Minimalist immediate mechanical intervention in acute ST-segment elevation myocardial infarction: is it time to redefine targets?
One of the greatest frustrations of the interventional cardiologist is that restoring flow down an occluded epicardial artery in patients with ST segment elevation myocardial infarction (STEMI) does not equate to normalisation of downstream microvascular flow. Far from being a rare occurrence, microvascular obstruction (MVO), which can be suspected from absence of ST-segment elevation resolution, angiographic scores (TIMI grade flow <3 and myocardial blush grade <3) or, more accurately, magnetic resonance imaging (MRI), occurs in 5–50% of STEMIs (1). Of note, MVO is a strong negative prognosticator associated with a higher prevalence of arrhythmias, pericardial effusion, tamponade, early congestive heart failure, adverse left ventricular remodelling, readmissions for heart failure and mortality (1).
In most cases STEMI is initiated by acute plaque rupture complicated by occlusive coronary thrombus. Disruption of this complicated culprit stenosis by ballooning and stent deployment during primary percutaneous intervention (PCI) might cause distal embolization. Over the last decade, a number of therapeutic strategies aimed to prevent such phenomenon and the subsequent plugging of the microcirculation have been tested (1). By far, the most tested approach has been manual thrombus aspiration (TA). Unfortunately the benefits of TA demonstrated in the TAPAS trial at 1 year (2) have not been replicated in the TASTE (3) and TOTAL (4) trials, possibly due to the limited power of these studies. Furthermore, there is an indication that there may be an increased risk of stroke with thrombus aspiration (4).
An alternative approach consists of limiting primary PCI to restoration of anterograde flow by wiring, aspiration thrombectomy and gentle ballooning, then, allowing antiplatelets and anticoagulants to decrease the thrombotic burden (5), and deferring stent deployment to a subsequent procedure. This deferred stenting strategy has been called the minimalist immediate mechanical intervention (MIMI) approach (6). Several trials have explored the potential benefit of deferred stenting in various settings. A meta-analysis of multiple non-randomised trials in STEMI and Non-STEMI (NSTEMI) and one randomised controlled trial (RCT) in NSTEMI concluded that delayed stent implantation was associated with better angiographic outcomes but was unable to draw conclusions about long term cardiac outcomes (7).
The first RCT studying deferred stenting in the setting of STEMI was DEFER-STEMI. Rather than randomising all STEMI patients to a deferred stenting approach, patients at high risk of no-reflow using clinical and angiographic criteria were selected in this single centre trial. These criteria included: a history of myocardial infarction; age ≥65 years; symptom duration >6 h; culprit coronary artery anomalies; heavy thrombus burden (TIMI grade 2 or higher); long lesion length (≥24 mm); small vessel diameter (≤2.5 mm) and clinical signs of acute microvascular injury after initial reperfusion with persistent ST-segment elevation >50%. One hundred and one subjects at high risk of no-reflow according to these criteria were randomised to immediate stenting (IS) or deferred stenting (DS) and were followed up for a minimum of 6 months. The deferred approach was shown to reduce no-/slow-reflow, distal embolization and intra-procedural thrombotic complications compared with immediate stenting. Final TIMI flow grade and myocardial blush grades were also better in the deferred stenting group. Furthermore, at 6 months, myocardial salvage (percentage of left ventricular mass) and salvage index measured with cardiac MRI were greater in the deferred stenting group. However, 2 patients experienced re-occlusion of the culprit artery prior to the second procedure, a complication which was not present in previous trials (8).
While DEFER-STEMI created expectations regarding potential long term benefits of a deferred stenting approach subsequent studies have offered conflicting results, casting doubts on the validity of the hypothesis. The MIMI trial, prospectively randomised 140 STEMI subjects in multiple centres to immediate stenting or deferred stenting. The primary endpoint was MVO expressed as the relative percentage of LV mass on the cardiac MRI a median of 5 days (interquartile range, 4–6 days) after the first procedure. There was a trend toward lower microvascular obstruction in the immediate stenting group compared with the deferred stenting group, which became significant after adjustment for the area at risk. Median LV ejection fraction, infarct weight and infarct size (% area at risk) did not differ between groups. These results suggest deferred stenting could increase MVO area size compared to immediate stenting. There was also no difference at 6 months in the rate of major cardiovascular event and cerebral events between the two arms (6).
Nearly simultaneously to the publication of the MIMI trial, the results of a third RCT of deferred stenting in STEMI, DANAMI 3-DEFER was published which focused on clinical outcomes (9). In DANAMI 3-DEFER, 1,215 STEMI patients were randomly assigned to immediate stenting or deferred stenting. DANAMI 3-DEFER not only has the largest patient population to date but also has the longest follow-up (median of 42 months). The primary composite endpoint of all-cause mortality, hospital admission for heart failure, recurrent myocardial infarction or unplanned revascularisation of the infarct-related artery occurred in 18% of immediate stenting patients and in 17% of the deferred stenting arm. In DANAMI 3-DEFER if there was <30% stenosis, stenting could be waived. However, it is important to note that 22% of patients randomised to deferred stenting had a stent implanted during the index procedure (cross-overs) and 2% required urgent revascularisation before the scheduled deferred procedure. DANAMI 3-DEFER concluded that deferring stenting in an unselected patient population is not beneficial (9).
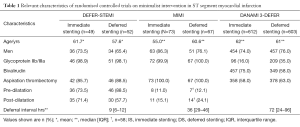
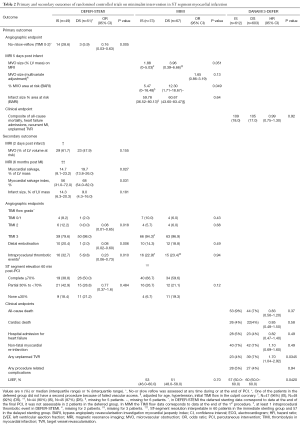
How should these discordant results between studies be interpreted? The first way to address this question is to analyse the differences between the three studies. Table 1 summarises differences in relevant characteristics and Table 2 summarise the outcomes which will be used as part of the following discussion.
Full table
Full table
The first question that has to be addressed is whether patients included in the 3 studies had a similar risk of atherothrombotic embolization and subsequent development of no reflow. Angiographic features that can identify subjects at higher risk of no reflow include: abrupt proximal cut-off pattern (without tapering); floating thrombus; accumulated thrombus proximal to occluded level; persistent dye staining distal to occluded level and infarct related artery with a lumen diameter of >4 mm (10). In this regard, it is important to highlight that DEFER-STEMI included only patients with a high risk of thrombotic embolization based on clinical and angiographic criteria, while MIMI and DANAMI 3-DEFER did not use similar high risk selection criteria but rather randomised all STEMIs regardless of MVO risk.
The second question is whether the endpoints used in the three studies were comparable. As Table 2 shows, only DANAMI 3-DEFER had a clinical primary endpoint, while the others had imaging-based endpoints (angiography or MRI). The MIMI investigators argue that MRI is a more sensitive indicator of MVO than the angiographic endpoint selected in the DEFER STEMI trial, which may depend on the operator’s opacification performance. However, MRI results were presented in DEFER-STEMI as secondary outcomes and showed benefit in the deferred stenting group (8). The timing of MRI in the two studies was different, while in the MIMI trial patients had an MRI within 3–8 days of acute MI, in DEFER-STEMI the MRI was performed twice, at 2 days and at 6 months. The MRI analysis at 6 months may have the added benefit of taking into account improvements in microcirculation due to non-sustained MVO resolving with time (6,8). These discussions on imaging-based primary endpoints are, in any case, overshadowed by the clinical results of DANAMI 3-DEFER. This is not only because angiographic or MRI findings are, ultimately, surrogates or predictors of clinical events, but also because the study population of DANAMI 3-DEFER was much larger (around ten times larger) than that of the other two trials (9).
A third aspect that deserves analysis is the heterogeneity in procedural aspects of the MIMI approach, such as optimal deferral time, use of aspiration thrombectomy and adjunctive pharmacological therapy. In DEFER-STEMI a period of 4–16 hours was recommended for deferred PCI. Allowing a longer time period did not result in any benefit in MIMI (median delay 36 hours) or DANAMI 3-DEFER (median delay of 3 days). The negative result in the MIMI trial could be due to the plaque stabilising effect of immediate stenting, which might limit further thrombosis and subsequent embolization (6). The low rates of pre and post-dilatation in the IS arm (11.0% and 15.1% respectively) could also contribute to decreased distal embolization in MIMI. These figures are in contrast with other minimalist intervention studies such as DEFER-STEMI, which had much higher pre and post dilatation rates (73.5% and 71.4% respectively) in the IS arm. As Table 1 shows, there were also relevant differences in the usage of GpIIb/IIIa inhibitors between the deferred stenting arm of DANAMI 3-DEFER (35%) compared to both arms of DEFER-STEMI (98%) and MIMI (close to 100%) trials.
Also, within each study important differences between study arms can be found. In the DEFER-STEMI trial the percentage of cases that were predilated was higher in the deferred stenting group (73.5% is vs. 88.5% in DS), conversely, post-dilatation was more prevalent in the IS group (71.4% is vs. 57.7% in DS). These differences could be important confounders. Finally, the fact that in the DANAMI-3 DEFER trial 22% of patients randomised to deferred stenting still had a stent implanted in the index procedure complicates the interpretation of results.
The applicability of the MIMI strategy has been studied in daily practice, out of the discussed randomized trials. Pascal et al. found the MIMI approach to be applicable and safe in every-day clinical practice and was used in around 20% of their STEMI patients (11). Although the actuarial freedom from major cardiovascular events in their series was significantly better with the MIMI approach at one year, that difference became non-significant after multivariate adjustment after a mean follow-up of 44 months. In this series despite the use of contemporary antiplatelets, acute coronary reocclusion occurred in one patient (1.8%) while the patient was still in the cath lab. Furthermore this strategy was associated with a significant reduction of the number of required stents (30% of the patients did not require stenting) (11). Our group reported on the safety of a stent-free approach during primary PCI in a series of STEMI patients, selected on the grounds of an excellent angiographic or intracoronary imaging result following thrombus aspiration (12).
Three further randomised trials are underway. INNOVATION (NCT02324348) which is studying the magnitude of microvascular obstruction and infarct size by MRI and MACCE up to 1 year in patients treated with standard primary PCI compared with patients having their stent implanted after 5–7 days. OPTIMASTRATEGY (NCT01462188) will be delaying stenting to 3–7 days and will be looking at myocardial blush grade on angiography, microvascular obstruction and infarct size on MRI and mortality at 6 months. PRIMACY (NCT01542385) which is delaying stent implantation to 4–7 days will be looking at clinical endpoints as primary outcomes.
Does microvascular obstruction equal microvascular plugging?
Yet, probably the most important pending question is whether the cornerstone of the above-discussed studies, the concept that plugging of the coronary microcirculation by debris dislocated for the culprit stenosis as the dominant cause of no-reflow, is correct. It has been shown that the microvascular obstruction pattern in MRI actually is mainly the result of intramyocardial haemorrhage, not of intraluminal obstruction (13). Furthermore, two recent studies based on intracoronary doppler and pressure have disclosed a strong relationship between zero flow pressure (an estimate of extravascular compression of the microcirculation) and the development and size of the MRI microvascular obstruction pattern (14,15). This constitutes a potential explanation to the overall clinical failure of treatment strategies addressing atherothrombotic embolization reported by randomized clinical trials.
Conclusions
The debate regarding the benefits of the MIMI approach is far from over. Selection of patients at highest risk of no-reflow is probably the way forward but further work needs to be done to identify the ideal mechanism of initial reperfusion, the ideal delay for repeat angiography and the ideal antiplatelet/anticoagulant strategy. It seems clear that for the time being, these new RCTs are unlikely to change the current practice of immediate stenting. Furthermore, based on recent research, the mechanistic concept behind the MIMI approach might be increasingly challenged, as its primary MRI endpoint might be largely the result of intramyocardial haemorrhage and extravascular compression rather than to microvascular plugging by atherothrombotic particles.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54:281-92. [Crossref] [PubMed]
- Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 2008;371:1915-20. [Crossref] [PubMed]
- Fröbert O, Lagerqvist B, Olivecrona GK, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med 2013;369:1587-97. [Crossref] [PubMed]
- Jolly SS, Cairns JA, Yusuf S, et al. Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 2016;387:127-35. [Crossref] [PubMed]
- Echavarría-Pinto M, Lopes R, Gorgadze T, et al. Safety and efficacy of intense antithrombotic treatment and percutaneous coronary intervention deferral in patients with large intracoronary thrombus. Am J Cardiol 2013;111:1745-50. [Crossref] [PubMed]
- Belle L, Motreff P, Mangin L, et al. Comparison of Immediate With Delayed Stenting Using the Minimalist Immediate Mechanical Intervention Approach in Acute ST-Segment-Elevation Myocardial Infarction: The MIMI Study. Circ Cardiovasc Interv 2016;9:e003388. [Crossref] [PubMed]
- Freixa X, Belle L, Joseph L, et al. Immediate vs. delayed stenting in acute myocardial infarction: a systematic review and meta-analysis. EuroIntervention 2013;8:1207-16. [Crossref] [PubMed]
- Carrick D, Oldroyd KG, McEntegart M, et al. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI). J Am Coll Cardiol 2014;63:2088-98. [Crossref] [PubMed]
- Kelbæk H, Høfsten DE, Køber L, et al. Deferred versus conventional stent implantation in patients with ST-segment elevation myocardial infarction (DANAMI 3-DEFER): an open-label, randomised controlled trial. Lancet 2016;387:2199-206. [Crossref] [PubMed]
- Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarct-related arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest 2002;122:1322-32. [Crossref] [PubMed]
- Pascal J, Veugeois A, Slama M, et al. Delayed Stenting for ST-Elevation Acute Myocardial Infarction in Daily Practice: A Single-Centre Experience. Can J Cardiol 2016;32:988-95. [Crossref] [PubMed]
- Escaned J, Echavarría-Pinto M, Gorgadze T, et al. Safety of lone thrombus aspiration without concomitant coronary stenting in selected patients with acute myocardial infarction. EuroIntervention 2013;8:1149-56. [Crossref] [PubMed]
- Betgem RP, de Waard GA, Nijveldt R, et al. Intramyocardial haemorrhage after acute myocardial infarction. Nat Rev Cardiol 2015;12:156-67. [Crossref] [PubMed]
- Teunissen PF, de Waard GA, Hollander MR, et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ Cardiovasc Interv 2015;8:e001786. [Crossref] [PubMed]
- Patel N, Petraco R, Dall'Armellina E, et al. Zero-Flow Pressure Measured Immediately After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Provides the Best Invasive Index for Predicting the Extent of Myocardial Infarction at 6 Months: An OxAMI Study (Oxford Acute Myocardial Infarction). JACC Cardiovasc Interv 2015;8:1410-21. [Crossref] [PubMed]


