Congenital anomalies of the IVC—embryological perspective and clinical relevance
Introduction
With the increased use of cross-sectional imaging, systemic venous anomalies are more frequently being recognized in asymptomatic patients. Accurate characterization of systemic venous anomalies plays a major role in the appropriate selection of a surgical approach or interventional procedure. In this article, we review common and uncommon inferior vena cava (IVC) anomalies. We describe the embryological basis and clinical implications of these anomalies, particularly from an interventional radiology perspective. We also discuss the complications and treatments of these anomalies.
Normal development/embryogenesis
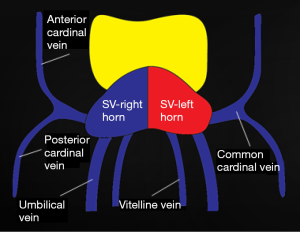
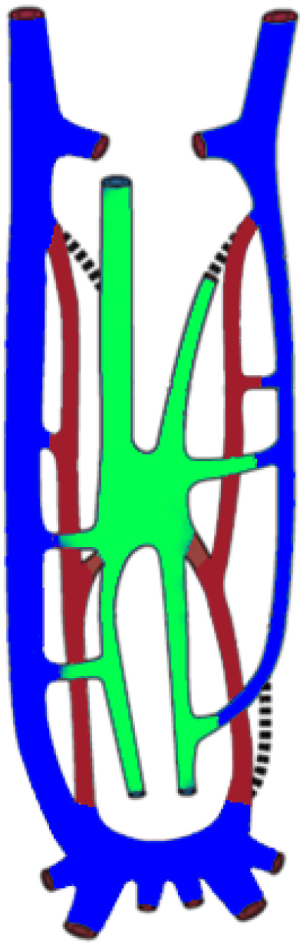
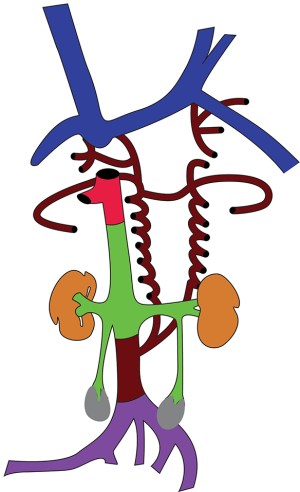
At 4 weeks of life, 3 distinct venous systems form: the vitelline system drains the gut, the umbilical system drains the placenta, and the cardinal system drains the rest of the embryo (Figure 1). The infrahepatic IVC develops from a set of 3 paired parallel veins appearing consecutively between 4 and 8 weeks of life, namely the posterior cardinal, subcardinal and supra cardinal veins (Figure 2). The suprahepatic IVC is derived from the cranial segment of the right vitelline vein. The retrohepatic segment is derived from an anastomosis between the cranial segment of the right subcardinal vein and the right vitelline vein (1). The infrahepatic/suprarenal IVC is derived from the right subcardinal vein (2). The renal collar is formed from anastomoses between the supra cardinal veins posteriorly and the subcardinal veins anteriorly, with the posterior limb regressing during development. On the right, the anterior limb is incorporated into the lateral wall of the renal segment of the IVC; and on the left, the anterior limb forms the normal adult left renal vein (3). The suprarenal portions of the right and left supra cardinal veins connect with the posterior cardinal veins, forming the azygos and hemiazygos systems, respectively. The infrarenal portions disappear on the left but form the infrarenal portion of the IVC on the right (2). The posterior cardinal veins mostly regress except for the distal portion, which later become the iliac confluence and future iliac veins, and the proximal portion, which joins the azygos and the hemiazygos veins (Figure 3).
Anomalies of the IVC
Abnormal drainage
In early fetal life, there is intercommunication between developing cardiac chambers and the systemic, pulmonary, and portal hepatic venous systems. Over time, these structures undergo complex regression and development. However, if these communications persist for too long, this can lead to unnatural preferential flows into one system, resulting in complex congenital anomalies.
IVC drainage into left atrium
In this anomaly, the IVC drains into the left instead of the right atrium. At 4 months of life, both the septum primum and septum secundum exist as separate membranes with free atrial communication via the ostium primum and ostium secundum. The sinus venosus communicates with the left and right atria through a left and right valve respectively; later, it migrates to the right, with the left valve disappearing (4). The right valve then atrophies, leaving only the Eustachian valve of the IVC and the Thebesian valve of the coronary sinus as remnants. If there is failure of regression of the portion that becomes the valve of the IVC and if it fuses with septum secundum, thus closing the foramen ovale, an IVC draining into the left atrium would be established. The blood from the IVC would be directed between the septum secundum and the septum primum and hence into the left atrium via the ostium primum (5).
Although usually asymptomatic, clinical signs may include dyspnea, cyanosis, clubbing and systemic emboli. On imaging, the IVC is positioned normally in the lower chest but then curves towards and joins the left atrium (Figure 4) (6). This anomaly could be confused with an inferior sinus venosus defect, in which the wall separating the IVC and the left atrium is absent, resulting in free communication. This is caused by underdevelopment of the septum secundum, leading to displacement/malpositioning of the septum primum (7).
In this anomaly, access into the right atrium through a femoral approach, as well as access into the IVC through a jugular approach is somewhat challenging. If the atrial septum is present (in 50%) (4), repair is achieved by entering the IVC from the right side, incising the septum, diverting blood flow to the right, and suturing the cut edges of the septal incision to the lateral wall of the IVC. If the atrial septum is absent, IVC blood is rerouted to the right atrium by reconstructing the atrial septum to the left side of the IVC orifice (6).
Pulmonary venous drainage into IVC
Anomalous pulmonary venous connection into the IVC can be total or partial. During development, blood returning from the lung buds drains into the splanchnic plexus, which communicates with paired cardinal veins and umbilicovitelline veins. This pulmonary venous connection normally involutes, a common pulmonary vein forms and joins the left atrium (8). If a splanchnic plexus communication with a cardinal or umbilicovitelline vein persists, some type of anomalous venous connection will occur (9).
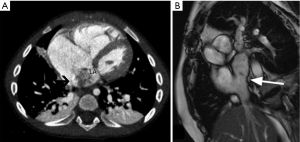
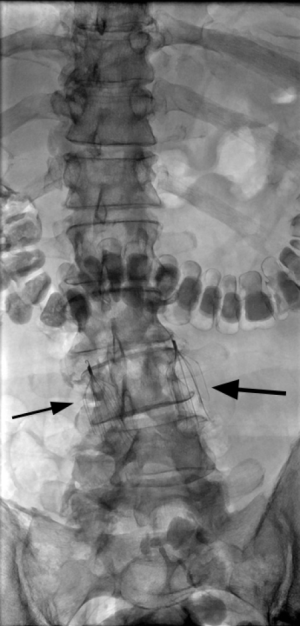
There are three types of total anomalous pulmonary venous connection (TAPVC). Type I is supracardiac and Type II is cardiac. In Type III TAPVC, which accounts for 26% of TAPVC, a common pulmonary vein drains below the diaphragm into the IVC or hepatic vein/azygos vein/portal vein (10). Due to compression of the draining vein by diaphragm, pulmonary edema may be seen (8). In partial anomalous pulmonary venous connection (PAPVC), at least one pulmonary vein drains to a systemic venous location, including the IVC. Scimitar syndrome is a rare condition, characterized by PAPVC, pulmonary hypoplasia and pulmonary artery hypoplasia/aplasia, usually on the right. The pulmonary vein drains into IVC, the azygos system, right atrium, portal vein, or a hepatic vein (8,11). The adult form of scimitar syndrome is asymptomatic, whereas infantile form presents with failure to thrive, pneumonia, pulmonary hypertension and heart failure (12). On radiograph, the scimitar sign may be seen, due to the anomalous vein running parallel to the right heart border, resembling a Turkish sword (Figure 5) (13).
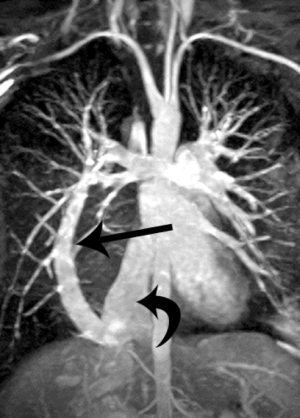
Interventional radiology procedures have been performed using detachable balloons, coils, or tissue adhesive to occlude the anomalous venous connection with good success rates (13). Indications for embolization are individualized but usually include symptomatology of pulmonary hypertension.
Abernethy malformation: portal vein draining into IVC
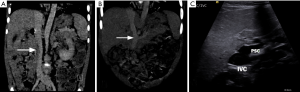
Abernethy malformation is a rare anomaly (14,15) characterized by the portal vein draining into the IVC. During the 5th week of life, the portal vein is constituted cranially from a segment of the prehepatic right vitelline vein, the intervitelline anastomosis, and caudally from the left vitelline vein (16). The right vitelline vein is a common conduit for the development of both the IVC and portal vein. The intervitelline vein anastomosis is interrupted by formation of early hepatic tissue at the site of this connection, and the cranial segment of the portal vein disappears (14). However, if there is an abnormal persistence of this connection, an Abernethy malformation may result. The hemodynamic thrust of the flow in the left umbilical vein, through the left then the right vitelline veins and back into the heart is also believed to be a cause (17).
There are two types of Abernethy malformation. In Type I, there is complete absence of portal vein (Figures 6,7), whereas in Type II, there is a hypoplastic portal vein with a partial portosystemic shunt perfusing the liver (14,15). Abernethy malformation may be asymptomatic or may present with acute hepatic decompensation or cirrhosis. Type I malformations is associated with congenital anomalies, especially cardiac, gastrointestinal, and genitourinary anomalies, as well as an increased risk of hepatocellular carcinoma (14). Type I is managed by liver transplant, whereas Type II shunt can be occluded either surgically or percutaneously using balloons or coils (14), in patients presenting with hepatic encephalopathy or bleeding varices (19-21). Once case report by Kuo described a staged treatment of a type II Abernethy by stent closing the Abernethy shunt while simultaneously creating a TIPS shunt. This TIPS shunt was later downsized, then totally closed (22).

Failure of development
Interruption of the IVC
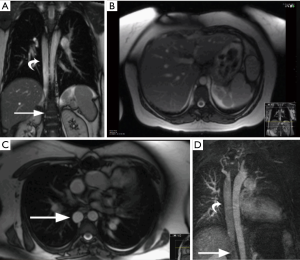
Interruption of IVC is characterized by absence of suprarenal/infrahepatic IVC, with a prevalence of 0.6% (23). This is caused by failure of the right subcarrdinal-hepatic anastomosis and atrophy of the right subcardinal vein. Consequently, blood is shunted from the suprasubcardinal anastomosis through the retrocrural azygos vein, which is partially derived from the right supra cardinal vein. This anomaly is associated with heterotaxy syndromes, polysplenia, atrioventricular septal defects, partial anomalous pulmonary venous connection, and pulmonary atresia (24).
On imaging, infrarenal IVC is intact, whereas the suprarenal portion of the IVC is absent (Figures 8,9). The post-hepatic segment of the IVC is present and receives the hepatic veins. The interrupted IVC rarely continues as the hemiazygos vein if there is a left IVC. Care should be taken to avoid misdiagnosis of this anomaly as a right-sided paratracheal mass or retrocrural adenopathy (23). In addition, accidental ligation of the azygos vein during surgery may be fatal; so venous cannulation for bypass surgery is performed by placing a large catheter in the SVC while hepatic flow is drained via a small catheter in the post hepatic IVC (6).

Absence of the infrarenal IVC
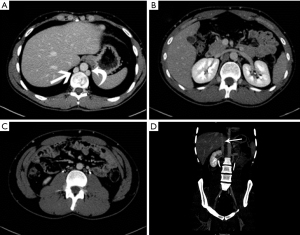
An absent infrarenal IVC is a rare anomaly resulting from failure of development of the posterior cardinal and supra cardinal veins, with preservation of the suprarenal segment of the IVC. Intrauterine or perinatal thrombosis has been proposed to cause this anomaly (26,27). Absence can be either complete or incomplete depending on the degree of involvement of the infrarenal IVC and the common iliac veins (Figure 10) (26). Absence of the infrarenal IVC may be asymptomatic or may present lower limb venous insufficiency, deep-vein thrombosis or varices (28). In cases of total absence of the IVC, blood returns usually through multiple collateral pathways, including the azygos/hemiazygos system, emptying into the SVC.
Failure of regression
Duplication of the IVC
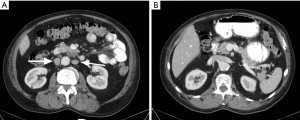
In this anomaly, there are bilateral IVCs due to the persistence of both right and left supra cardinal veins (Figure 11). The prevalence of this anomaly is 1% to 3% (29), and the most common form is one in which 2 distinct IVCs arise from each iliac vein (30). Usually, the left IVC ends at the level of the left renal vein, crossing over to join the right IVC (3). There may be significant asymmetry in the sizes of the left and right veins if the left IVC crosses over at a lower level than the renal vein (29). Care should be taken to avoid mistaking this anomaly for adenopathy (27). Duplication of the IVC should be suspected in cases of recurrent pulmonary embolism after placement of an IVC filter. This condition is treated by placing filters in both IVCs (31) (Figure 12), placing a filter in the suprarenal IVC (32), or performing coil embolization of the smaller IVC (33).
Circumaortic left renal vein
In this anomaly, the left renal vein encircles the abdominal aorta. During IVC development, anastomotic communications between subcardinal and supra cardinal channels form a collar of veins encircling the aorta. The ventral portion of this circumaortic collar persists as the normal left renal vein, and the dorsal vein normally regresses. Circumaortic vein results if there is persistence of the dorsal collar (Figure 13). The incidence of this anomaly is 1.5% to 16% (34-36).
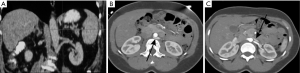
Care should be taken to avoid misdiagnosis of this condition as retroperitoneal adenopathy (27). The major clinical significance of this anomaly is in preoperative planning before nephrectomy and in renal vein catheterization for venous sampling. Compression of the renal vein by the aorta can cause hematuria, abdominal/flank pain or “nutcracker” phenomenon, which entails hypertension, hematuria, and ureteric varices (37). In this anomaly, placing the IVC filter below the lower renal vein may be a challenge since adequate distance may not be available between the lower renal vein and the iliac vein. Therefore, either a suprarenal IVC filter or 2 filters in either iliac vein may have to be placed. However, Fang et al. (38) reported that the frequency of pulmonary embolism in patients who had filters placed at or in between the 2 left renal veins was not significantly different from those who underwent infrarenal or suprarenal filter placement.
Combined etiology
In patients with these anomalies, certain sections of developing primitive systemic veins fail to develop while others fail to regress.
Left IVC
A left IVC results from regression of the right supra cardinal vein with persistence of the left supra cardinal vein. The prevalence of this anomaly is 0.2% to 0.5% (29). In incomplete left-IVC, the left common iliac vein ascends as a duplicated left IVC and drains into the left renal vein. The vein then crosses the aorta anteriorly and joins the right IVC in a normal fashion. In complete left-IVC, the left IVC receives the left renal vein and continues as a preaortic trunk that travels obliquely and empties into the right IVC (Figure 14) (39).
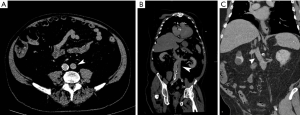
Left IVC can be misdiagnosed as a left-sided para-aortic adenopathy (40). This anomaly must also be considered when interventional radiologists are placing an IVC filter, especially through a transjugular approach (26), and when repairing aortic aneurysms (41). In patients with chronic central venous occlusions in the chest requiring long-term central venous catheters, access into the infrarenal IVC via a translumbar approach is a viable alternative (42). A catheter placed into the left infrarenal IVC will take a double curve as it travels via the left renal vein into the suprarenal IVC. This left IVC approach may pose complications similar to a left SVC approach when placing a filter, such as higher incidences of chronic occlusions, due to the anatomical difference in location and the curve that the anomalous vein takes as it goes centrally towards the right heart.
Retrocaval ureter
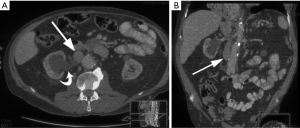
In retrocaval ureter, the ureter is posterior and medial to the IVC, which can cause ureteral compression leading to complications such as hydronephrosis or recurrent urinary tract infections (27). This is caused by abnormal persistence of the right subcardinal vein positioned ventral to the ureter in the definitive IVC, as a result of which the developing right ureter courses behind and medial to the IVC (Figure 15). This anomaly has incidence of 0.06% to 0.17% and is more common in men (43). This anomaly almost always occurs on the right, but when occurring on the left, it is usually associated with either a partial or complete situs inversus or a left-sided IVC (43). On imaging, retrocaval ureter appears like a fish hook or a reverse J, with the proximal ureter appearing at the level of the lumbar pedicles (44). Surgical relocation of the ureter anterior to the IVC is the treatment of choice (29).
Retroaortic left renal vein
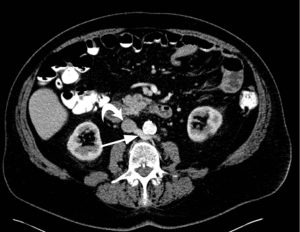
This anomaly is caused by persistence of dorsal portion of the renal collar and regression of the ventral collar, resulting in the left renal vein coursing posterior to the aorta (Figure 16) (2). This anomaly has an incidence of 0.8% to 3.7% (34). This is usually asymptomatic, but may cause hematuria and abdominal/flank pain (37) due to compression of left renal vein (45,46). Knowledge of this anomaly is essential before renal and adrenal venography, renal venous renin sampling, left renal transplant and splenorenal shunt. Failure to recognize this anomaly may lead to severe hemorrhage (47).
Conclusions
IVC anomalies are commonly encountered due to increased use of cross sectional imaging modalities. Knowledge of the embryology helps in understanding the spectrum of these anomalies and their clinical implications. It also helps in designing an effective treatment plan from an interventional standpoint.
Acknowledgements
Rukmini Komarlu M.D. for contribution of “Scimitar” syndrome MIP image. Ms. Megan Griffiths for her assistance in preparing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Le Borgne J, Paineau J, Hamy A, et al. Interruption of the inferior vena cava with azygos termination associated with congenital absence of portal vein. Surg Radiol Anat 2000;22:197-202. [Crossref] [PubMed]
- Guttmann G, Endean E (2010) Embryology. In: Cronenwett J, Johnston W (eds) Rutherford's Vascular Surgery, 7th ed. Volume 1. Philadelphia: Saunders/Elsevier;15-30. Available online: https://www-clinicalkey-com.ccmain.ohionet.org/#!/content/book/3-s2.0-B9781455753048000029
- Malaki M, Willis AP, Jones RG. Congenital anomalies of the inferior vena cava. Clin Radiol 2012;67:165-71. [Crossref] [PubMed]
- Burri H, Vuille C, Sierra J, et al. Drainage of the inferior vena cava to the left atrium. Echocardiography 2003;20:185-9. [Crossref] [PubMed]
- Black H, Smith GT, Goodale WT. Anomalous inferior vena cava draining into the left atrium associated with intact interatrial septum and multiple pulmonary arteriovenous fistulae. Circulation 1964;29:258-67. [Crossref] [PubMed]
- Mazzucco A, Bortolotti U, Stellin G, et al. Anomalies of the systemic venous return: a review. J Card Surg 1990;5:122-33. [Crossref] [PubMed]
- Prasad D, Snyder C, Ashwath R. Septum primum malposition defect and inferior sinus venosus defect: a rare association. Cardiol Young 2015;25:1389-92. [Crossref] [PubMed]
- Dillman JR, Yarram SG, Hernandez RJ. Imaging of pulmonary venous developmental anomalies. AJR Am J Roentgenol 2009;192:1272-85. [Crossref] [PubMed]
- Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007;115:103-8. [Crossref] [PubMed]
- Karamlou T, Gurofsky R, Al Sukhni E, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation 2007;115:1591-8. [Crossref] [PubMed]
- White CS, Baffa JM, Haney PJ, et al. MR imaging of congenital anomalies of the thoracic veins. Radiographics 1997;17:595-608. [Crossref] [PubMed]
- Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and considerations for clinicians. Clin Anat 2014;27:1234-43. [Crossref] [PubMed]
- Midyat L, Demir E, Aşkin M, et al. Eponym. Scimitar syndrome. Eur J Pediatr 2010;169:1171-7. [Crossref] [PubMed]
- Kwapisz L, Wells MM, AlJudaibi B. Abernethy malformation: congenital absence of the portal vein. Can J Gastroenterol Hepatol 2014;28:587-8. [Crossref] [PubMed]
- Hu GH, Shen LG, Yang J, et al. Insight into congenital absence of the portal vein: is it rare? World J Gastroenterol 2008;14:5969-79. [Crossref] [PubMed]
- Marks C. Developmental basis of the portal venous system. Am J Surg 1969;117:671-81. [Crossref] [PubMed]
- Lassau JP, Bastian D. Organogenesis of the venous structures of the human liver: a hemodynamic theory. Anat Clin 1983;5:97-102. [Crossref]
- Ghandour A, Partovi S, Karuppasamy K, et al. Microcatheter in superior mesenteric vein/splenic vein confluence, with no flow distally into the portal circulation. Asvide 2016;3:521. Available online: http://www.asvide.com/ articles/1296
- Potts JR 3rd, Henderson JM, Millikan WJ Jr, et al. Restoration of portal venous perfusion and reversal of encephalopathy by balloon occlusion of portal systemic shunt. Gastroenterology 1984;87:208-12. [PubMed]
- Ikeda S, Sera Y, Yoshida M, et al. Successful coil embolization in an infant with congenital intrahepatic portosystemic shunts. J Pediatr Surg 1999;34:1031-2. [Crossref] [PubMed]
- Tannuri U, Galvão F, Leal AJ, et al. Congenital absence of the portal vein: a complex disease with multiple manifestations and types of treatment. Eur J Pediatr Surg 2011;21:269-72. [Crossref] [PubMed]
- Kuo MD, Miller FJ, Lavine JE, et al. Exploiting phenotypic plasticity for the treatment of hepatopulmonary shunting in Abernethy malformation. J Vasc Interv Radiol 2010;21:917-22. [Crossref] [PubMed]
- Ginaldi S, Chuang VP, Wallace S. Absence of hepatic segment of the inferior vena cava with azygous continuation. J Comput Assist Tomogr 1980;4:112-4. [Crossref] [PubMed]
- Schultz CL, Morrison S, Bryan PJ. Azygos continuation of the inferior vena cava: demonstration by NMR imaging. J Comput Assist Tomogr 1984;8:774-6. [Crossref] [PubMed]
- Ghandour A, Partovi S, Karuppasamy K, et al. Interruption of the suprarenal portion of the IVC with azygos continuation. Asvide 2016;3:522. Available online: http://www.asvide.com/ articles/1297
- Petik B. Inferior vena cava anomalies and variations: imaging and rare clinical findings. Insights Imaging 2015;6:631-9. [Crossref] [PubMed]
- Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics 2000;20:639-52. [Crossref] [PubMed]
- Dougherty MJ, Calligaro KD, DeLaurentis DA. Congenitally absent inferior vena cava presenting in adulthood with venous stasis and ulceration: a surgically treated case. J Vasc Surg 1996;23:141-6. [Crossref] [PubMed]
- Phillips E. Embryology, normal anatomy, and anomalies. In: Ferris EJ, Hipona FA, Kahn PC, Phillips E, Shapiro JH (eds) Venography of the Inferior Vena Cava and its Branches. Baltimore: Williams & Wilkins 1969:1-32.
- Pineda D, Moudgill N, Eisenberg J, et al. An interesting anatomic variant of inferior vena cava duplication: case report and review of the literature. Vascular 2013;21:163-7. [Crossref] [PubMed]
- Moubarak G, Schleich JM, Daubert JC. Long-term efficacy of two vena cava filter implants for congenital duplicated inferior vena cava. Arch Cardiovasc Dis 2009;102:77-8. [Crossref] [PubMed]
- Sartori MT, Zampieri P, Andres AL, et al. Double vena cava filter insertion in congenital duplicated inferior vena cava: a case report and literature review. Haematologica 2006;91:ECR30. [PubMed]
- Smith DC, Kohne RE, Taylor FC. Steel coil embolization supplementing filter placement in a patient with a duplicated inferior vena cava. J Vasc Interv Radiol 1992;3:577-80. [Crossref] [PubMed]
- Karkos CD, Bruce IA, Thomson GJ, et al. Retroaortic left renal vein and its implications in abdominal aortic surgery. Ann Vasc Surg 2001;15:703-8. [Crossref] [PubMed]
- Nonami Y, Yamasaki M, Sato K, et al. Two types of major venous anomalies associated with abdominal aneurysmectomy: a report of two cases. Surg Today 1996;26:940-4. [Crossref] [PubMed]
- Trigaux JP, Vandroogenbroek S, De Wispelaere JF, et al. Congenital anomalies of the inferior vena cava and left renal vein: evaluation with spiral CT. J Vasc Interv Radiol 1998;9:339-45. [Crossref] [PubMed]
- Cuéllar i Calàbria H, Quiroga Gómez S, Sebastià Cerqueda C, et al. Nutcracker or left renal vein compression phenomenon: multidetector computed tomography findings and clinical significance. Eur Radiol 2005;15:1745-51. [Crossref] [PubMed]
- Fang AS, Morita S, Gill GS, et al. Clinical outcomes of inferior vena cava filter placement in patients with renal vein anomalies. Ann Vasc Surg 2014;28:318-23. [Crossref] [PubMed]
- Ng WT, Ng SS. Double inferior vena cava: a report of three cases. Singapore Med J 2009;50:e211-3. [PubMed]
- Siegfried MS, Rochester D, Bernstein JR, et al. Diagnosis of inferior vena cava anomalies by computerized tomography. Comput Radiol 1983;7:119-23. [Crossref] [PubMed]
- Mathews R, Smith PA, Fishman EK, et al. Anomalies of the inferior vena cava and renal veins: embryologic and surgical considerations. Urology 1999;53:873-80. [Crossref] [PubMed]
- Salgado OJ, Urdaneta B, Colmenares B, et al. Right versus left internal jugular vein catheterization for hemodialysis: complications and impact on ipsilateral access creation. Artif Organs 2004;28:728-33. [Crossref] [PubMed]
- Uthappa MC, Anthony D, Allen C. Case report: retrocaval ureter: MR appearances. Br J Radiol 2002;75:177-9. [Crossref] [PubMed]
- Talner LB, O’Reilly P, Wasserman NF. Specific causes of urinary obstruction. In: Pollack HM, McClennan BL (eds) Clinical Urography, 2nd ed. Philadelphia: Saunders; 2000;1967-2136.
- Beinart C, Sniderman KW, Saddekni S, et al. Left renal vein hypertension: a cause of occult hematuria. Radiology 1982;145:647-50. [Crossref] [PubMed]
- Nishimura Y, Fushiki M, Yoshida M, et al. Left renal vein hypertension in patients with left renal bleeding of unknown origin. Radiology 1986;160:663-7. [Crossref] [PubMed]
- Brancatelli G, Galia M, Finazzo M, et al. Retroaortic left renal vein joining the left common iliac vein. Eur Radiol 2000;10:1724-5. [Crossref] [PubMed]


