
Influence of sodium-glucose cotransporter 2 inhibitors on the triglyceride-glucose index in acute myocardial infarction patients with type 2 diabetes mellitus
Highlight box
Key findings
• In acute myocardial infarction (AMI) patients with type 2 diabetes mellitus (T2DM), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2-i) was associated with an improvement of triglyceride-glucose index and a lower risk of major adverse cardiovascular events (MACEs) during 11 months follow-up.
What is known and what is new?
• Recent clinical studies have confirmed that SGLT2-i can attenuate left ventricular end-diastolic diameter and reduce MACEs in AMI patients with T2DM. The mechanisms responsible for their beneficial effects including reducing infarct size, anti-inflammation, improving cardiac nerve activity.
• Our research on SGLT2-i focuses on these metabolic-lipid effects, indicating a new cardio-protective mechanism of SGLT2-i in the context of AMI.
What is the implication, and what should change now?
• Our research regarding SGLT2-i centers on these metabolic-lipid effects, suggesting a novel cardio-protective mechanism of SGLT2-i in the setting of AMI. Consequently, the application of SGLT2-i should be implemented in AMI patients with T2DM as soon as possible.
Introduction
As a novel oral anti-hyperglycemic agent, sodium-glucose cotransporter 2 inhibitors (SGLT2-i) have been demonstrated to improve cardiovascular and renal outcomes in heart failure patients with or without type 2 diabetes mellitus (T2DM) (1-4). In addition, recent clinical studies have confirmed that SGLT2-i can attenuate left ventricular end-diastolic diameter (LVDD) and reduce major adverse cardiovascular events (MACEs) in acute myocardial infarction (AMI) patients with T2DM (5,6). The mechanisms responsible for the beneficial effects including reducing infarct size, mitigating acute myocardial ischemia/reperfusion (I/R) injury, increasing left ventricular function, anti-inflammation, improving cardiac nerve activity (7-10).
The fasting triglyceride-glucose (TyG) index has emerged as a new indicator of insulin resistance (IR), consistent with the high insulin-glucose clamp test for IR diagnosis (11,12). Interestingly, the significance of TyG index in cardiovascular diseases (CVDs) has been increasingly recognized in recent years. Extensive studies have demonstrated a close relationship between elevated TyG index and the risk of heart failure (13), myocardial infarction (14), and atrial fibrillation (15).
A recent study confirmed that TyG index can serve as a predictive factor for in-hospital mortality in non-diabetic patients with AMI (16). Additionally, another study has identified that SGLT2-i can reduce the TyG index in T2DM patients (17). Therefore, this study aimed to evaluate the influence of SGLT2-i on the TyG index in AMI patients with T2DM. We presented this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-287/rc).
Methods
Study design
A single-center retrospective study on consecutive patients with AMI and T2DM admitted to the chest pain center of the Second Affiliated Hospital of Anhui Medical University from January 2020 to January 2023 was conducted. AMI and T2DM were identified by International Classification of Diseases (ICD) codes from inpatient medical settings and validated by medical record review. Inclusion criteria for the study were as follows: diagnosed with a first AMI with or without ST-segment elevation and T2DM following European Society of Cardiology guidelines (18-20); never received SGLT2-i within 3 months or before. Exclusion criteria encompassed severe liver and or kidney dysfunction; cardiogenic shock; infection; age below 18 years old; type 1 diabetes mellitus; diabetes ketoacidosis; incomplete information; deaths in the hospital. We reviewed all patients’ previous outpatient follow-up records, including laboratory tests and telephone follow-ups, to ensure medication adherence within 7 to 15 months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for the study was obtained from the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (approval No. YX2023-071). Individual consent for this retrospective analysis was waived.
Study population
There were 180 patients with AMI and T2DM who met the inclusion and exclusion criteria for this study. Of these, 101 had ST-segment elevation myocardial infarction (STEMI) and 79 had non-STEMI (NSTEMI). Based on the choice of post-admission glucose control strategy, the patients were divided into the control group (79 patients who received sulfonylureas, α-glucosidase inhibitors, metformin, or other oral anti-diabetic drugs) and the SGLT2-i group (101 patients who received dapagliflozin or canagliflozin). During hospitalization, a comprehensive medical and family history, physical examination, standard laboratory tests, 12-lead electrocardiogram (ECG), and transthoracic echocardiography were performed for each patient. Follow-up laboratory tests and transthoracic echocardiography were arranged at 12 months after discharge. If a MACE occurred during the follow-up period, including recurrent myocardial infarction, heart failure hospitalization, ischemic stroke, or cardiovascular death, the follow-up was stopped.
Blood test
All patients underwent blood testing immediately upon their initial medical contact for the examination of white blood cell (WBC), N-terminal pro-B-type natriuretic peptide (NT-proBNP), cardiac troponin I (cTnI). And fasting vein blood was collected the next morning after admission and placed in an anticoagulant tube for examination. Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), fasting blood glucose (Glu), glycosylated hemoglobin (HbAlc), and other biochemical indicators were detected using enzyme-linked immunosorbent assay, while the fasting TyG calculated using the formula ln [TG (mg/dL) × plasma glucose (mg/dL)/2].
Echocardiographic parameters
Two-dimensional transthoracic echocardiography was conducted to assess left ventricular ejection fraction (LVEF) using a Philips iE33 device (Philips, Andover, MA, USA) at baseline (before discharge) and 6 months after discharge by two echocardiographers with 10 years of professional experience. Image acquisitions and measurements were performed in accordance with the guidelines of the European Association of Echocardiography and the American Society of Echocardiography (21).
Percutaneous coronary intervention (PCI)
PCI was uniformly performed in each patient primary or selectively depended on the current guidelines (18,19) using standard technique, primarily via the radial artery approach. Assessment of coronary artery lesions was conducted utilizing the Gensini Score (22). The primary objective of PCI was to reinstate normal blood flow in the infarct-related artery (IRA), achieved through either stent implantation or balloon dilatation alone, contingent upon the experienced operator’s discretion, who performs more than 200 coronary interventions per year. Evaluation of IRA blood flow was based on the Thrombolysis in Myocardial Infarction (TIMI) score. Other significant non-culprit lesions of ≥70% severity was addressed during the index hospitalization or selectively managed in consultation with the attending physician and the patient.
Statistical analysis
The data analyses were conducted using R version 4.22. Propensity score matching (PSM) was utilized to achieve baseline balance for patient characteristics. The propensity score was calculated through a logistic regression model, and the covariates adjusted in the model included all clinical, biochemical, and angiographic parameters based on nearest neighbor matching. The normality of continuous variable distribution was assessed using the Shapiro-Wilk test. Normally distributed data were analyzed using t-tests and presented as mean ± standard deviation, while non-normally distributed data were analyzed using the Wilcoxon rank sum test and presented as median with interquartile range or represented as M (P25, P75). Categorical variables were analyzed using the Chi-square test or Fisher’s exact test and described in terms of frequency and percentage (%). Between the SGLT2-i and non-SGLT2-i cohorts, Kaplan-Meier (KM) curves of MACE were compared using the log-rank test. Univariate and Multivariate Cox regression were performed to detect any independent risk factors by computing the hazard ratio (HR) with a 95% confidence interval (CI). A threshold of a two-sided 0.05 was regarded as statistically significant for P value.
Results
The course of study
During the period from January 2020 to January 2023, a total of 245 patients diagnosed with AMI and T2DM were admitted to our hospital. Following the application of exclusion criteria, a study cohort comprising 180 patients was identified. Post-admission glucose control strategy was as follows: those receiving other antidiabetic drugs and those receiving SGLT2-i. Among these patients, 101 patients (56.1%) belonged to the SGLT2-i group (Figure 1).

Clinical, biochemical, and angiographic parameters before PSM
Among the 180 enrolled patients, 79 (43.89%) patients treated with other oral antidiabetic agents, constituting control group, while the remaining 101 patients with SGLT2-i formed SGLT2-i group. A comparison of clinical, biochemical, and angiographic parameters between the two groups is presented in Table 1.
Table 1
| Variables | Control group (n=79) | SGLT2-i group (n=101) | t/W/χ2 | P |
|---|---|---|---|---|
| Male | 56 (70.89) | 73 (72.28) | 0.042 | 0.84 |
| Age (years) | 68.00 (56.50, 75.00) | 63.00 (54.00, 69.00) | 4,823.5* | 0.02 |
| STEMI | 41 (51.90) | 60 (59.41) | 1.014 | 0.31 |
| Hypertension | 51 (64.56) | 65 (64.36) | 1.113 | 0.98 |
| Heart rate (bpm) | 75.00 (70.00, 81.00) | 75.00 (69.00, 80.00) | 4,190.5 | 0.56 |
| Insulin use | 40 (50.63) | 29 (28.71) | 9.010* | 0.003 |
| BMI (kg/m2) | 27.38±6.22 | 26.32±7.08 | 1.051 | 0.85 |
| Smoker | 40 (50.63) | 53 (52.48) | 0.496 | 0.81 |
| Drinker | 22 (27.85) | 35 (34.65) | 0.949 | 0.33 |
| Dual antiplatelet drugs | 79 (100.00) | 101 (100.00) | – | – |
| ACEI/ARB/ARNI | 56 (70.89) | 76 (75.25) | 0.431 | 0.51 |
| Stains | 79 (100.00) | 101 (100.00) | – | – |
| Sulfonylureas | 29 (36.71) | 44 (43.56) | 0.864 | 0.35 |
| Metformin | 43 (54.43) | 67 (66.34) | 2.644 | 0.10 |
| α-glucosidase inhibitors | 28 (35.44) | 49 (48.51) | 3.094 | 0.08 |
| DPP-4 inhibitors | 12 (15.19) | 23 (22.77) | 1.627 | 0.20 |
| Patients undergoing PPCI | 58 (73.42) | 77 (76.24) | 0.188 | 0.67 |
| Time of DTB in patients undergoing PPCI (min) | 86.23±32.27 | 84.12±37.14 | 0.400 | 0.69 |
| Gensini score | 53.82±30.24 | 44.36±25.57 | 2.273* | 0.02 |
| Glu (mmol/L) | 8.08 (6.50, 9.10) | 8.10 (6.950, 11.33) | 3,363.5 | 0.07 |
| HbAlc (mmol/L) | 7.30 (6.65, 8.10) | 7.50 (6.50, 8.70) | 3,447.5 | 0.12 |
| Cr (μmol/L) | 89.00 (67.50, 134.00) | 76.00 (61.00, 92.00) | 5,055.5* | 0.002 |
| WBC (×109/L) | 7.25 (6.17, 9.07) | 7.61 (6.42, 9.67) | 3,550.5 | 0.21 |
| TC (mmol/L) | 3.93 (3.27, 4.77) | 4.60 (3.64, 5.22) | 3,073.0* | 0.008 |
| TG (mmol/L) | 1.33 (0.98, 1.87) | 1.47 (1.14, 2.29) | 3,458.5 | 0.13 |
| TyG | 9.09±0.79 | 9.31±0.74 | −1.921 | 0.06 |
| LDL-C (mmol/L) | 2.55±1.07 | 2.78±0.88 | −1.613 | 0.11 |
| ALT (U/L) | 27.00 (18.00, 43.00) | 30.00 (21.00, 47.00) | 3,546.0 | 0.20 |
| cTnI (ng/dL) | 1.06 (0.100, 5.46) | 3.43 (0.49, 84.10) | 2,872.0* | 0.001 |
| NT-proBNP (pg/mL) | 907.00 (907.00, 1,001.50) | 907.00 (834.00, 907.00) | 4,224.5 | 0.47 |
| LVDD (mm) | 47.50 (46.00, 51.50) | 47.50 (45.00, 52.00) | 4,227.5 | 0.49 |
| EF (%) | 60.00 (55.50, 61.00) | 60.00 (51.00, 62.00) | 4,034.0 | 0.90 |
Normally distributed data were presented as mean ± standard deviation; non-normally distributed data were presented as median (interquartile range) or represented as M (P25, P75); n (%) for categorical variables. *, P<0.05; the remaining P values were all >0.05. Smoker: more than 5 cigarettes per day, continuously or for more than 6 months. Drinker: more than 50 g per day, continuously or for more than 6 months. Dual antiplatelet drugs: aspirin + clopidogrel or aspirin + ticagrelor. DPP-4 inhibitors: including saxagliptin, sitagliptin. SGLT2-i, sodium-glucose cotransporter 2 inhibitors; STEMI, acute ST elevation myocardial infarction; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ARNI, angiotensin receptor-neprilysin inhibitor; PPCI, primary percutaneous coronary intervention; DTB, door to balloon dilation; Glu, fasting blood glucose; HbAlc, glycosylated hemoglobin; Cr, serum creatinine; WBC, white blood cell; TC, total cholesterol; TG, triglyceride; TyG, triglyceride-glucose; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVDD, left ventricular end-diastolic diameter; EF, ejection fraction.
Before matching, there were no significant differences in the TyG index between the two groups. However, there were statistical disparities in age, proportion of insulin use, Gensini score, serum creatinine (Cr), TC, and cTnI as demonstrated in Table 1.
Post-propensity scores matching results
To balance potential confounders and minimize selection bias, we utilized R 4.22 for coarsening precise matching in a one-to-one manner. Consequently, control group remained 32 patients, and SGLT2-i group remained 37 patients. The cohorts were well-balanced based on baseline clinical, biochemical, and angiographic parameters, with a standardized difference of <10% after matching as showed in Table 2.
Table 2
| Variables | Control group (n=32) | SGLT2-i group (n=37) | t/W/χ2 | P |
|---|---|---|---|---|
| Male | 27 (84.38) | 24 (64.86) | 3.387 | 0.07 |
| Age (years) | 67.00 (56.75, 71.00) | 59.00 (55.00, 69.00) | 688.0 | 0.25 |
| STEMI | 15 (46.88) | 23 (62.16) | 1.621 | 0.20 |
| Hypertension | 18 (56.25) | 21 (56.76) | 0.002 | 0.97 |
| Heart rate (bpm) | 78.00 (71.50, 82.50) | 75.00 (70.00, 80.00) | 689.0 | 0.24 |
| Insulin | 9 (28.13) | 10 (27.03) | 0.010 | 0.92 |
| BMI (kg/m2) | 27.12±5.13 | 25.93±6.08 | 0.871 | 0.39 |
| Smoker | 18 (56.25) | 17 (45.95) | 0.729 | 0.39 |
| Drinker | 14 (43.75) | 16 (43.24) | 0.002 | 0.97 |
| Dual antiplatelet drugs | 32 (100.00) | 37 (100.00) | – | – |
| ACEI/ARB/ARNI | 30 (93.75) | 34 (91.89) | 0.088 | 0.77 |
| Stain | 32 (100.00) | 37 (100.00) | – | – |
| Sulfonylureas | 22 (68.75) | 21 (56.76) | 1.051 | 0.31 |
| Metformin | 24 (75.00) | 28 (75.68) | 0.004 | 0.95 |
| α-glucosidase inhibitors | 16 (50.00) | 22 (59.46) | 0.621 | 0.43 |
| DPP-4 inhibitors | 6 (18.75) | 10 (27.03) | 0.660 | 0.42 |
| Patients undergoing PPCI | 21 (65.63) | 26 (70.27) | 0.170 | 0.68 |
| Time of DTB in patients undergoing PPCI (min) | 85.26±29.16 | 86.23±27.31 | −0.143 | 0.89 |
| Gensini score | 54.13±24.36 | 52.26±23.14 | 0.327 | 0.75 |
| Glu (mmol/L) | 8.08 (6.56, 8.11) | 8.08 (6.47, 10.38) | 476.0 | 0.16 |
| HbAlc (mmol/L) | 7.49±1.27 | 7.71±1.23 | −0.722 | 0.47 |
| Cr (μmol/L) | 77.50 (69.50, 95.25) | 73.00 (61.00, 92.00) | 685.0 | 0.26 |
| WBC (×109/L) | 7.21 (6.11, 8.91) | 7.40 (6.44, 9.71) | 526.5 | 0.43 |
| NLR | 5.94±1.23 | 6.11±1.08 | −0.611 | 0.54 |
| TC (mmol/L) | 4.02±0.91 | 3.93±0.81 | 0.413 | 0.68 |
| TG (mmol/L) | 1.37 (0.91, 1.92) | 1.40 (1.07, 2.29) | 546.0 | 0.58 |
| TyG | 9.09±0.79 | 9.31±0.74 | −1.194 | 0.24 |
| LDL-C (mmol/L) | 2.31±0.81 | 2.35±0.68 | −0.231 | 0.82 |
| ALT (U/L) | 33.50 (25.00, 51.50) | 30.00 (22.00, 41.00) | 693.5 | 0.22 |
| cTnI (ng/dL) | 0.47 (0.02, 3.56) | 2.14 (0.25, 20.10) | 445.0 | 0.08 |
| NT-proBNP (pg/mL) | 907.00 (581.25, 924.25) | 907.00 (446.00, 907.00) | 618.0 | 0.77 |
| LVDD (mm) | 47.50 (45.75, 51.25) | 47.50 (44.00, 50.00) | 619.0 | 0.74 |
| EF (%) | 60.00 (50.00, 62.00) | 60.00 (58.00, 62.00) | 573.0 | 0.82 |
Normally distributed data were presented as mean ± standard deviation; non-normally distributed data were presented as median (interquartile range) or represented as M (P25, P75); n (%) for categorical variables. P values were all >0.05. Smoker: more than 5 cigarettes per day, continuously or for more than 6 months. Drinker: more than 50 g per day, continuously or for more than 6 months. Dual antiplatelet drugs: aspirin + clopidogrel or aspirin + ticagrelor. DPP-4 inhibitors: including saxagliptin, sitagliptin. SGLT2-i, sodium-glucose cotransporter 2 inhibitors; STEMI, acute ST elevation myocardial infarction; BMI, body mass index; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ARNI, angiotensin receptor-neprilysin inhibitor; PPCI, primary percutaneous coronary intervention; DTB, door to balloon dilation; Glu, fasting blood glucose; HbAlc, glycosylated hemoglobin; Cr, serum creatinine; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; TC, total cholesterol; TG, triglyceride; TyG, triglyceride-glucose; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVDD, left ventricular end-diastolic diameter; EF, ejection fraction.
Follow-up results
Laboratory tests
The median follow-up period was 11 months in both cohorts, with the maximum being 14 months. Laboratory tests and transthoracic echocardiography were re-evaluated in the outpatient department. The comparison of follow-up results between the two groups after matching confirmed statistically significant differences in TG reduction, TyG index reduction, WBC reduction, and LVDD reduction in the SGLT2-i group, as indicated in Table 3.
Table 3
| Variables | Control group (n=32) | SGLT2-i group (n=37) | t/W/χ2 | P |
|---|---|---|---|---|
| Follow-up time (months) | 10.56±2.34 | 11.02±2.73 | −0.745 | 0.46 |
| Glu (mmol/L) | 8.08 (6.56, 8.11) | 8.08 (6.59, 8.20) | 577 | 0.80 |
| HbAlc (mmol/L) | 7.45 (6.68, 8.08) | 7.45 (6.70, 8.30) | 571.5 | 0.82 |
| TC (mmol/L) | 4.03±0.81 | 4.04±1.01 | −0.045 | 0.96 |
| TG (mmol/L) | 2.60±3.07 | 1.42±1.01 | 2.206* | 0.03 |
| TyG | 9.07±0.31 | 8.74±0.35 | 4.116** | <0.001 |
| LDL-C (mmol/L) | 2.36±0.75 | 2.29±0.76 | 0.384 | 0.70 |
| Cr (μmol/L) | 77.50 (69.50, 95.25) | 77.00 (67.00, 94.00) | 612 | 0.76 |
| WBC (×109/L) | 8.72±1.59 | 7.37±1.63 | 3.460* | 0.001 |
| NLR | 6.34±1.89 | 5.72±1.67 | 1.447 | 0.15 |
| ALT (U/L) | 33.50 (25.00, 51.50) | 33.00 (25.00, 49.00) | 589.5 | 0.78 |
| cTnI (mg/dL) | 0.26 (0.00, 2.03) | 0.14 (0.00, 1.43) | 734.0 | 0.54 |
| NTproBNP (pg/mL) | 204.00 (108.75, 280.25) | 125.00 (65.00, 236.00) | 718.5 | 0.58 |
| LVDD† (mm) | 1.36±2.11 | −0.53±1.46 | 4.250** | <0.001 |
| EF† (%) | 0.25 (−2.00, 2.00) | 1.00 (−7.00, 4.00) | 629.5 | 0.67 |
Normally distributed data were presented as mean ± standard deviation; non-normally distributed data were presented as median (interquartile range) or represented as M (P25, P75). †, represented improvements from baseline values, with positive values representing increases and negative values representing decreases. *, P<0.05; **, P<0.01. SGLT2-i, sodium-glucose cotransporter 2 inhibitors; Glu, fasting blood glucose; HbAlc, glycosylated hemoglobin; TC, total cholesterol; TG, triglyceride; TyG, triglyceride-glucose; LDL-C, low-density lipoprotein cholesterol; Cr, serum creatinine; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; ALT, alanine aminotransferase; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide; LVDD, left ventricular end-diastolic diameter; EF, ejection fraction.
Incidence of MACE
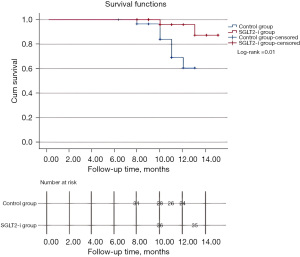
During the follow-up period, control group had a higher incidence of MACEs compared to SGLT2-i group with a significant difference observed between the two groups (log-rank =0.01) as illustrated in Figure 2. The specific details included: in the control group there were occurrences such as recurrent myocardial infarction (2 cases), hospitalization for heart failure (5 cases), and cardiovascular death (1 case); while SGLT2-i group had occurrences included recurrent myocardial infarction (1 case) and hospitalization for heart failure (1 case).
Influence of SGLT2-i on the TyG index
To evaluate the impact of SGLT2-i on the TyG index in patients with AMI and T2DM, we conducted a comparison of the TyG index between the two groups during follow-up, as well as an analysis of changes in the TyG index before and after treatment within each group. Interestingly, we observed a decrease in the TyG index in the SGLT2-i group following treatment (8.74±0.35 vs. 9.31±0.74, P<0.001), as well as a significant reduction compared to the non-SGLT2-i group at the end of the follow-up period (8.74±0.35 vs. 9.07±0.31, P<0.001). In contrast, there was no notable difference in the control group before and after treatment (9.07±0.31 vs. 9.09±0.79, P=0.89) as showed in Figure 3.

Cox regression analysis of risk of MACE in AMI patients with T2DM
Cox regression analysis was used to analyze the factors affecting MACE incidence in AMI patients with T2DM. Univariate analysis showed that usage of SGLT2-i (HR =0.172; 95% CI: 0.035–0.852; P=0.03), Cr (HR =0.959; 95% CI: 0.922–0.999; P=0.04), LDL (HR =0.431; 95% CI: 0.193–0.964; P=0.04), TyG at baseline (HR =2.808; 95% CI: 1.453–5.424; P=0.002), and changes of TyG (TyG at follow-up minus TyG at baseline) (HR =2.102; 95% CI: 1.238–3.569; P=0.006) were associated with the risk of MACE. However, multivariate analysis showed only usage of SGLT2-i was associated with the risk of MACE (HR =0.077; 95% CI: 0.009–0.682; P=0.02) (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Usage of SGLT2-i | 0.172 (0.035–0.852) | 0.03 | 0.077 (0.009–0.682) | 0.02 | |
| Cr | 0.959 (0.922–0.999) | 0.04 | 0.956 (0.897–1.018) | 0.16 | |
| LDL-C | 0.431 (0.193–0.964) | 0.04 | 0.405 (0.130–1.262) | 0.12 | |
| TyG at baseline | 2.808 (1.453–5.424) | 0.002 | 0.359 (0.034–3.764) | 0.39 | |
| Changes of TyG | 2.102 (1.238–3.569) | 0.006 | 4.408 (0.548–35.483) | 0.16 | |
MACE, major adverse cardiovascular event; HR, hazard ratio; CI, confidence interval; SGLT2-i, sodium-glucose cotransporter 2 inhibitors; Cr, serum creatinine; LDL-C, low-density lipoprotein cholesterol; TyG, triglyceride-glucose; changes of TyG, TyG at follow-up minus TyG at baseline.
Discussion
IR refers to the reduced sensitivity of insulin-sensitive tissues to insulin (23), leading to impaired biological activity of insulin and subsequent elevation of blood sugar levels. This metabolic disturbance results in dysfunctional adipose tissue and is associated with various diseases such as obesity, inflammation, dyslipidemia, reactive oxygen species (ROS) production, atherosclerosis, endothelial dysfunction, and hypertension, all contributing to the development of CVDs (24,25). Clinical trials have demonstrated that IR is linked to poor short-term and long-term outcomes in patients with AMI (16,26), and there are currently no approved hypoglycemic agents specifically targeting IR (27). While SGLT2-i have been shown to benefit CVD patients with T2DM, it remains uncertain whether the benefits include improvement in IR.
In this study, there were statistically significant differences in related indicators between the two groups before PSM. After PSM, there were no statistically significant differences in baseline clinical indicators and laboratory tests between the two groups, indicating comparability. The follow-up time did not differ significantly between the two groups. The results demonstrated that SGLT2-i could improve TyG index (IR) within 6–12 months while maintaining similar blood glucose control levels compared to other antidiabetic drugs in AMI patients with T2DM. Furthermore, this study confirmed that the reduction of LVDD in SGLT2-i group was statistically significant (P<0.01), in accordance with previous studies (28,29). There was a difference in MACE rate between the groups (P<0.05), consistent with previous studies’ findings (6-9). Additionally, univariate analysis showed that usage of SGLT2-i, TyG at baseline, and changes of TyG were associated with the risk of MACE. However, multivariate analysis showed only usage of SGLT2-i was associated with the risk of MACE, which may attribute to the small size of the sample. Therefore, we conclude that SGLT2-i can improve the prognosis and cardiac function of AMI patients with T2DM within 6–12 months after myocardial infarction onset by improving TyG index (IR) and systemic inflammatory response when compared to other antidiabetic drugs; these improvements may be beneficial for long-term patient prognosis, while larger sample sizes and longer follow-up studies are needed to confirm these results.
The mechanisms underlying the potential cardiovascular benefits of SGLT2-i drugs remain unclear, as SGLT2 has not been shown to be expressed in human cardiomyocytes and is mainly expressed in proximal tubular cells. While increased diuresis/natriuresis, improved glycemic control, decreased blood pressure, weight loss, improved vascular function, altered tissue sodium handling, ameliorated myocardial ischemia-reperfusion injury, and reduced myocardial infarction size, reduced the risk of sudden cardiac death and ventricular arrhythmias may and ameliorated iron deficiency and increased iron levels in the heart may play a role (30-33), other mechanisms are still being explored. The TyG index has been confirmed by numerous clinical trials as a predictor of MACEs in patients with AMI (16,34). However, no drug has been proven to improve the TyG index. A recent retrospective observational study involving 143 patients with T2DM found that dapagliflozin and empagliflozin were both effective in reducing the TyG index (17). Nevertheless, such efficacy of SGLT2-i in patients with T2DM and AMI has not been confirmed by clinical studies. This study represents the first retrospective analysis of AMI patients with T2DM to confirm that SGLT2-i can improve the TyG index in these patients, suggesting a potential new mechanism for SGLT2-i drugs to benefit these individuals.
The mechanisms of action of SGLT2-i on TyG index remain unclear. A study involving 143 patients with T2DM confirmed that SGLT2-i could improve the TyG index by reducing TG levels (17), and Matsubayashi et al. reported that increased hepatic insulin clearance (HIC) was significantly associated with reduced TG via the SGLT2-i tofogliflozin compared with placebo (35). However, another study involving 60 male patients with new-onset T2DM demonstrated significant improvements in blood glucose and TyG index in the metformin + SGLT2-i group compared to metformin alone (36). Similar to the former one, the SGLT2-i group also showed a significant reduction in TG levels at the end of follow-up in our study, while there were no significant changes in glucose and other lipid parameters. In addition, the SGLT2-i have been proved to induce a metabolic switch away from the consumption of energy-inefficient glucose towards the utilization of free fatty acids and ketone bodies as conclusively demonstrated both in animals’ models and in humans (28,37).
Inflammation plays a crucial role in the development and progression of coronary atherosclerosis, including endothelial injury, plaque rupture, and thrombosis (38,39). The SGLT2-i agent has been demonstrated anti-inflammatory effects (9,40). Therefore, it is plausible that SGLT2-i drugs may also have anti-inflammatory effects beneficial for AMI patients with T2DM. Additionally, this study confirmed that SGLT2-i can further decrease LVDD compared with other hypoglycemic drugs, which is consistent with previous studies (5-10).
Our trial is subject to certain limitations. Firstly, as a single-center, retrospective study spanning a period of 3 years, there may be some deviation in the selection of patients despite stringent adherence to predefined inclusion and exclusion criteria, like its non-interventional nature may lead to loss of sample size and incomplete data collection. Additionally, while the proportion of patients underwent primary PCI (PPCI) is similar in both groups, not all patients received PPCI, potentially impacting the difference in patency time and consequently affecting the results. Secondly, the sample size of the trial is relatively small and only NT-proBNP and transthoracic echocardiography were employed for evaluating cardiac function, these methods may lack precision compared to cardiac magnetic resonance (CMR) imaging or single-photon emission computed tomography (SPECT). Lastly, the follow-up period was relatively short; an extended follow-up might have led to different conclusions. Therefore, it is imperative to conduct a large-scale prospective randomized controlled trial to validate these findings.
Conclusions
In AMI patients with T2DM, the use of SGLT2-i was associated with an improvement of TyG index and a lower risk of MACE during 11 months follow-up. Our findings offer new insights into the cardio-protective mechanisms of SGLT2-i in the context of AMI.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-287/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-287/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-287/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-287/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for the study was obtained from the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (approval No. YX2023-071). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021;385:1451-61. [Crossref] [PubMed]
- Varzideh F, Kansakar U, Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother 2021;7:e67-8. [Crossref] [PubMed]
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995-2008. [Crossref] [PubMed]
- Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Empagliflozin improves quality of life in nondiabetic HFrEF patients. Sub-analysis of the EMPATROPISM trial. Diabetes Metab Syndr 2022;16:102417. [Crossref] [PubMed]
- Wang K, Wang L, Hu GQ, et al. Effect of sodium-glucose cotransporter 2 inhibitor on clinical parameters and prognosis of patients with acute myocardial infarction complicated with type 2 diabetes mellitus. China Medicine 2024;19:171-5. [PubMed]
- Paolisso P, Bergamaschi L, Gragnano F, et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol Res 2023;187:106597. [Crossref] [PubMed]
- Andreadou I, Bell RM, Bøtker HE, et al. SGLT2 inhibitors reduce infarct size in reperfused ischemic heart and improve cardiac function during ischemic episodes in preclinical models. Biochim Biophys Acta Mol Basis Dis 2020;1866:165770. [Crossref] [PubMed]
- Lahnwong C, Palee S, Apaijai N, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol 2020;19:91. [Crossref] [PubMed]
- Paolisso P, Bergamaschi L, Santulli G, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol 2022;21:77. [Crossref] [PubMed]
- Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 2020;19:148. [Crossref] [PubMed]
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214-23. [PubMed]
- Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract 2011;93:e98-e100. [Crossref] [PubMed]
- Xu L, Wu M, Chen S, et al. Triglyceride-glucose index associates with incident heart failure: A cohort study. Diabetes Metab 2022;48:101365. [Crossref] [PubMed]
- Tian X, Zuo Y, Chen S, et al. Triglyceride-glucose index is associated with the risk of myocardial infarction: an 11-year prospective study in the Kailuan cohort. Cardiovasc Diabetol 2021;20:19. [Crossref] [PubMed]
- Zhang Y, Wang L, Qi J, et al. Correlation between the triglyceride-glucose index and the onset of atrial fibrillation in patients with non-alcoholic fatty liver disease. Diabetol Metab Syndr 2023;15:94. [Crossref] [PubMed]
- Guo J, Ji Z, Carvalho A, et al. The triglycerides-glucose index and the triglycerides to high-density lipoprotein cholesterol ratio are both effective predictors of in-hospital death in non-diabetic patients with AMI. PeerJ 2022;10:e14346. [Crossref] [PubMed]
- Imre E, Gunhan HG, Erel P, et al. SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: a real-world retrospective observational study. Minerva Endocrinol (Torino) 2023;48:295-304. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367. [Crossref] [PubMed]
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255-323. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [Crossref] [PubMed]
- Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 2001;109:S135-48. [Crossref] [PubMed]
- Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol 2019;234:8152-61. [Crossref] [PubMed]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270-87. [Crossref] [PubMed]
- Drwiła-Stec D, Rostoff P, Gajos G, et al. Predictive value of metabolic score for insulin resistance and triglyceride glucose-BMI among patients with acute myocardial infarction in 1-year follow-up. Coron Artery Dis 2023;34:314-9. [Crossref] [PubMed]
- Kosmas CE, Bousvarou MD, Kostara CE, et al. Insulin resistance and cardiovascular disease. J Int Med Res 2023;51:3000605231164548. [Crossref] [PubMed]
- Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol 2019;73:1931-44. [Crossref] [PubMed]
- Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized Trial of Empagliflozin in Nondiabetic Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol 2021;77:243-55. [Crossref] [PubMed]
- Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci 2020;5:632-44. [Crossref] [PubMed]
- Santos-Gallego CG, Requena-Ibáñez JA, Picatoste B, et al. Cardioprotective Effect of Empagliflozin and Circulating Ketone Bodies During Acute Myocardial Infarction. Circ Cardiovasc Imaging 2023;16:e015298. [Crossref] [PubMed]
- Oates CP, Santos-Gallego CG, Smith A, et al. SGLT2 inhibitors reduce sudden cardiac death risk in heart failure: Meta-analysis of randomized clinical trials. J Cardiovasc Electrophysiol 2023;34:1277-85. [Crossref] [PubMed]
- Angermann CE, Santos-Gallego CG, Requena-Ibanez JA, et al. Empagliflozin effects on iron metabolism as a possible mechanism for improved clinical outcomes in non-diabetic patients with systolic heart failure. Nat Cardiovasc Res 2023;2:1032-43. [Crossref] [PubMed]
- Luo E, Wang D, Yan G, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol 2019;18:150. [Crossref] [PubMed]
- Matsubayashi Y, Yoshida A, Suganami H, et al. Association of increased hepatic insulin clearance and change in serum triglycerides or β-hydroxybutyrate concentration via the sodium/glucose-cotransporter 2 inhibitor tofogliflozin. Diabetes Obes Metab 2020;22:947-56. [Crossref] [PubMed]
- Lin J, Zhan L, Chen Z, et al. The effect of SGLT2i on the GH/IGF1 axis in newly diagnosed male T2D patients - a prospective, randomized case-control study. Endocrine 2024;84:203-12. [Crossref] [PubMed]
- Santos-Gallego CG, Mayr M, Badimon J. SGLT2 Inhibitors in Heart Failure: Targeted Metabolomics and Energetic Metabolism. Circulation 2022;146:819-21. [Crossref] [PubMed]
- Crea F, Libby P. Acute Coronary Syndromes: The Way Forward From Mechanisms to Precision Treatment. Circulation 2017;136:1155-66. [Crossref] [PubMed]
- Libby P, Hansson GK. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J Am Coll Cardiol 2019;74:1594-607. [Crossref] [PubMed]
- Heerspink HJL, Perco P, Mulder S, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019;62:1154-66. [Crossref] [PubMed]


