of the DVT/PE was continued with anticoagulation alone. The patient was maintained on therapeutic heparin anticoagulation. Given the patient’s threatened limb, the vascular surgery team performed an urgent open surgical axillary embolectomy and perfusion of the left upper extremity was restored. The following day, to prevent further embolization, the patient was brought to the catheterization lab for evaluation of the intracardiac shunt. Intracardiac echocardiography (ICE) confirmed presence of a large PFO which was closed without complication using a 30 mm Gore Cardioform device. ICE performed after device placement demonstrated no evidence of residual shunt.
The PERT and PERT meeting infrastructure allowed for the rapid coordination of multiple teams to generate a thoughtful, coordinated and comprehensive treatment plan. The PERT system brought together the primary clinical teams with multiple subspecialists quickly and simultaneously to discuss the patient. A complex clinical plan was developed and performed in a deliberate, logical order with little time wasted and with minimal interruption of anticoagulation. The day after his PFO closure, the patient was ambulating without evidence of hemodynamic compromise or hypoxia. He was transitioned to rivaroxaban and discharged home. In searching for a primary etiology for his venous thromboembolism (VTE), he underwent age specific cancer related screening. He had an elevated prostate specific antigen and work up revealed prostate cancer. He was treated with external beam radiation therapy (EBRT) followed by long-term androgen deprivation therapy. Regarding his DVT/PE, he did well with progressive clinical improvement, and he has returned to his active lifestyle without any limitations.
Discussion
There are a number of recently published articles describing how PERT programs rapidly initiate and implement thoughtful treatment plans for patients with severe PE (3,4). Currently, promising novel therapeutic approaches and tools are being developed to treat PE. However there are few guidelines and limited data to support their use in patients with severe PE. Therefore in a field where much clinical decision making is left to the role of “expert opinion,” the ability to swiftly organize local expert opinion into a single conversation performed at the onset of a patient’s care may be invaluable.
The cases presented here demonstrate that PE can be a complex diagnosis that often presents in the midst of difficult medical scenarios. PERT programs provide an infrastructure which immediately and simultaneously engages multiple experts to determine the best course of action for PE patients. Each consultant contributes relevant and vital information about a patient’s clinical situation and perils. Neurologists and neurosurgeons can provide critical information and guidance on the bleeding risk associated with intracranial masses and strokes. Oncologists can comment on the prognosis of patients whose underlying malignancy is contributing to their hypercoagulable state. Obstetricians and gynecologists can offer insights into the heightened risks of anticoagulation and hemodynamic challenges of submassive PE in pregnant patients and fetuses. These are only a few examples of the many subspecialists who are critical in the decision making process for patients with PE. Each clinician can and must be an integral part of an individual’s particular “PERT”. The multi-disciplinary activation, conference call and PERT infrastructure provide the opportunity to include each expert’s valuable input from the start of a patient’s care.
The emergence and utilization of PERT programs are in their infancy. Although we have preliminary reports from multiple institutions on the value of PERTs, the presumed benefits and impact of this multi-specialty care require further evaluation (3,4). In addition to the outcomes of mortality, chronic thromboembolic pulmonary hypertension and functional status post PE, the PERT community needs to examine if PERTs improve clinical targets such as time to or completeness of anticoagulation, utilization of guideline directed therapies, duration of hospital stay and quality of life. Furthermore, the infrastructure for PERTs needs to be solidified. With various specialists involved in a clinical discussion, decisions regarding medicolegal responsibility and reimbursement for time spent are emerging and call for further investigation. The emergence of multi-disciplinary online-facilitated consultations also raises important questions about how (or if) the expertise of a given facility’s PERT can be extended to provide acute consultation to other regional facilities that lack a PERT. The availability of such expertise-sharing technologies has similarly been discussed in its role in the treatment of acute aortic syndromes (11).
Although there is much work ahead, PERT programs systems are an exciting new endeavor. The care of PE has reached the modern age before that of many other conditions. It has rapidly become a model for multi-disciplinary care and utilization of groundbreaking infrastructure and strategies. It can serve not only as a foundation to constantly improve the treatment of PE patients, but also as a model for the multi-disciplinary treatment of other acute complex clinical conditions.
Acknowledgements
The authors wish to acknowledge and thank all of the members of MGH PERT for their ongoing support and commitment to the program.
Footnote
Conflicts of Interest: Kenneth Rosenfield discloses the following relationships. Consultant: Cardinal Health, SurModics. Grants/Contracts: Abbott Vascular, Atrium, Lutonix/BARD, The Medicines Company. Equity: Access Closure, Inc., AngioDynamics/Vortex. Personal Compensation: Cook, HCRI, The Medicines Company. Board Member: VIVA Physicians. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005;3:1611-7. [Crossref] [PubMed]
- Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract (1995) 2014;42:31-7. [Crossref] [PubMed]
- Kabrhel C, Rosovsky R, Channick R, et al. A Multidisciplinary Pulmonary Embolism Response Team: Initial 30-Month Experience With a Novel Approach to Delivery of Care to Patients With Submassive and Massive Pulmonary Embolism. Chest 2016;150:384-93. [Crossref] [PubMed]
- Dudzinski DM, Piazza G. Multidisciplinary Pulmonary Embolism Response Teams. Circulation 2016;133:98-103. [Crossref] [PubMed]
- Ahearn GS, Hadjiliadis D, Govert JA, et al. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: a case report and review of treatment options. Arch Intern Med 2002;162:1221-7. [Crossref] [PubMed]
- Turrentine MA, Braems G, Ramirez MM. Use of thrombolytics for the treatment of thromboembolic disease during pregnancy. Obstet Gynecol Surv 1995;50:534-41. [Crossref] [PubMed]
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86. [Crossref] [PubMed]
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92. [Crossref] [PubMed]
- Piazza G, Hohlfelder B, Jaff MR, et al. Successful treatment of massive pulmonary embolism in the 38th week of pregnancy. Ann Thorac Surg 2004;77:694-5. [Crossref] [PubMed]
- Tobis J, Shenoda M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012;60:1722-32. [Crossref] [PubMed]
- Schoenhagen P, Roselli EE, Harris CM, et al. Online network of subspecialty aortic disease experts: Impact of "cloud" technology on management of acute aortic emergencies. J Thorac Cardiovasc Surg 2016;152:39-42. [Crossref] [PubMed]


