
Energy loss and adults with congenital heart disease: a novel marker of cardiac workload beyond right ventricular size
Introduction
Right ventricular (RV) dysfunction is critical in most adults with congenital heart disease (ACHD) after biventricular repair; however, the condition in many young adolescents remains asymptomatic even after reaching the criteria for surgery. If a good chance of invasive treatment is missed, ventricular aggravation is unavoidable. In particular, the RV easily gets affected from volume and/or pressure overload; therefore, proactive detection and treatment of fragile ventricles are essential. In tetralogy of Fallot (TOF), there is an expert consensus on the optimal timing of pulmonary valve replacement (PVR) for significant pulmonary regurgitation (PR) based on RV size (1-5). Magnetic resonance imaging (MRI) measurement is considered as a ‘gold standard’; however, the evaluation of RV size and function is more challenging than expected, as the following reasons.
First, the reproducibility of the MRI RV measurements is not always perfect. It is sometimes challenging for different imaging specialists in clinical practice to detect temporal changes in serial images. Secondly, RV deterioration in so-called restrictive physiology is challenging to be assessed, because RV size and ejection fraction (EF) do not reach the cut-off values for surgery. We are still contemplating whether restrictive physiology affects positively or negatively on RV deterioration. Previous reports have suggested a favourable influence on RV dilatation and exercise capacity in adolescence (6-8); however, restrictive physiology in a broad sense causes various cardiac events and symptoms (6-8). To proactively treat this condition, borderline cases of restrictive physiology should be identified. Combined valvular diseases, such as PR, pulmonary stenosis (PS), and tricuspid regurgitation (TR), also exist in this condition, which is challenging evaluated using conventional modalities. Moreover, RV systolic function tends to be overestimated owing to the Frank-Starling law of the heart. Therefore, a novel parameter beyond RV size and EF is required to detect potential cardiac burden. Finally, some ACHDs have a relatively hypoplastic RV at birth, such as pulmonary atresia with intact ventricular septum (PAIVS), which sometime shows significant RV diastolic dysfunction in adulthood. After reaching adulthood, RV myocardium is chronically stretched, and RV size and function pretend to be ‘normal’ due to the volume overload of PR and TR, leading to significant RV diastolic dysfunction. In such CHDs, it is difficult to determine a clear cut-off value of RV size and function for invasive treatments.
Overall, additional information on ACHD after biventricular repair is required. The reason why adjunctive information is required is as follows: (I) to evaluate the severity of cardiac burden in symptomatic patients with normal RV size and EF, (II) to determine the optimal timing of invasive treatments in asymptomatic ones, and (III) to detect proactively a potential cardiac burden leading to ventricular deterioration, from a fluid dynamics perspective.
What is energy loss (EL) by using 4D flow MRI?
EL using 4D flow MRI is a novel non-invasive flow visualization method. No contrast medium is needed. Currently, several clinical applications for the comprehensive assessment of haemodynamics are available, including echocardiography vector flow mapping (VFM), 4D flow MRI, and simulation medicine in computational fluid dynamics (CFD) (9-11). A 4D flow MRI can detect a flow condition in real time. EL is the energy dissipated by blood viscosity, and evaluates the cardiac workload related to the prognosis of heart failure (9-13). The advantage is that EL is an independent parameter of the current heart failure or cardiac remodeling state, such as the chamber size or ventricular wall motion. This parameter is based on intuitive and clear physiological concepts, which are suitable for in vivo flow measurements using inner velocity profiles.
In 2011, Itatani et al. derived a novel method of calculating EL without pressure information (9,10). Because this parameter is mathematically derived as shown in the following equations, the accuracy of EL as an energy level drop should be conceptually proven, and this conceptual proof has already been performed with numerical experiments.
EL is defined as the drop in energy level from the inlets to the outlets (9,10).
where ρ is the density of the blood and P is the pressure.
At the stenotic site, the kinetic pressure increased with flow acceleration, the static pressure decreased, and the total pressure remained constant. However, with flow deceleration distal to the stenosis site, the kinetic pressure decreased substantially, and the static pressure recovered to some extent. This phenomenon is called pressure recovery, and the total pressure decreases according to the amount of EL by the inefficient jet flow or vortex flow distal to the stenotic site (Figure 1A,1B).

Finally, Itatani uses this equation (9,10):
This requires only the flow velocity distribution and does not include a pressure term; thus, it is applicable to noninvasive flow visualisation methods using echocardiography and MRI. Cardiac catheterization for a pressure-volume (PV) loop is not needed. EL has been reported as a useful parameter of cardiac workload that predicts ventricular deterioration in myocardium, valvular disease, and CHD (13-17). In aortic stenosis (AS), EL is an important predictor of cardiac events (12,14). In aortic regurgitation (AR), symptomatic patients with AR and a small left ventricle (LV) showed elevated EL, suggesting that unfavourable turbulent flow in a small LV leads to inefficient haemodynamics (18,19). EL is considered a marker of potential cardiac workload, even when cardiac function and volume are preserved. This single parameter is clinically useful and conceptually includes both preload and afterload caused by the diseased turbulent flow and is amenable to systolic and diastolic analyses separately.
Various types of unique flows, including vorticity and helicity, occur inside the ventricle and large vessels in CHD, and it is challenging to distinguish between physiologically normal and abnormal flows. A comprehensive quantitative parameter to estimate the unfavourable flow structure is required when physicians consider the pathophysiology of diseases and their long-term negative effects on CHDs.
EL in ACHD after biventricular repair
Even with very good TOF, EL is known to be higher than that in normal controls (20,21); therefore, it is inappropriate to compare absolute values to healthy controls. It is better to follow the temporal changes and assess postoperative improvement in each patient.
In our previous study on mid-term outcomes in TOF with PR, 4D flow MRI-derived EL and RV workloads were reported (15,20,21). Our conclusions were as follows: (I) high EL, particularly high diastolic EL, was a powerful predictor of cardiac events in adult TOF with PR, and (II) higher diastolic EL was also related to lower RVEF and deteriorated RV function in adult TOF with PS and PR. TOF with significant PR cases (transannular patch repair) showed a biphasic EL/CO curve and a markedly elevated diastolic EL as well as systolic EL (Figure 2A,2B). Several previous studies have also reported that diastolic EL was obviously elevated in TOF compared with other simple CHDs, such as atrial septal defects (21), particularly, in the right ventricular outflow tract (RVOT) region, but not the RV inflow area (21). In a swine with PS and PR study, intraventricular pressure was maintained at a relatively low level owing to the compensation by RV dilatation in young pigs with PR; however, there was early diastolic intraventricular kinetic energy (KE) elevation due to PR, and the atrial contraction was unfortunately not efficient because of the same timing (late diastolic). Old swine with PS and PR showed poor ventricular compliance, continuously high intraventricular pressure, and low KE, suggesting inefficient energy consumption within one heartbeat, suggesting significant EL (22). A similar phenomenon can occur in adults with TOF.

Here, we introduce a typical case of TOF in an adult patient. A 43-year-old man with TOF presented with breathlessness and general fatigue. He had undergone pulmonary valvuloplasty at 4 years of age. Echocardiography revealed a severe PR, mild-moderate PS and moderate TR. The RV had increased in size [indexed RV end-diastolic volume (RVEDV) of 138 mL/m2 and indexed RV end-systolic volume (RVESV) of 68 mL/m2 on MRI] with normal systolic function. His 4D flow MRI showed a markedly elevated EL. His exercise capacity was reduced and peak VO2 was 62% of the predicted value on cardiopulmonary testing. Brain natriuretic peptide (BNP) was mildly elevated. We considered his condition to be restrictive physiology in a broad band; therefore, he underwent percutaneous PVR with Harmony valve of 25 mm. After 3 months, he showed improvement in New York Heart Association (NYHA) class 1, and EL was significantly reduced (Figure 3).

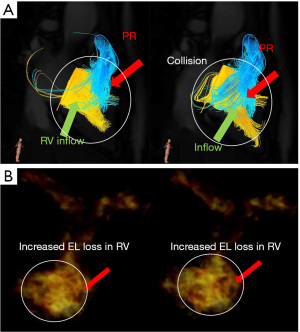
Other factors associated with EL elevation, such as intraventricular flow collisions, should also be considered. Symptomatic patients with AR show a smaller LV volume and higher EL (18,19); the vortex interaction between the transmitral inflow and the AR jet results in an undesirable EL (18,19). The same phenomenon can occur in right-heart fluid dynamics. In cases with a small and stiffened RV, such as PAIVS, the PR and tricuspid inflow may easily cause vortex interactions, resulting in energy inefficiency in the right heart. Herein, we describe a typical case of adult PAIVS. A 41-year-old man with PAIVS presented with breathlessness and mild peripheral oedema. The patients underwent Brock procedure at 1 month of age and RVOT reconstruction at 1 year of age. Echocardiography revealed moderate PR, mild PS and moderate TR, which did not meet the PVR criteria. However, 4D flow MRI showed markedly elevated EL in the RV with diastolic collisions of the PR and TR (Figure 4). The patient was administered diuretics.
Clinical application and limitation of EL measurement by using 4D flow MRI
Possible clinical application of EL is (I) to follow the temporal changes in each patient and (II) to calculate the cardiac burden using pressure data from catheterisation. Although EL appears to be an ideal marker of haemodynamics from a fluid dynamics perspective, EL measurement using 4D flow MRI has some limitations. EL is dependent on stroke volume (SV) and heart rate (9,10), and EL divided by cardiac output (CO), EL/CO, is recommended. Some previous studies have also recommended dividing EL by KE, EL/KE. Currently, no official guidelines are available. When considering the workload, a PV loop is necessary to determine the percentage of the total EL. Now, the simple estimation is as follows: we assumed 4 L/min of CO, 100/10 mmHg of LV pressure, and a rectangular workload from the PV loop in an adult with TOF. Total ventricular workload (mW) (1 mmHg = 133.322 Pa) = 4/1,000/60×(100−10)×133.322=799.8 mW. Other studies have reported approximately 400–900 mW of total ventricular workload (23). From this calculation, we assume that over 50–100 mW (more than approximately 5–10%) of EL cannot be ignored (23). If EL is less than 10 mW (approximately 1–2%), there is a small possibility of long-term negative effects on ventricular function. Colorado group reported that right heart EL in relatively good TOF against total input RV mechanical power was 4.7% (24). They considered that <5% of the EL was within the normal range in adult TOF, which is compatible with our previous results. Using this method, we can assess the severity of the cardiac burden in adults with CHD, which can be compared with other patients.
Now the normal value of EL is still chaotic, because the assessed values are different depending on modalities (transthoracic echocardiography, transesophageal echocardiography, 4D flow MRI, and CFD), vendors, timings of assessments (systolic EL, diastolic EL, average EL), the unit of assessments (mW, mW/m, mW/m3), method of correction (EL/CO, EL/SV, indexed EL, EL/KE), and anatomical features (biventricular repair, and Fontan circulation, systemic/pulmonary ventricle). No studies have reported EL and long-term outcomes; therefore, the clinical importance of this novel marker is still unknown. However, further studies are required to confirm this novel technique from a fluid dynamics perspective.
Conclusions
The advantage of EL measurement is to detect cardiac overload which integrates both afterload and preload beyond ventricular size and function in ACHD from a fluid dynamic perspective, even though it is still compensated. EL may shed light on cardiac burden assessment beyond RV size in ACHD; however, flow dynamics software is still being developed, both technically and methodologically, and its clinical impact on long-term outcomes remains unknown. Therefore, further studies are warranted.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing. 4D flow images were technically supported by Mr. Hiroshi Hamano (MR Clinical Application Specialist, Services & Solutions Delivery, Health Systems, Philips Japan, Ltd.) and Mr. Kohei Suzuki and Mr. Shohei Miyazaki (Cardio flow design Inc.).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Harald Kaemmerer) for the series “Current Management Aspects of Adult Congenital Heart Disease (ACHD): Part VI” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-296/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-296/coif). The series “Current Management Aspects of Adult Congenital Heart Disease (ACHD): Part VI” was commissioned by the editorial office without any funding or sponsorship. Y.S. reports funding from Grands-in-Aid for Scientific Research (KAKEN). Keiichi Itatani reports funding from Japan Agency for Medical Research (AMED), Grands-in-Aid for Scientific Research (KAKEN), and Japan Science and Technology Agency (JST) for his blood flow imaging research and clinical research of adult congenital heart surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heng EL, Gatzoulis MA, Uebing A, et al. Immediate and Midterm Cardiac Remodeling After Surgical Pulmonary Valve Replacement in Adults With Repaired Tetralogy of Fallot: A Prospective Cardiovascular Magnetic Resonance and Clinical Study. Circulation 2017;136:1703-13. [Crossref] [PubMed]
- Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation 2013;128:1855-7. [Crossref] [PubMed]
- Lee C, Kim YM, Lee CH, et al. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol 2012;60:1005-14. [Crossref] [PubMed]
- Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation 2007;116:545-51. [Crossref] [PubMed]
- Therrien J, Provost Y, Merchant N, et al. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol 2005;95:779-82. [Crossref] [PubMed]
- Kutty S, Valente AM, White MT, et al. Usefulness of Pulmonary Arterial End-Diastolic Forward Flow Late After Tetralogy of Fallot Repair to Predict a "Restrictive" Right Ventricle. Am J Cardiol 2018;121:1380-6. [Crossref] [PubMed]
- Mercer-Rosa L, Fogel MA, Paridon SM, et al. Revisiting the End-Diastolic Forward Flow (Restrictive Physiology) in Tetralogy of Fallot: An Exercise, Echocardiographic, and Magnetic Resonance Study. JACC Cardiovasc Imaging 2018;11:1547-8. [Crossref] [PubMed]
- Lam YY, Kaya MG, Goktekin O, et al. Restrictive right ventricular physiology: its presence and symptomatic contribution in patients with pulmonary valvular stenosis. J Am Coll Cardiol 2007;50:1491-7. [Crossref] [PubMed]
- Itatani K, Sekine T, Yamagishi M, et al. Hemodynamic Parameters for Cardiovascular System in 4D Flow MRI: Mathematical Definition and Clinical Applications. Magn Reson Med Sci 2022;21:380-99. [Crossref] [PubMed]
- Itatani K, Miyazaki S, Furusawa T, et al. New imaging tools in cardiovascular medicine: computational fluid dynamics and 4D flow MRI. Gen Thorac Cardiovasc Surg 2017;65:611-21. [Crossref] [PubMed]
- Loke YH, Yildiran IN, Capuano F, et al. Tetralogy of Fallot regurgitation energetics and kinetics: an intracardiac flow analysis of the right ventricle using computational fluid dynamics. Int J Cardiovasc Imaging 2024;40:1135-47. [Crossref] [PubMed]
- Bahlmann E, Gerdts E, Cramariuc D, et al. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013;127:1149-56. [Crossref] [PubMed]
- Shiina Y, Inai K, Nagao M. Non-physiological Aortic Flow and Aortopathy in Adult Patients with Transposition of the Great Arteries after the Jatene Procedure: A Pilot Study Using Echo Planar 4D Flow MRI. Magn Reson Med Sci 2021;20:439-49. [Crossref] [PubMed]
- Garcia D, Pibarot P, Dumesnil JG, et al. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation 2000;101:765-71. [Crossref] [PubMed]
- Shibata M, Itatani K, Hayashi T, et al. Flow Energy Loss as a Predictive Parameter for Right Ventricular Deterioration Caused by Pulmonary Regurgitation After Tetralogy of Fallot Repair. Pediatr Cardiol 2018;39:731-42. [Crossref] [PubMed]
- Takigami M, Itatani K, Nakanishi N, et al. Evaluation using a four-dimensional imaging tool before and after pulmonary valve replacement in a patient with tetralogy of Fallot: a case report. J Med Case Rep 2019;13:30. [Crossref] [PubMed]
- Nabeta T, Itatani K, Miyaji K, et al. Vortex flow energy loss reflects therapeutic effect in dilated cardiomyopathy. Eur Heart J 2015;36:637. [Crossref] [PubMed]
- Kainuma A, Itatani K, Akiyama K, et al. Preoperative Left Ventricular Energy Loss in the Operating Theater Reflects Subjective Symptoms in Chronic Aortic Regurgitation. Front Surg 2022;9:739743. [Crossref] [PubMed]
- Stugaard M, Koriyama H, Katsuki K, et al. Energy loss in the left ventricle obtained by vector flow mapping as a new quantitative measure of severity of aortic regurgitation: a combined experimental and clinical study. Eur Heart J Cardiovasc Imaging 2015;16:723-30. [Crossref] [PubMed]
- Shiina Y, Nagao M, Itatani K, et al. 4D flow MRI-derived energy loss and RV workload in adults with tetralogy of Fallot. J Cardiol 2024;83:382-9. [Crossref] [PubMed]
- Loke YH, Capuano F, Cleveland V, et al. Moving beyond size: vorticity and energy loss are correlated with right ventricular dysfunction and exercise intolerance in repaired Tetralogy of Fallot. J Cardiovasc Magn Reson 2021;23:98. [Crossref] [PubMed]
- Fernandes JF, Hammel JM, Zhou J, et al. Right ventricular energetics and power in pulmonary regurgitation vs. stenosis using four dimensional phase contrast magnetic resonance. Int J Cardiol 2018;263:165-70. [Crossref] [PubMed]
- Itatani K, Miyaji K, Ohara K, et al. Computational Fluid Dynamic Simulations on Fontan Circulation. Pediatric Cardiology and Cardiac Surgery 2010;26:39-48.
- McLennan D, Schäfer M, Barker AJ, et al. Abnormal flow conduction through pulmonary arteries is associated with right ventricular volume and function in patients with repaired tetralogy of Fallot: does flow quality affect afterload? Eur Radiol 2023;33:302-11. [Crossref] [PubMed]


