
Investigating the causal relationship between genetically determined metabolites and ischemic stroke functional outcomes: a Mendelian randomization study
Highlight box
Key findings
• This study used a Mendelian randomization (MR) framework to investigate the causal relationships between plasma metabolites and functional outcomes after ischemic stroke, utilizing genome-wide association study (GWAS) data for analysis. The inverse-variance weighted method initially identified 59 metabolites with potential causal effects on recovery. After applying false discovery rate corrections, one metabolite remained significant (P<0.05). Sensitivity tests further validated the robustness of these findings.
What is known and what is new?
• The metabolic underpinnings of ischemic stroke recovery have been increasingly recognized with metabolomics providing key insights into disturbances following stroke. Previous research primarily relied on observational studies or limited cohort analyses to link metabolic profiles to stroke severity and outcomes.
• This study advanced understandings by leveraging a MR framework to firmly establish causal relationships between genetically determined metabolites and ischemic stroke functional outcomes. We identified several metabolites, such as X-17146, that had a robust causal impact on recovery processes. Notably, this study is among the first to apply MR in conjunction with comprehensive GWAS data, significantly enhancing the specificity and reliability of the metabolic biomarkers linked to functional outcomes.
What is the implication, and what should change now?
• Our findings underscore the potential of targeted metabolic interventions in enhancing stroke rehabilitation strategies. Additionally, the integration of metabolomic profiling in routine clinical assessments could pave the way for more precise, personalized therapeutic strategies, ultimately improving the functional outcomes and the quality of life of stroke survivors.
Introduction
Ischemic stroke is one of the most prevalent neurological disorders worldwide, with high incidence and mortality rates. According to the World Health Organization, approximately 15 million people suffer a stroke annually (1). Of these, about one-fifth die within a month, and over two-thirds sustain varying degrees of long-term disability (2). Understanding the factors that influence post-stroke outcomes is crucial for optimizing stroke management and improving patient prognosis.
Metabolomics, the study of small-molecule metabolites in biological systems, has become a vital tool in clinical research (3). This discipline helps quantify metabolic changes due to disease, environmental factors, or interventions, providing insights into crucial disease mechanisms. In clinical settings, metabolomics aids in the identification of disease biomarkers, the understanding of pathogenesis, and the development of personalized medicine approaches. For instance, it allows clinicians to predict disease progression and optimize treatments (4). In stroke research, metabolomics has provided significant insights into biochemical disturbances ischemic stroke by identifying metabolic profiles linked to stroke severity and outcomes (5), including C-reactive protein, aquaporin, and so on. However, these studies have largely relied on specific population samples or large cohort metabolite screenings, which has led to a gap in the comprehensive analysis of broad populations.
Mendelian randomization (MR) uses genetic variants as instrumental variables (IVs) to estimate the causal effects of exposures like metabolites on outcomes such as ischemic stroke recovery (6). This approach mimics the conditions of a randomized controlled trial by leveraging the random assortment of genes at conception, thus providing higher-quality evidence than observational studies (7). Integrating MR with metabolomics allows researchers to infer causal relationships between metabolic disturbances and clinical outcomes by linking genetic variations to metabolic profiles, effectively minimizing confounding and reverse causation issues (8). Additionally, the ability of MR to use existing genetic and metabolic data for large-scale analyses makes it an efficient tool for exploring complex interactions in diseases like stroke, helping to identify new therapeutic targets and deepen understanding of disease mechanisms (9) (10), This study aimed to explore the causal relationships between genetically determined metabolites and functional recovery after stroke through MR (Figure S1). We present this article in accordance with the STROBE-MR reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-369/rc).
Methods
Data sources
This research employed a MR approach to explore the causal links between plasma metabolites and the functional outcomes of ischemic stroke patients. Outcome data were derived from the comprehensive Genetics of Ischaemic Stroke Functional Outcome (GISCOME) genome-wide association study (GWAS), which examined 6,165 patients from Europe, the United States of America, and Australia (Table 1) (11). The primary endpoint of the study was the assessment of functional recovery using the modified Rankin Scale (mRS). The mRS as close as possible to 90 days (60–190 days permitted) was selected to assess functional outcome (12). A mRS score of 0–2 indicated a good functional outcome (n=3,741), while a mRS score of 3–6 represented a poor functional outcome ischemic stroke (n=2,280). Independent GWAS analyses were performed in each participating study, and the results were combined through meta-analysis. Building on this data, the researchers conducted further analyses on these patients, adjusting for ancestry, age, sex and stroke severity using the National Institutes of Health Stroke Scale (NIHSS) (13). Meanwhile, the study classified cases into small vessel disease (referred to as lacunar stroke) and other subtypes (referred to as non-lacunar stroke) based on the subtype classification in TOAST (Trial of ORG 10172 in Acute Stroke Treatment). A total of 992 patients had lacunar stroke, 3,991 patients had non-lacunar stroke, and 1,182 patients lacked this information. In the present study, the adjusted group was used for the primary MR analysis. While the models without adjustment for the baseline NIHSS (the baseline group) were used for sensitivity testing, which provided a means to verify the robustness of our results without the adjustments for stroke severity and other variables. This approach enhanced the reliability of our findings by demonstrating their consistency across different conditions.
Table 1
| Consortium | Trait | Cases | Primary endpoints |
|---|---|---|---|
| GISCOME | Functional outcomes in ischemic stroke patients | 6,165 | mRS score recorded from 60 to 190 days ischemic stroke |
GISCOME, Genetics of Ischaemic Stroke Functional Outcome; mRS, modified Rankin Scale.
To source our exposure data, we curated genetic instruments for 1,091 plasma metabolites, of which 241 were categorized as unknown or ‘partially’ characterized molecules, along with 309 metabolite ratios. This was achieved through a GWAS involving 8,299 participants of European descent from the Canadian Longitudinal Study on Aging (CLSA) cohort (10). To our knowledge, this represents the most comprehensive analysis of human metabolites conducted to date. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Selection of IVs
In this research, the methodology for selecting IVs was rigorously defined to ensure their reliability and robustness. Initially, single nucleotide polymorphisms (SNPs) were chosen based on their strong association with exposure as evidenced by a genome-wide significance level of P<1×10−6. To guarantee adequate representation within the population, only SNPs with a minor allele frequency above 0.01 were considered significant. Following this, linkage disequilibrium (LD) score regression was conducted to eliminate SNPs under an LD threshold of R2<0.001 across a span of 10,000 kb to ensure no horizontal pleiotropy and confirm the relevance of the genetic instruments to the exposures studied. Further, if the selected IVs were absent in the outcome summary data, proxy SNPs demonstrating high LD (R2>0.8) with the initial IVs were used as replacements. Finally, to assess the strength of each SNP as an IV, the F statistic was calculated using the following equation: F = R2 × (N − 2) / (1 − R2), where R2 is the proportion of variance in the exposure accounted for by the SNP, and N is the sample size. An F value exceeding 10 was necessary to confirm the robustness and reliability of the IVs.
Statistical analysis
In this investigation, a MR analysis was conducted using a suite of robust methods to deduce causal connections between plasma metabolites and ischemic stroke functional outcomes (the adjusted group). The primary analytical method used was the inverse-variance weighted (IVW) method, which integrates the effect estimates from each IV within a meta-analysis construct, providing a precision-weighted average of causal estimates (14). As a first line of analysis, the IVW method presupposes the validity of all IVs.
Supplementing the primary method, MR-Egger regression was employed to identify and adjust for any potential pleiotropic effects, with its intercept offering insights into the existence of horizontal pleiotropy (15). Further, the weighted-median technique was applied to ensure that the findings remain consistent even if up to half of the IVs were invalid (16). The simple-mode and weighted-mode methods and the Wald ratio were additionally implemented to corroborate the primary findings (17).
Following the preliminary MR analyses, any significant associations revealed by the IVW method (PIVW<0.05) were meticulously scrutinized. Separate analyses were conducted for the 1,091 identified metabolites and 309 metabolic ratios. To manage the risks of multiple comparisons, a false discovery rate (FDR) correction was employed to refine the P values to pinpoint the statistically significant metabolites and metabolic ratios linked to ischemic stroke functional outcomes (18).
Sensitivity testing
To validate the integrity of the MR results, comprehensive sensitivity analyses were conducted. Initially, the same exposures were subjected to an additional MR analysis in the baseline group. Subsequently, the intersection was identified to determine which metabolites exhibited significant causal relationships in both the NIHSS-adjusted group and the baseline group.
A leave-one-out approach was adopted, whereby each IV was sequentially omitted from the analysis to gauge its impact on the overall causal estimate (19). This technique was instrumental in detecting any IVs that might unduly skew the findings. Cochran’s Q test was used to assess heterogeneity among the IVs to identify variability that could suggest pleiotropy or other biases (20,21). Additional sensitivity testing included MR-Egger regression to further assess and adjust for horizontal pleiotropy, using the intercept to detect directional pleiotropy (22). Collectively, these sensitivity tests enhanced the reliability of the MR results, addressing potential biases and affirming the causal relationships between the plasma metabolites and ischemic stroke functional outcomes. All the MR analyses were performed using the TwoSampleMR (version 0.5.6), MR (version 0.5.1), and MRPRESSO (version 1.0) packages in R (version 4.2.3).
Results
Selection of IVs
The overall study workflow is shown in Figure 1. Under the premise that P<1×10−6, the number of IVs with the ratio of 1,091 metabolites to 309 metabolites was not less than 3, and a total of 6,034 IVs were screened (table available at https://cdn.amegroups.cn/static/public/cdt-24-369-1.xlsx). Importantly, the minimum F statistics for the validity test were all above 10 (table available at https://cdn.amegroups.cn/static/public/cdt-24-369-1.xlsx); thus, weak instrumental bias was unlikely to occur.

MR analysis
In this study, MR was employed to investigate the influence of plasma metabolite levels on functional outcomes following a stroke (table available at https://cdn.amegroups.cn/static/public/cdt-24-369-2.xlsx). Using the IVW method, our study identified 59 metabolites with potentially causal relationships to ischemic stroke functional outcomes (Table 2 and Figure 2). These metabolites were categorized into protective and harmful factors based on their association with recovery.
Table 2
| Exposure | nSNP | B | SE | PRAW | PFDR | OR (95% CI) |
|---|---|---|---|---|---|---|
| X-17146 levels | 16 | −0.7249 | 0.1707 | <0.001 | 0.02 | 0.48 (0.35–0.68) |
| ADP to EDTA ratio | 22 | 0.3949 | 0.1129 | 0.001 | 0.14 | 1.48 (1.19–1.85) |
| C20:2 levels | 25 | 0.3353 | 0.1044 | 0.001 | 0.71 | 1.40 (1.14–1.71) |
| Phosphate to threonine ratio | 28 | −0.3863 | 0.1285 | 0.003 | 0.41 | 0.68 (0.53–0.87) |
| Picolinate levels | 19 | 0.3508 | 0.1217 | 0.004 | >0.99 | 1.43 (1.12–1.80) |
| X-15461 levels | 24 | 0.3936 | 0.1434 | 0.006 | >0.99 | 1.48 (1.12–1.96) |
| AMP to citrate ratio | 22 | −0.4317 | 0.1582 | 0.006 | 0.65 | 0.65 (0.48–0.89) |
| X-12261 levels | 14 | −0.3679 | 0.1379 | 0.008 | >0.99 | 0.69 (0.53–0.91) |
| X-22834 levels | 20 | −0.4089 | 0.1548 | 0.008 | >0.99 | 0.66 (0.49–0.90) |
| 3-CMPFP levels | 25 | 0.3237 | 0.1230 | 0.009 | >0.99 | 1.38 (1.09–1.76) |
| 5,6-dihydrothymine levels | 16 | −0.4646 | 0.1801 | 0.010 | >0.99 | 0.63 (0.44–0.89) |
| C20:3n3 or 6 levels | 28 | 0.2011 | 0.0808 | 0.01 | >0.99 | 1.22 (1.04–1.43) |
| 2-hydroxypalmitate levels | 17 | −0.4298 | 0.1737 | 0.01 | >0.99 | 0.65 (0.46–0.91) |
| X-23974 levels | 20 | 0.3883 | 0.1580 | 0.01 | >0.99 | 1.47 (1.08–2.01) |
| Ceramide (d18:1/24:1) levels | 28 | 0.2508 | 0.1025 | 0.01 | >0.99 | 1.29 (1.05–1.57) |
| 3-acetylphenol sulfate levels | 20 | 0.4006 | 0.1644 | 0.01 | >0.99 | 1.50 (1.08–2.06) |
| X-23654 levels | 38 | −0.2429 | 0.0997 | 0.01 | >0.99 | 0.78 (0.65–0.95) |
| Methyl vanillate sulfate levels | 23 | 0.2563 | 0.1054 | 0.02 | >0.99 | 1.29 (1.05–1.59) |
| Tryptophan betaine levels | 33 | −0.2587 | 0.1076 | 0.02 | >0.99 | 0.77 (0.63–0.95) |
| Homostachydrine levels | 27 | 0.3168 | 0.1321 | 0.02 | >0.99 | 1.37 (1.06–1.78) |
| 17:1n7 levels | 20 | 0.3962 | 0.1666 | 0.02 | >0.99 | 1.49 (1.07–2.06) |
| Phosphate to 5-oxoproline ratio | 22 | 0.4346 | 0.1831 | 0.02 | >0.99 | 1.54 (1.08–2.21) |
| Glucose-to-mannose ratio | 23 | −0.2676 | 0.1137 | 0.02 | >0.99 | 0.77 (0.61–0.96) |
| Uridine levels | 19 | 0.3689 | 0.1573 | 0.02 | >0.99 | 1.45 (1.06–1.97) |
| ADP to citrate ratio | 20 | 0.2759 | 0.1185 | 0.02 | >0.99 | 1.32 (1.04–1.66) |
| Alpha-hydroxyisovalerate levels | 19 | 0.2605 | 0.1119 | 0.02 | >0.99 | 1.30 (1.04–1.62) |
| HVA levels | 24 | 0.2911 | 0.1254 | 0.02 | >0.99 | 1.34 (1.05–1.71) |
| N-palmitoyl-sphinganine (d18:0/16:0) levels | 22 | 0.3277 | 0.1422 | 0.02 | >0.99 | 1.39 (1.05–1.83) |
| X-24801 levels | 32 | −0.2465 | 0.1070 | 0.02 | >0.99 | 0.78 (0.63–0.96) |
| Alanine levels | 20 | 0.3655 | 0.1591 | 0.02 | 0.98 | 1.44 (1.06–1.97) |
| ADP to valine ratio | 19 | 0.2664 | 0.1164 | 0.02 | 0.98 | 1.31 (1.04–1.64) |
| Sphingomyelin (d18:1/20:1, d18:2/20:0) levels | 23 | −0.2839 | 0.1241 | 0.02 | 0.96 | 0.75 (0.59–0.96) |
| Dihomo-linolenoyl-choline levels | 25 | 0.2870 | 0.1266 | 0.02 | 0.98 | 1.33 (1.04–1.71) |
| 1-(1-enyl-palmitoyl)-2-linoleoyl-GPE (p-16:0/18:2) levels | 20 | 0.3347 | 0.1483 | 0.02 | 0.97 | 1.40 (1.04–1.87) |
| N-acetylcitrulline levels | 16 | −0.1673 | 0.0742 | 0.02 | 0.94 | 0.85 (0.73–0.98) |
| Taurolithocholate 3-sulfate levels | 20 | −0.3128 | 0.1399 | 0.03 | 0.95 | 0.73 (0.56–0.96) |
| Phosphocholine levels | 27 | −0.2887 | 0.1296 | 0.03 | 0.94 | 0.75 (0.58–0.97) |
| Salicylate to oxalate (ethanedioate) ratio | 14 | 0.4016 | 0.1827 | 0.03 | >0.99 | 1.49 (1.04–2.14) |
| Hypotaurine levels | 26 | 0.2895 | 0.1320 | 0.03 | 0.990 | 1.34 (1.03–1.73) |
| 2-linoleoylglycerol (18:2) levels | 23 | 0.2757 | 0.1260 | 0.03 | 0.98 | 1.32 (1.03–1.67) |
| X-23680 levels | 19 | −0.2622 | 0.1199 | 0.03 | 0.95 | 0.77 (0.61–0.97) |
| X-24556 levels | 24 | −0.2525 | 0.1156 | 0.03 | 0.93 | 0.78 (0.62–0.97) |
| Ceramide (d18:1/17:0, d17:1/18:0) levels | 22 | 0.2709 | 0.1249 | 0.03 | 0.94 | 1.31 (1.03–1.67) |
| Mannose levels | 23 | 0.2599 | 0.1200 | 0.03 | 0.92 | 1.30 (1.03–1.64) |
| Isovalerylglycine levels | 21 | −0.2668 | 0.1240 | 0.03 | 0.92 | 0.77 (0.60–0.98) |
| ADP to pantothenate ratio | 14 | 0.3284 | 0.1538 | 0.03 | >0.99 | 1.39 (1.03–1.88) |
| X-12701 levels | 12 | 0.3898 | 0.1834 | 0.03 | 0.96 | 1.48 (1.03–2.12) |
| Pyrraline levels | 22 | 0.3100 | 0.1461 | 0.03 | 0.94 | 1.36 (1.02–1.82) |
| Umbelliferone sulfate levels | 21 | 0.3025 | 0.1436 | 0.04 | 0.96 | 1.35 (1.02–1.79) |
| C17 levels | 22 | 0.3003 | 0.1441 | 0.04 | 0.99 | 1.35 (1.02–1.79) |
| ADP to FAD ratio | 23 | 0.1992 | 0.0961 | 0.04 | >0.99 | 1.22 (1.01–1.47) |
| 3-CMPFP levels | 16 | 0.4383 | 0.2115 | 0.04 | 0.99 | 1.55 (1.02–2.35) |
| Arachidate (20:0) levels | 20 | −0.3313 | 0.1617 | 0.04 | >0.99 | 0.72 (0.52–0.99) |
| X-25271 levels | 15 | 0.3665 | 0.1817 | 0.04 | >0.99 | 1.44 (1.01–2.06) |
| Serine to alpha-ketobutyrate ratio | 16 | 0.3461 | 0.1722 | 0.045 | >0.99 | 1.41 (1.01–1.98) |
| C8-DC levels | 21 | −0.3269 | 0.1635 | 0.046 | >0.99 | 0.72 (0.52–0.99) |
| X-23648 levels | 20 | −0.3005 | 0.1507 | 0.046 | >0.99 | 0.74 (0.55–0.99) |
| Carnitine C14 levels | 19 | 0.3708 | 0.1868 | 0.047 | >0.99 | 1.45 (1.00–2.09) |
| Histidine to phosphate ratio | 26 | −0.3231 | 0.1642 | 0.049 | >0.99 | 0.72 (0.52–1.00) |
17:1n7, 10-heptadecenoate; 3-CMPFP, 3-carboxy-4-methyl-5-pentyl-2-furanpropionate; ADP, adenosine 5’-diphosphate; AMP, adenosine 5’-monophosphate; C17, margaroylcarnitine; C20:2, dihomo-linoleoylcarnitine; C20:3n3 or 6, dihomo-linolenoyl carnitine; C8-DC, suberate; CI, confidence interval; EDTA, ethylenediaminetetraacetic acid; FAD, flavin adenine dinucleotide; FDR, false discovery rate; HVA, homovanillate; IVW, inverse-variance weighted; MR, Mendelian randomization; nSNP, number of single nucleotide polymorphisms; OR, odds ratio; RAW, raw data; SE, standard error.
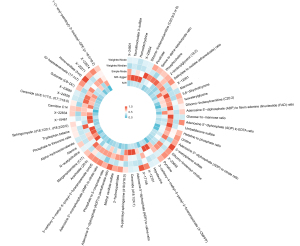
The harmful factors included the adenosine 5’-diphosphate (ADP) to ethylenediaminetetraacetic acid (EDTA) ratio, which was positively associated with worse outcomes [odds ratio (OR) =1.48, 95% confidence interval (CI): 1.19–1.85, P=0.001]; dihomo-linoleoylcarnitine (C20:2) levels, which were also negatively associated with a better recovery (OR =1.40, 95% CI: 1.14–1.71, P=0.001); X-15461 levels, which appeared to reduce recovery prospects (OR =1.48, 95% CI: 1.12–1.96, P=0.006); picolinate levels, which appeared to be similarly detrimental (OR =1.43, 95% CI: 1.12–1.80, P=0.004); and 3-carboxy-4-methyl-5-pentyl-2-furanpropionate (3-CMPFP) levels, which were also associated with negative recovery outcomes (OR =1.38, 95% CI: 1.09–1.76, P=0.009).
Conversely, the protective factors included X-17146 levels, which were positively associated with recovery (OR =0.48, 95% CI: 0.35–0.68, P<0.001); the phosphate to threonine ratio, which indicated a beneficial effect (OR =0.68, 95% CI: 0.53–0.87, P=0.003); the adenosine 5’-monophosphate (AMP) to citrate ratio, which was associated with positive outcomes (OR =0.65, 95% CI: 0.48–0.89, P=0.006); and X-12261 and X-22834 levels, which both demonstrated protective associations (OR =0.69, 95% CI: 0.53–0.91, P=0.008 and OR =0.66, 95% CI: 0.49–0.90, P=0.008, respectively). It is particularly noteworthy that the positive causal links between X-17146 and ischemic stroke functional outcomes (OR =0.48, 95% CI: 0.35–0.68, P<0.001) persisted even after the FDR correction (PFDR=0.02).
Sensitivity testing
In the sensitivity testing, we repeated the MR analysis in the baseline group (without adjustment for age, sex, ancestry, and stroke severity assessed by the NIHSS) to confirm that the causal relationships between the identified plasma metabolites and ischemic stroke functional outcomes were consistent. The findings are summarized in Table 3 in which eight metabolites are listed that showed positive and significant causal relationships in both the adjusted group and the baseline group. The risk factors included the phosphate to 5-oxoproline ratio (OR =2.29, 95% CI: 1.05–4.99, P=0.04) and the salicylate to oxalate (ethanedioate) ratio (OR =1.88, 95% CI: 1.09–3.24, P=0.02). These findings suggest that these metabolites have a harmful impact on recovery. Conversely, the protective factors included tryptophan betaine levels (OR =0.66, 95% CI: 0.49–0.90, P=0.008), taurolithocholate 3-sulfate levels (OR =0.60, 95% CI: 0.42–0.87, P=0.008), phosphocholine levels (OR =0.67, 95% CI: 0.47–0.97, P=0.03), and the histidine to phosphate ratio (OR =0.56, 95% CI: 0.35–0.89, P=0.01). The dual significance of these results emphasizes the potential influence of these metabolites on recovery outcomes, reaffirming their importance regardless of adjustments. The complete results for the baseline group are detailed in table available at https://cdn.amegroups.cn/static/public/cdt-24-369-3.xlsx.
Table 3
| Exposure | NIHSS-adjusted group | Baseline group | |||
|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | ||
| X-22834 levels | 0.008 | 0.6644 (0.4905–0.8999) | 0.049 | 0.57 (0.32–1.00) | |
| Tryptophan betaine levels | 0.02 | 0.7721 (0.6253–0.9533) | 0.008 | 0.66 (0.49–0.90) | |
| Phosphate to 5-oxoproline ratio | 0.02 | 1.5443 (1.0785–2.2112) | 0.04 | 2.29 (1.05–4.99) | |
| Taurolithocholate 3-sulfate levels | 0.03 | 0.7314 (0.5560–0.9622) | 0.008 | 0.60 (0.42–0.87) | |
| Phosphocholine levels | 0.03 | 0.7492 (0.5812–0.9658) | 0.03 | 0.67 (0.47–0.97) | |
| Salicylate to oxalate (ethanedioate) ratio | 0.03 | 1.4942 (1.0444–2.1376) | 0.02 | 1.88 (1.09–3.24) | |
| Mannose levels | 0.03 | 1.2969 (1.0250–1.6408) | 0.03 | 1.39 (1.04–1.85) | |
| Histidine to phosphate ratio | 0.049 | 0.7239 (0.5247–0.9987) | 0.01 | 0.56 (0.35–0.89) | |
CI, confidence interval; MR, Mendelian randomization; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Cochran’s Q test, including two statistical methods (the IVW and MR-Egger methods), was employed to assess the heterogeneity in the association between the plasma metabolites and ischemic stroke functional outcomes (table available at https://cdn.amegroups.cn/static/public/cdt-24-369-4.xlsx). In the analysis results, all the P values were greater than 0.05, which indicated that there was a lack of significant evidence for heterogeneity across the study (Table S1). The range of the Q values varied from a minimum of 5.591283 (for 3-CMPFP levels) to a maximum of 36.70919 (for homostachydrine levels).
In the analysis in which Egger’s regression was employed to evaluate potential pleiotropy, the intercept values for the identified plasma metabolites varied from −0.0906 to 0.0833 with all the P values exceeding 0.05 (Table S2). This suggests an absence of significant pleiotropy across the study results. A full list of the MR-Egger results is provided in table available at https://cdn.amegroups.cn/static/public/cdt-24-369-5.xlsx. Additionally, the leave-one-out sensitivity analysis further confirmed the robustness of these findings. Finally, across all MR methods, we found no evidence for a causal effect of 59 metabolites on overall ischemic stroke risk, providing evidence to support that their association with the functional outcome may not be attributed to collider bias (table available at https://cdn.amegroups.cn/static/public/cdt-24-369-6.xlsx).
Discussion
This study employed a MR framework to explore the causal relationships between plasma metabolites and ischemic stroke functional outcomes, using GWAS data for the analysis. Initially, the IVW method identified 59 metabolites with potential causal impacts on recovery. After applying FDR corrections, one metabolite remained significantly associated with ischemic stroke functional outcomes (P<0.05). Sensitivity tests, including Cochran’s Q test, MR-Egger regression, and leave-one-out analyses, further validated the robustness of these findings. This study marks an advancement in stroke research; it was the first to integrate metabolomics with GWAS data to explore the causal relationships between plasma metabolites and ischemic stroke functional outcomes. This approach not only enhanced our understanding of the metabolites involved in stroke recovery but also identified potential biomarkers, investigated their underlying mechanisms, and established a foundation for future research. Indeed, these findings could lead to improvements in clinical treatments.
A feature of our study is the performance of the MR analysis in both the adjusted and baseline groups. Notably, we identified eight metabolites or metabolic ratios that exhibited significant associations in both groups. This approach further ensured the robustness and generalizability of our findings, highlighting the consistent impact of these metabolites across different settings.
After applying FDR corrections to control for multiple comparisons and reduce the risk of type I errors, only one metabolite remained statistically significant. This stringent correction method often results in a reduced number of significant findings, primarily due to its adjustment for the cumulative probability of observing false positives across multiple tests, which is particularly crucial in studies with large datasets and in which numerous hypotheses are being tested (18). Unfortunately, the single metabolite that retained significance after FDR adjustment, referred to as X-17146, is currently an unidentified compound. The unknown status of X-17146 limits our ability to fully understand its biological functions and implications in stroke recovery. The continued exploration of X-17146 may provide critical information that could help tailor post-stroke treatment strategies more effectively, ultimately improving rehabilitation outcomes.
The relationship between metabolic factors and ischemic stroke functional recovery is increasingly recognized as crucial, given the significant influence of metabolic health on rehabilitation outcomes. Various studies have examined this interplay, each focusing on different metabolic aspects and their impacts on recovery ischemic stroke, often targeting specific patient populations. For example, Knops et al. concentrated on ischemic stroke patients, exploring how changes in body composition and metabolic profiles are correlated with skeletal muscle functional capacity. Their research established a foundation for linking metabolic alterations with functional recovery, suggesting that changes in body composition ischemic stroke could critically affect rehabilitation outcomes (23). Extending on this exploration, Pradillo et al. investigated the effects of metabolic syndrome on vascular function and recovery in aged rats, highlighting how pre-existing metabolic conditions can alter recovery trajectories through changes in angiogenesis and vascular health, thus underscoring the vital role of metabolic syndrome components in modulating crucial biological processes for recovery in an elderly population (24). This study affirmed that metabolic dysregulation is intimately linked with stroke recovery. Wang et al. took a broader approach by examining a spectrum of metabolic dysfunctions, including amino acid metabolism, lipid metabolism, and oxidative stress, and their associations with ischemic stroke depression (25). By analyzing metabolomic data, they revealed the complex mechanisms by which metabolic pathways impact mental health outcomes in stroke survivors. This study addressed various metabolites; however, its focus was primarily on mental health disorders rather than overall functional recovery.
Building on these insights, our research initially identified 59 potential genetically causal metabolites and ratios that span a broad spectrum of metabolic functions. These included amino acids, lipids, carbohydrates, and other small-molecule metabolites that play diverse roles in the body’s biochemical pathways, underlying their potential significance in ischemic stroke recovery processes. The variability in results across the different MR methods, such as the IVW, MR-Egger regression, simple-mode, weighted-median, and weighted-mode methods, and the Wald ratio, underscores the importance of method selection and its impact on causal inferences. Each method has its unique assumptions and limitations: IVW assumes no pleiotropy and can be biased if some instruments are invalid; MR-Egger regression allows for pleiotropic effects and tests for directional pleiotropy but has wider CIs; the simple-mode and weighted-mode methods are robust to outliers and invalid instruments by focusing on the most common causal estimates; the weighted-median method provides a balance by being accurate even if up to 50% of the data comes from invalid instruments; and the Wald ratio, used in two-sample MR, assumes each SNP affects the outcome only through its effect on the exposure (17,26). These methodological differences highlight the complexities of drawing robust conclusions and the necessity of employing multiple MR methods to validate findings and understand the nuances of causal inference in genetic epidemiology.
Mannose, despite its roles in biological processes like protein glycosylation, may exert detrimental effects ischemic stroke. Dysregulated mannose metabolism could potentially lead to improper protein function and disrupted cellular communication, hindering tissue repair and recovery (27). Furthermore, mannose’s involvement in inflammatory processes could exacerbate secondary damage in the brain by promoting pro-inflammatory pathways (28). Furthermore, mannose’s involvement in inflammatory processes could exacerbate secondary damage in the brain by promoting pro-inflammatory pathways (29). Conversely, taurolithocholate, despite being cytotoxic under specific conditions, may also exhibit protective properties (30). It has been suggested that its ability to modulate mitochondrial function could, in certain contexts, limit oxidative stress and support adaptive cellular responses, aiding recovery ischemic stroke (31). While elevated levels are generally associated with damage, controlled modulation might reduce neuronal stress in ischemic conditions, offering potential therapeutic insights. Betaine, particularly its metabolite tryptophan betaine, might have protective effects ischemic stroke. By influencing tryptophan metabolism, it could enhance serotonin production, which plays a critical role in neurological recovery and mood stabilization, key factors in improving ischemic stroke outcomes (32). Furthermore, its general role in lowering homocysteine levels may indirectly protect cardiovascular and neural functions (33). Elevated phosphocholine, rather than solely indicating cellular breakdown, could reflect an adaptive response aimed at maintaining cell membrane integrity during ischemic stress (34). Phosphocholine might act to stabilize neuronal membranes, supporting structural and functional recovery of affected tissue. Adiponectin, a hormone linked to metabolic health, also plays a role in recovery by affecting insulin sensitivity and inflammation (35,36). Managing adiponectin levels through weight control and pharmacological agents like metformin can enhance insulin response and overall metabolic function (37). By strategically managing these metabolites, it is possible to support essential biological processes involved in stroke recovery, such as enhancing neuroplasticity, reducing inflammation, and improving metabolic health. These findings may be beneficial in improving functional outcomes and the overall quality of life of stroke survivors.
Research has emphasized the importance of addressing metabolic issues to effectively enhance functional recovery after a stroke. Aquilani et al. approached the problem from a nutritional perspective, suggesting that metabolic impairments common in stroke patients are crucial in determining rehabilitation success, and advocating for targeted nutritional interventions as part of standard care (38). Similarly, Mele et al. explored the potential therapeutic effects of statins in a stroke rehabilitation cohort, highlighting how lipid-lowering agents might improve functional outcomes through their cardiovascular protective effects (39).
Our results align with these findings, emphasizing that specific metabolites play critical roles in ischemic stroke recovery and can be modulated through targeted interventions. For instance, amino acids, such as phenylalanine, are vital for protein synthesis and muscle recovery, essential components of the rehabilitation process (40). These amino acids can be regulated by dietary adjustments or supplements to ensure optimal levels for tissue repair. Additionally, uridine, which is crucial for RNA synthesis, can be supplemented through dietary sources like brewer’s yeast to enhance cognitive function and neuronal health, which are often compromised following a stroke. Fatty acids, including heptadecenoate (17:1n7), significantly affect inflammation and cellular health (41). Adjusting dietary fatty-acid intake can modulate these processes, which are critical for recovery and functional restoration ischemic stroke.
The early treatment of stroke currently faces several challenges, including limited knowledge about individual variability in response to treatment, and a lack of personalized therapeutic strategies (42). The findings from our MR study indicate potential targets for intervention that could enhance ischemic stroke recovery by addressing specific metabolic dysfunctions. The early identification and modulation of influential metabolites could lead to more tailored and effective treatment approaches, potentially improving functional outcomes. Looking forward, incorporating metabolomic profiling into routine clinical assessment ischemic stroke could allow for more precise adjustments in therapeutic strategies based on individual metabolic needs, paving the way for personalized medicine in stroke care.
However, the findings of our study must be considered in light of several limitations. First, the generalizability of our results might be constrained by population-specific factors, as genetic diversity across different ethnicities could influence metabolite levels and their impact on health outcomes. Second, while our study provides a foundational understanding of the association between metabolites and stroke outcomes, further refinement in metabolomics is necessary to identify and characterize the specific roles of these metabolites. Finally, MR is based on genetic variants, which reflect the impact of metabolites over a lifetime. As metabolites may vary significantly over time, the results of our study may not be a good representative of metabolite changes during recovery after stroke. Future research should also integrate basic experimental validations to confirm the causal pathways suggested by our MR analyses. This would involve detailed biochemical and physiological studies to elucidate how these metabolites influence recovery mechanisms at a molecular level, ensuring that any clinical applications developed from our findings are both effective and safe.
Conclusions
This study used a MR framework to investigate the causal relationships between plasma metabolites and ischemic stroke functional outcomes. Our research highlights the substantial potential of integrating metabolomic data with MR to identify crucial metabolites that impact ischemic stroke recovery. By pinpointing specific metabolic pathways, our findings open up new avenues for targeted interventions that could significantly improve rehabilitation outcomes.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE-MR reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-369/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-369/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-369/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Anju SJ. Posterior circulation stroke characteristics [POCSTROCH study]: An observational COHORT study. Vellore: Christian Medical College; 2018.
- Aderemi AV, Ayeleso AO, Oyedapo OO, et al. Metabolomics: A Scoping Review of Its Role as a Tool for Disease Biomarker Discovery in Selected Non-Communicable Diseases. Metabolites 2021;11:418. [Crossref] [PubMed]
- Qiu S, Cai Y, Yao H, et al. Small molecule metabolites: discovery of biomarkers and therapeutic targets. Signal Transduct Target Ther 2023;8:132. [Crossref] [PubMed]
- Ke C, Pan CW, Zhang Y, et al. Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: a systematic review. Metabolomics 2019;15:152. [Crossref] [PubMed]
- Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA 2017;318:1925-6. [Crossref] [PubMed]
- Neeland IJ, Kozlitina J. Mendelian Randomization: Using Natural Genetic Variation to Assess the Causal Role of Modifiable Risk Factors in Observational Studies. Circulation 2017;135:755-8. [Crossref] [PubMed]
- Liu X, Tong X, Zou Y, et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat Genet 2022;54:52-61. [Crossref] [PubMed]
- Liu H, Zhang X, Zhou Y, et al. Association between blood pressure and different antihypertensive drugs with outcome after ischemic stroke: A Mendelian randomization study. Int J Stroke 2023;18:1247-54. [Crossref] [PubMed]
- Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 2023;55:44-53. [Crossref] [PubMed]
- Söderholm M, Pedersen A, Lorentzen E, et al. Genome-wide association meta-analysis of functional outcome after ischemic stroke. Neurology 2019;92:e1271-83. [Crossref] [PubMed]
- Maguire JM, Bevan S, Stanne TM, et al. GISCOME - Genetics of Ischaemic Stroke Functional Outcome network: A protocol for an international multicentre genetic association study. Eur Stroke J 2017;2:229-37. [Crossref] [PubMed]
- Spilker J, Kongable G, Barch C, et al. Using the NIH Stroke Scale to assess stroke patients. The NINDS rt-PA Stroke Study Group. J Neurosci Nurs 1997;29:384-92. [Crossref] [PubMed]
- Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol 2023;47:314-31. [Crossref] [PubMed]
- Lin Z, Deng Y, Pan W. Combining the strengths of inverse-variance weighting and Egger regression in Mendelian randomization using a mixture of regressions model. PLoS Genet 2021;17:e1009922. [Crossref] [PubMed]
- Scholz FW. Weighted median regression estimates. Ann Statist 1978;6:603-9. [Crossref]
- Minelli C, Del Greco M F, van der Plaat DA, et al. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol 2021;50:1651-9. [Crossref] [PubMed]
- Storey JD. False Discovery Rate. In: Lovric M. editor. International Encyclopedia of Statistical Science. Berlin, Heidelberg: Springer; 2011:504-8.
- VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, et al. Methodological challenges in mendelian randomization. Epidemiology 2014;25:427-35. [Crossref] [PubMed]
- Pereira TV, Patsopoulos NA, Salanti G, et al. Critical interpretation of Cochran’s Q test depends on power and prior assumptions about heterogeneity. Res Synth Methods 2010;1:149-61. [Crossref] [PubMed]
- Cohen JF, Chalumeau M, Cohen R, et al. Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol 2015;68:299-306. [Crossref] [PubMed]
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 2017;32:377-89. [Crossref] [PubMed]
- Knops M, Werner CG, Scherbakov N, et al. Investigation of changes in body composition, metabolic profile and skeletal muscle functional capacity in ischemic stroke patients: the rationale and design of the Body Size in Stroke Study (BoSSS). J Cachexia Sarcopenia Muscle 2013;4:199-207. [Crossref] [PubMed]
- Pradillo JM, Hernández-Jiménez M, Fernández-Valle ME, et al. Influence of metabolic syndrome on post-stroke outcome, angiogenesis and vascular function in old rats determined by dynamic contrast enhanced MRI. J Cereb Blood Flow Metab 2021;41:1692-706. [Crossref] [PubMed]
- Wang M, Gui X, Wu L, et al. Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: a metabonomics study. BMC Neurol 2020;20:250. [Crossref] [PubMed]
- Brion MJ, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 2013;42:1497-501. [Crossref] [PubMed]
- Kollmann K, Pohl S, Marschner K, et al. Mannose phosphorylation in health and disease. Eur J Cell Biol 2010;89:117-23. [Crossref] [PubMed]
- Dhanalakshmi M, Sruthi D, Jinuraj KR, et al. Mannose: a potential saccharide candidate in disease management. Med Chem Res 2023;32:391-408. [Crossref] [PubMed]
- Sun AH, Collette JR, Sifers RN. The cytoplasmic tail of human mannosidase Man1b1 contributes to catalysis-independent quality control of misfolded alpha1-antitrypsin. Proc Natl Acad Sci U S A 2020;117:24825-36. [Crossref] [PubMed]
- Kusaczuk M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019;8:1471. [Crossref] [PubMed]
- Eggink HM, Tambyrajah LL, van den Berg R, et al. Chronic infusion of taurolithocholate into the brain increases fat oxidation in mice. J Endocrinol 2018;236:85-97. [Crossref] [PubMed]
- Pajares MA, Pérez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci 2006;63:2792-803. [Crossref] [PubMed]
- Zawieja E, Drabińska N, Jeleń H, et al. Betaine supplementation modulates betaine concentration by methylenetetrahydrofolate reductase genotype, but has no effect on amino acid profile in healthy active males: A randomized placebo-controlled cross-over study. Nutr Res 2024;127:63-74. [Crossref] [PubMed]
- Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol 2013;48:20-38. [PubMed]
- Aquilani R, Boselli M, Paola B, et al. Is stroke rehabilitation a metabolic problem? Brain Inj 2014;28:161-73. [Crossref] [PubMed]
- Mele C, Maggioni G, Giordano A, et al. A Retrospective Study on Statins and Post-stroke Patients: What About Functional Outcome and Follow-Up in a Stroke Rehabilitation Cohort? Front Neurol 2021;12:744732. [PubMed]
- Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, et al. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am J Clin Nutr 2020;112:303-17. [PubMed]
- Calder PC. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J Parenter Enteral Nutr 2015;39:18S-32S. [PubMed]
- Winzer C, Wagner O, Festa A, et al. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care 2004;27:1721-7. [PubMed]
- Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 2005;6:13-21. [Crossref] [PubMed]
- Su JR, Lu ZH, Su Y, et al. Relationship of Serum Adiponectin Levels and Metformin Therapy in Patients with Type 2 Diabetes. Horm Metab Res 2016;48:92-8. [Crossref] [PubMed]
- Richards LG, Cramer SC. Therapies Targeting Stroke Recovery. Stroke 2023;54:265-9. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


