
Aneurysm of the fetal right ventricular free wall progressing to hypoplastic right heart syndrome: prenatal diagnosis, maternal digoxin therapy, and successful surgical intervention—a case report
Highlight box
Key findings
• Right ventricular free wall aneurysm may be accompanied by severe tricuspid valve stenosis and right ventricular free wall akinesia, which may lead to reduced right ventricular output.
• The fetalHQ® software is a useful tool for the assessment of ventricular contractility disorders.
• The natural course of hemodynamic changes accompanying tricuspid stenosis and aneurysm of the right ventricular free wall may lead to hypoplastic right heart syndrome (HRHS) with the development of ductal-dependent pulmonary circulation.
What is known and what is new?
• Aneurysm of the fetal right ventricular free wall is a rare clinical entity, data on the natural progression during fetal life is limited, with available knowledge primarily based on a small number of case reports and series.
• We described the natural course of this anomaly, the results of digoxin therapy, postnatal diagnosis and management, the method of cardiac surgery after delivery, and a 3-month follow-up after the surgical procedure.
What is the implication, and what should change now?
• Correct recognition, understanding, and monitoring of the changes are important for making decisions about prenatal and postnatal management.
• Certainly, a description of similar cases would allow for a better understanding of the pathophysiology and perhaps some regularities in the future. Further follow-up of the patient is also necessary.
Introduction
Background
Congenital right ventricular aneurysm (CVA) and diverticulum (CVD) are rare cardiac diseases that can be diagnosed prenatally. CVAs are usually not associated with other anomalies. CVD can also be associated with abnormalities in midline body structures, including the sternum, pericardium, heart (such as atrial or ventricular septal defects), diaphragm, and abdominal wall. Aneurysms are generally composed of fibrotic tissue, exhibit impaired contractility, and have broad-based attachments to the ventricular cavity (1-6). A little is known about the etiology of the defect. An important known factor is coronary artery maldevelopment (stenosis or hypoplasia of the coronary artery, coronary fistulas). Ventricular aneurysms may change in size and shape over time, with a tendency to progressively enlarge, warranting ongoing monitoring. In contrast, a diverticulum contains all myocardial layers, demonstrates near-normal contractility, is generally small with a narrow neck, and tends to remain stable in size (7,8). The differential diagnosis for right ventricular CVA includes Uhl’s anomaly, a rare congenital condition characterized by the partial or complete absence of right ventricular muscle (9). The hemodynamic impact of a ventricular aneurysm during fetal life depends on its size (10), the extent of myocardium involved, and its location. It may impair myocardial contractility, resulting in reduced cardiac output, overall cardiac dysfunction, and in some cases, fetal hydrops (10-15). Additionally, a mass effect on adjacent structures, such as the lungs, can contribute to pulmonary hypoplasia. Large aneurysms with thin, scarred walls carry a risk of rupture, potentially leading to fetal demise (16-18). Ischemic areas within the myocardium may further compromise valve function, causing tricuspid or mitral regurgitation, and increase the risk of arrhythmias (3,8,13,19,20). Knowledge about the natural progression of ventricular aneurysms or diverticula detected in fetal life remains limited, with most insights coming from a small number of case reports and series (19-23).
Rationale and knowledge gap
Although the literature describes cases of fetal right ventricular (RV) free wall aneurysms and subsequent postnatal follow-up (1,11,22,23), there is a lack of information on the natural evolution of hemodynamic changes during fetal life. There are also limited descriptions of myocardial contractility disorders accompanying a fetal RV free wall aneurysm. Zhao et al. first used fetalHQ® software for prenatal evaluation of the shape and contractility of congenital ventricular aneurysms (24). We described the natural evolution of a rare clinical entity with respect to hemodynamic changes, in particular myocardial contractility abnormalities, using two-dimensional speckle tracking echocardiography (2D-STE).
Objective
This case report describes the natural progression of a rare fetal condition involving a RV free wall aneurysm with critical pulmonary stenosis, progressing to hypoplastic right heart syndrome (HRHS). The condition was closely monitored using advanced fetal imaging and managed with prenatal digoxin therapy, culminating in a successful first-stage surgical intervention after birth. The fetalHQ® software provided objective metrics for assessing right and left ventricular function and tracking disease progression. This data supported prenatal decision-making and facilitated counselling with the family regarding the evolving condition and postnatal management strategies. We described the results of digoxin therapy, postnatal diagnosis and management, a diagnostic approach, and successful first-stage surgery after delivery with a 3-month follow-up. We also presented a first-trimester prenatal counseling in such entities, which emphasized the multifactorial nature of the condition, the expected progression of RV hypoplasia and failure, and the need for postnatal surgical staged interventions. We present this case in accordance with the CARE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-477/rc).
Case presentation
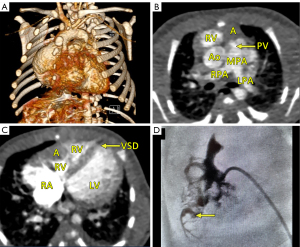
This report presents the case of a fetus in whom an aneurysm of the RV free wall with critical pulmonary stenosis was diagnosed during ultrasound examination in the first trimester, at 13 weeks of gestational (w. g.). At the time of diagnosis, severe tricuspid valve stenosis with severe tricuspid regurgitation, critical pulmonary valve stenosis, pericardial effusion, ascites (Video 1, Figure 1A), and secondary reversal of flow in the ductus venosus a-wave was found. Additionally, a small muscular interventricular leak was noted. The coexistence of ductus venosus agenesis with ductus venosus-systemic shunt (DVSS) (Figure 1B) was detected, with no other abnormalities of the portosystemic system. No genomic imbalance was demonstrated by microarray-based comparative genomic hybridization (aCGH) based on amniotic fluid samples. There was no reported family history of congenital heart disease, and all routine prenatal screening results were normal. Serial echocardiographic measurements demonstrated a persistently small tricuspid valve annulus with a Z-score of −3.2 at 21 weeks, without significant growth in subsequent evaluations. The right ventricle displayed reduced end-diastolic area (0.47 cm2 at 21 weeks, increasing marginally to 1.01 cm2 at 31 weeks), with a corresponding decline in right ventricle to left ventricle area ratio (RV/LVAR) from 0.69 to 0.55, indicating progressive hypoplasia. With progressive impairment of RV function, an evolution from critical stenosis to pulmonary atresia was observed. With progressive pulmonary valve dysfunction, retrograde flow through the ductus arteriosus became increasingly visible, from partial retrograde flow seen at 27 w. g. (Figure 1C) to complete retrograde flow at 35 w. g. coinciding with no antegrade flow across the pulmonary valve, confirming ductal-dependent pulmonary circulation. The tricuspid valve was thickened and dysplastic, with severe stenosis and regurgitation confirmed postnatally. To address cardiac insufficiency, digoxin therapy was initiated at 13 w. g. The total loading dose of 1,200 micrograms orally in the first 24 hours was followed by a maintenance dose of 375 micrograms per day. Therapy resulted in significant improvement, with resolution of ascites and reduction of pericardial effusion observed within two weeks. Regular maternal follow-up included weekly monitoring of serum digoxin levels (target range, 0.5–2 ng/mL) and electrocardiographic evaluations to rule out toxicity. No maternal adverse effects were observed during therapy. This treatment was continued until delivery, resulting in the stabilization of fetal hydrops and a significant reduction in pericardial effusion. Echocardiographic imaging suggested a double-chambered appearance of the RV (Figures 1D,2A) with the aneurysm forming the anterior and superior chamber and the functional RV positioned inferiorly. This configuration likely disrupted the RV’s pressure gradients and flow dynamics, contributing to the absence of effective forward flow through the pulmonary valve and the progression to functional pulmonary atresia. In the second trimester, in addition to routine functional assessment, fetalHQ® software was used to monitor the size and contractility of the RV and left ventricle (LV) in terms of global and segmental strain (25-29). Fractional shortening of each of the 24 RV segments revealed akinesis of the free wall of the RV (Figures 2B-2D,3, Video 2), with an abnormal myocardial performance index (MPI) of 0.68 (normal value <0.55). The RV fractional area change (FAC) was −1.68% (−9.90 Z-score), and the pulmonary flow/systemic flow ratio (Qp/Qs) was reduced to the value of 0.863 (normal value 1.4). Motion vector analysis was used to assess RV contractility abnormalities, revealing contraction of the opposing helical tracts of the interventricular septum, contraction of the circular fibers of the LV, and displacement of the stiff, akinetic RV along the interventricular septum. We described the stiff, akinetic ventricle as a “frozen ventricular sign” (Figure 2B-2D, Video 3). The LV contractility, global sphericity index, and end-diastolic diameter for each of the 24 right left ventricular segments were normal (Figure 3, Table 1). At 27 weeks, the LV global longitudinal strain (GLS) was noted to be −10.33%, which was disproportionately low compared to its values at 21 weeks (−23.88%) and 31 weeks (−28.00%). Despite this, the LV FAC remained normal at 42.83%, supporting normal systolic function. This isolated deviation in GLS may reflect transient hemodynamic variation or technical variability during measurement. The LV MPI showed a decreasing trend during gestation (0.52 at 21 weeks to 0.34 at 31 weeks), likely reflecting maturational improvement in myocardial performance, consistent with preserved LV function throughout pregnancy. With increasing gestational age, in the 2nd and 3rd trimesters, the aneurysm of the right ventricle remained relatively stable in size (3 mm × 3 mm × 7 mm, in 21 w. g.), the fetus developed features of fetal growth restriction (FGR) while maintaining normal peripheral Doppler readings. Reduction of segmental transverse strain for each of the 24 segments reflecting impaired RV free wall contractility. Furthermore, the GLS for the RV remained persistently, extremely low throughout pregnancy. Despite this, an increase in RV stroke volume was noted (from 0.693 mL at 21 weeks to 2.190 mL at 31 weeks), suggesting some degree of compliance, likely due to volume overload caused by severe tricuspid regurgitation. The Qp/Qs ratio progressively decreased (0.863 at 21 weeks to 0.510 at 31 weeks), reflecting the hemodynamic shift toward ductal-dependent pulmonary circulation (Figure 3, Video 3, Table 1). Serial echocardiographic measurements demonstrated a persistently small tricuspid valve annulus with a Z-score of −3.2 at 21 weeks, without significant growth in subsequent evaluations. The right ventricle displayed reduced end-diastolic area (0.470 cm2 at 21 weeks, increasing marginally to 1.010 cm2 at 31 weeks), with a corresponding decline in RV/LVAR from 0.69 to 0.55, indicating progressive hypoplasia. The tricuspid valve was thickened and dysplastic, with severe stenosis and regurgitation confirmed postnatally. A female newborn weighing 2,480 g was born in good condition at 39 w. g via planned cesarean section. Apgar scores were 8 and 9 at 1 and 5 minutes, respectively. The newborn exhibited mild central cyanosis, with oxygen saturation of 85% on room air, and was hemodynamically stable. Prostaglandin E1 infusion was initiated to maintain ductal patency. The baby was admitted to the neonatal intensive care unit (NICU) for monitoring and planning of surgical intervention. Postnatal echocardiography showed a slightly enlarged right atrium and a hypoplastic tricuspid valve annulus measuring 5 mm, with trace flow into the hypoplastic, bi-chambered right ventricle. Pulmonary atresia was confirmed at the valve level, with a pulmonary artery ring of 7.3 mm and a pulmonary trunk diameter of 7.6 mm. The aneurysm was akinetic and showed no significant changes in size or morphology compared to prenatal findings. No evidence of rupture or thrombus formation was noted during the neonatal period, and the aneurysm is being closely monitored during follow-up for potential long-term complications. Good mitral and aortic valve function and small muscular ventricular septal defect of approximately 3 mm was confirmed. The pulmonary trunk and its branches were not underdeveloped. Computed tomography (CT) angiography confirmed a small right ventricle with a hypoplastic cavity, a small interventricular septal defect, and pulmonary trunk and branch diameters of 8.5 mm and 3.5 mm, respectively (Figure 4A-4C). The neonate was stable and remained in good clinical condition. Finally, a systemic-pulmonary shunt (Blalock-Taussig anastomosis) was carried out. During the surgery, the aneurysm was left untouched. After 3 months of postsurgical follow-up, the patient remained stable and required anti-platelet therapy with daily doses of acetylsalicylic acid. As preparation for a second stage of treatment, a cardiac catheterization was carried out, which revealed poor filling of the hypoplastic right ventricle with a small aneurysmal outpouching (4–5 mm) and numerous sinusoids. A fistula was identified between the distal right coronary artery (RCA) and the right ventricle, with no continuity between the proximal and distal RCA (Figure 4D). Information from this episode of care, organized in a timeline format, is presented in Figure S1.
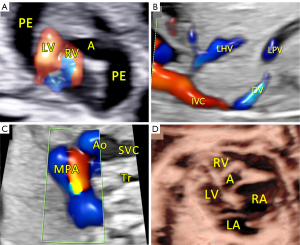
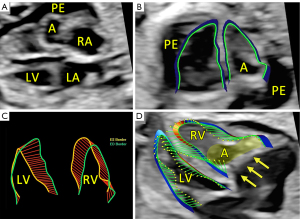
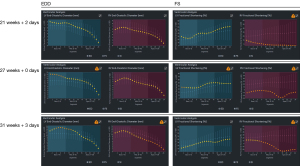
Table 1
| Parameters | Gestational age | ||
|---|---|---|---|
| 21 weeks + 2 days | 27 weeks + 0 days | 31 weeks + 3 days | |
| EFW (Hedlock) (g) | 345 | 665 | 1148 |
| MPI | |||
| LV (reference value) | 0.52 (<0.55) | 0.43 (<0.55) | 0.34 (<0.55) |
| RV (reference value) | 0.68 (<0.55) | 1.01 (<0.55) | 0.63 (<0.55) |
| SV (mL) | |||
| LV | 0.803 | 2.040 | 4.290 |
| RV | 0.693 | 1.300 | 2.190 |
| Qp/Qs (reference value) | 0.863 (1.4) | 0.637 (1.4) | 0.510 (1.4) |
| FAC | |||
| LV (%) | 51.90 | 42.83 | 45.30 |
| LV Z-score | –0.09 | –0.71 | –0.06 |
| RV (%) | –1.68 | –8.55 | –0.01 |
| RV Z-score | –9.90 | –8.07 | –7.79 |
| GLS (%) | |||
| LV | –23.88 | –10.33 | –28.00 |
| RV | 2.620 | 0.700 | 4.100 |
| EDA (cm2) | |||
| LV | 0.680 | 1.360 | 2.290 |
| RV | 0.470 | 0.870 | 1.010 |
| RV/LVAR | 0.69 | 0.63 | 0.55 |
EFW, estimated fetal weight; EDA, end-diastolic area; FAC, fractional area change; GLS, global longitudinal strain; HRHS, hypoplastic right heart syndrome; LV, left ventricle; MPI, myocardial performance index; Qp/Qs, pulmonary flow/systemic flow; RV, right ventricle; RV/LVAR, right ventricle to left ventricle area ratio; SV, stroke volume.
All procedures conducted in this case adhered to the ethical standards set by the institutional and/or national research committees and followed the principles of the Helsinki Declaration (revised in 2013). Written informed consent for the publication of this case report and accompanying images was obtained from the patient’s parents. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
A RV free wall aneurysm may result in a very different prognosis. Any hemodynamic disturbances do not accompany some cases of CAV and may remain asymptomatic during pregnancy and postnatally. Some CAVs lead to hemodynamic instability and, consequently, death. We described a case of an aneurysm of the free wall of the RV detected during the first-trimester ultrasound scan, presenting signs of early cardiac failure. Because of that digoxin therapy was introduced with successful results preventing from intrauterine fetal demise. The aneurysm coexisted with severe tricuspid valve stenosis and akinesia of the free wall of the RV. We demonstrated the usefulness of fetalHQ® software for the assessment of RV contractility disorders. We presented the natural course of hemodynamic changes leading to HRHS with the development of ductal-dependent pulmonary circulation.
Strengths and limitations
This report highlights the intricate interplay between structural defects and hemodynamics in fetal heart development. The progression of tricuspid stenosis to RV hypoplasia, coronary fistula, and aneurysm formation reflects a sequential disruption of normal cardiac development and emphasizes the multifactorial nature of HRHS. We described the natural prenatal course of the disease, the results of digoxin therapy, postnatal diagnosis and management, the cardiac surgery applied after delivery, and a 3-month follow-up after the surgical procedure. Our observations may be influenced by potential biases, and controlling for confounding factors in this case was not feasible, which could impact the interpretation of findings. Further research is needed to validate these results before they can be generalized to a broader population. Nonetheless, this report provides a valuable basis for future large-scale investigations.
Similar studies review
Athiel et al. described three cases of RV free wall aneurysms (23). Two of them, diagnosed in the first trimester, were accompanied by massive pericardial effusion at the time of diagnosis, which is the most common symptom accompanying CVA (1,8,23). In one case, the effusion resolved spontaneously, and the fetus did not show any hemodynamic disturbances, remaining completely asymptomatic until the age of 3 years, without any medical intervention. In the second case, at 15 weeks of gestation, the pregnancy was terminated. In the third case, the diagnosis was made in the second trimester, and the patient remained asymptomatic, without any medical intervention before or after birth. Hirose et al. detailed a case involving a 22-week fetus diagnosed with a significant left ventricular aneurysm, which was associated with hydrops fetalis, manifesting as subcutaneous edema, pericardial and peritoneal effusions (22). The aneurysm had a thin wall, displayed hypokinesia, and progressively enlarged with advancing gestation, exerting compressive effects on the lung. Despite the gradual deterioration of left ventricular function, reflected by an increasing Tei index, hydrops resolved by 32 weeks of gestation, likely due to maternal digoxin therapy and effective compensation by the right ventricle, as evidenced by retrograde flow in the distal aortic arch through the patent ductus arteriosus. In our case, similar to the case described by Hirose, the application of digoxin therapy significantly improved myocardial contractility, leading to the remission of ascites and edema. Similarly, despite digoxin treatment, the presence of a free-walled aneurysm progressively worsened the contractility of the chamber in which it was located. Zhao et al. first used fetalHQ® software for prenatal evaluation of the shape and contractility of congenital ventricular aneurysms (24). Among the 10 analyzed cases of CVA and CVD, Zhao et al. reported only one case of RV free wall aneurysm, the course of which was not described due to the termination of pregnancy.
This case differs from previously reported asymptomatic cases of RV aneurysm in terms of its early onset and severe associated hemodynamic consequences. While asymptomatic cases often involve isolated aneurysms without functional compromise, this patient exhibited severe tricuspid valve dysfunction, functional pulmonary atresia, and progression to HRHS, and probably would not survive if digoxin was not administered in the first trimester of pregnancy. The akinetic aneurysm contributed to reduced RV output and ductal-dependent circulation. The use of advanced imaging techniques such as fetalHQ® software and speckle tracking also provided unique insights into hemodynamic progression and myocardial function not typically documented in prior reports. The cardiac catheterization provided unique information about the potential etiology of the aneurysm.
Explanations of findings
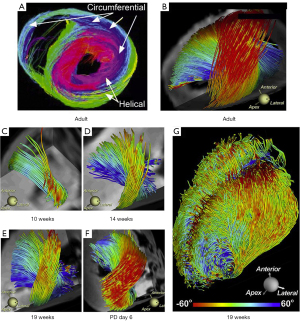
To comprehend the contractility and complex geometry of the right ventricle, it is essential to examine the orientation of the muscle fibers that form the ventricular walls. Mekkaoui et al. analyzed the fiber orientation of the myocardium. They identified two opposing helical fiber tracts extending from the base to the apex of the lateral wall of the LV, along with a nonhelical circumferential fiber tract (30). The fiber orientation in the fetal heart, similar to that of the adult heart, was not present at 10 w. g. but resembled an adult orientation by 19 weeks (Figure 5). Buckberg et al. explored the helical architecture of the ventricles, supporting Torrent-Guasp’s theory that the heart is formed by two continuous muscle bands: the basal loop, containing transverse fibers that wrap around both ventricles, and the apical loop, made up of right- and left-handed helices intersecting at a 60-degree angle to create an apical vortex (31,32). The right- and left-handed helices play a key role in generating opposing contraction forces between the base and apex of each ventricle, facilitating effective blood ejection and filling. Due to the specific orientation of myocardial fibers, the interventricular septum primarily supports the function of the LV. Research on the right ventricle suggests that its longitudinal shortening from base to apex results from the coiling and contraction of the apical helix, with minimal involvement from the longitudinal and circumferential fibers of the lateral wall. Interestingly, studies in both adults and animal models have shown that RV failure does not occur when the free wall loses function. However, heart failure does develop when the interventricular septum becomes ischemic (33-35). In this case, we visualized complete RV akinesis caused by an aneurysm of the RV free wall. The function of the RV, initially compromised by the aneurysm, improved after maternal digoxin therapy, which contributed to the resolution of fetal hydrops. The parallel circulation model of the fetus allowed for partial compensation of the dysfunctional RV by the LV, which progressively increased in size and assumed a more spherical shape due to volume overload as it partially took over the function of the failing RV.
While the temporal relationship between the RV free wall aneurysm and tricuspid valve pathology remains uncertain, the primary abnormality is most likely severe tricuspid valve stenosis. This condition likely disrupted blood flow into the RV during early embryogenesis, reducing ventricular filling and myocardial stress, which are critical for normal RV development. This restriction in blood flow not only led to RV hypoplasia, but also predisposed the fetus to the development of coronary fistulae. The resulting myocardial ischemia may have contributed to the formation of the aneurysm in the free wall of the RV.
The RV free wall aneurysm, in turn, further compounded the functional deterioration of the RV by reducing its effective contractile function. The gradual loss of RV output ultimately progressed to HRHS. The progression to pulmonary atresia, with the absence of antegrade flow across the pulmonary valve, marked a critical turning point in the condition. However, by the time the pulmonary valve became fully obstructed, the branches of the pulmonary artery had already developed sufficiently to sustain pulmonary circulation. After the development of pulmonary atresia, retrograde flow through the ductus arteriosus maintained further growth of the pulmonary artery branches, ensuring continued pulmonary perfusion despite the loss of antegrade flow.
This sequence of events illustrates the interplay between tricuspid valve dysfunction and the formation of the RV aneurysm, as well as the critical role of fetal compensatory mechanisms. The use of advanced imaging techniques, such as fetalHQ® software, enabled detailed assessment of both RV and LV function, providing objective data to track the progression of the disease. These insights supported prenatal decision-making and facilitated counselling with the family, helping them understand the evolving condition and potential outcomes.
Implications and actions needed
From a clinical point of view, the key issue in the described case in the context of postnatal prognosis is maintaining the flow through the pulmonary arteries. RV free wall aneurysm may be accompanied by both tricuspid stenosis and RV contractility disorders. Each of these leads to a decrease in RV cardiac output. The introduction of digoxin therapy improved the contractility of both ventricles and in the case of the RV, cardiac output is generated by the contraction of the interventricular septum, with a little contribution of circular fibers of the RV free wall. This would explain why a stiff, akinetic right ventricle generates stroke volume, but also explains the presence of significant tricuspid valve regurgitation that appears during the ventricle contraction. It explains that the systolic function of RV was maintained in our case. As with the LV aneurysm described by Hirose, over time, despite digoxin therapy, the presence of the aneurysm caused the RV to be unable to generate sufficient flow to maintain pulmonary valve patency, ultimately leading to the development of HRHS. The fetal “parallel” circulatory model allows for partial compensation of impaired contractile function in one ventricle by the other, normally functioning ventricle. Reverse flow from the aortic arch maintained normal flow through the pulmonary arteries. The ductal-dependent pulmonary circulation required prostaglandin therapy after delivery. Certainly, a description of similar cases would allow for a better understanding of the pathophysiology and perhaps some regularities in the future. Further follow-up of the patient is also necessary.
Conclusions
Congenital RV aneurysms are rare but significant anomalies with potential for severe hemodynamic consequences. Multidisciplinary approaches integrating advanced imaging techniques, prenatal counseling, and tailored postnatal management are essential for optimizing outcomes.
Acknowledgments
We are very grateful to the patient’s parents. We also acknowledge Kazimierz Pitynski, MD, PhD, for creating favorable research conditions for working on this manuscript and Joanna Baran for the graphic design.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-477/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-477/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-477/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ohlow MA, Lauer B, Brunelli M, Geller JC. Ventricular aneurysms are different from ventricular diverticula! Circ J 2013;77:276. [Crossref] [PubMed]
- Morin C, Ponzio A, Guirgis M, et al. Prenatal diagnosis of congenital ventricular aneurysm and diverticulum: Prenatal features and perinatal management. Prenat Diagn 2022;42:428-34. [Crossref] [PubMed]
- Witters I, Boshoff D, De Catte L, et al. Pericardial effusion in the first trimester of pregnancy. Prenat Diagn 2011;31:215-7. [Crossref] [PubMed]
- McAuliffe FM, Hornberger LK, Johnson J, et al. Cardiac diverticulum with pericardial effusion: report of two new cases treated by in-utero pericardiocentesis and a review of the literature. Ultrasound Obstet Gynecol 2005;25:401-4. [Crossref] [PubMed]
- Krasemann T, Gehrmann J, Fenge H, et al. Ventricular aneurysm or diverticulum? Clinical differential diagnosis. Pediatr Cardiol 2001;22:409-11. [Crossref] [PubMed]
- Paoletti D, Robertson M. Prenatal diagnosis of a left ventricular diverticulum. Australas J Ultrasound Med 2012;15:112-4. [Crossref] [PubMed]
- Gowda M, Bharathi S, Thiagarajan M, et al. Prenatal diagnosis of fetal right and left congenital ventricular aneurysms. J Matern Fetal Neonatal Med 2018;31:2367-70. [Crossref] [PubMed]
- Ohlow MA, von Korn H, Lauer B. Characteristics and outcome of congenital left ventricular aneurysm and diverticulum: Analysis of 809 cases published since 1816. Int J Cardiol 2015;185:34-45. [Crossref] [PubMed]
- Vaujois L, van Doesburg N, Raboisson MJ. Uhl's anomaly: a difficult prenatal diagnosis. Cardiol Young 2015;25:580-3. [Crossref] [PubMed]
- Sharma JR, Oforl-Amanfo G, Marboe C, et al. Congenital left ventricular aneurysm with pericardial effusion: prenatal diagnosis, surgical management and follow-up. Pediatr Cardiol 2002;23:458-61. [Crossref] [PubMed]
- Williams JA, Collardey KR, Treadwell MC, et al. Prenatally diagnosed right ventricular outpouchings: a case series and review of the literature. Pediatr Cardiol 2009;30:840-5. [Crossref] [PubMed]
- Azancot A, Diehl R, Dorgeret S, et al. Isolated pericardial effusion in the human fetus: a report of three cases. Prenat Diagn 2003;23:193-7. [Crossref] [PubMed]
- El Kady D, Gerscovich EO, Moon-Grady A, et al. Congenital cardiac left ventricular aneurysm with pericardial effusion: early prenatal diagnosis and intervention. J Ultrasound Med 2005;24:1011-5. [Crossref] [PubMed]
- Garcia Rodriguez R, Rodriguez Guedes A, Garcia Delgado R, et al. Prenatal Diagnosis of Cardiac Diverticulum with Pericardial Effusion in the First Trimester of Pregnancy with Resolution after Early Pericardiocentesis. Case Rep Obstet Gynecol 2015;2015:154690. Erratum in: Case Rep Obstet Gynecol 2017;2017:6207658. [Crossref] [PubMed]
- Monteiro Pereira Leite MDF, Pereira Júnior JP, Verona Barreto Farias C, et al. Fetal left ventricular giant aneurysm: three-dimensional virtual and printed models. Ultrasound Obstet Gynecol 2024;64:127-8. [Crossref] [PubMed]
- Koshiishi T, Osada H, Hata A, et al. Prenatal rupture of right ventricular diverticulum: a case report and review of the literature. Prenat Diagn 2007;27:1154-7. [Crossref] [PubMed]
- Bernasconi A, Delezoide AL, Menez F, et al. Prenatal rupture of a left ventricular diverticulum: a case report and review of the literature. Prenat Diagn 2004;24:504-7. [Crossref] [PubMed]
- Prendiville TW, Ravekes WJ, Spevak PJ. Fetal myocardial injury progressing to ventricular septal rupture and aneurysm formation. Prenat Diagn 2011;31:405-6. [Crossref] [PubMed]
- Marijon E, Ou P, Fermont L, et al. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J Thorac Cardiovasc Surg 2006;131:433-7. [Crossref] [PubMed]
- Papagiannis J, Van Praagh R, Schwint O, et al. Congenital left ventricular aneurysm: clinical, imaging, pathologic, and surgical findings in seven new cases. Am Heart J 2001;141:491-9. [Crossref] [PubMed]
- Johnson JA, Ryan G, Toi A, et al. Prenatal diagnosis of a fetal ventricular diverticulum associated with pericardial effusion: successful outcome following pericardiocentesis. Prenat Diagn 1996;16:954-7. [Crossref] [PubMed]
- Hirose A, Maeno Y, Suda K, et al. Serial hemodynamic assessment using Doppler echocardiography in a fetus with left ventricular aneurysm presented as fetal hydrops. J Perinatol 2013;33:486-9. [Crossref] [PubMed]
- Athiel Y, Barrois M, Bault JP, et al. Fetal diagnosis of right cardiac ventricular aneurysms: A report of three cases. J Gynecol Obstet Hum Reprod 2018;47:481-5. [Crossref] [PubMed]
- Zhao L, Wu P, Jiao X, et al. Characteristics and outcomes of fetal ventricular aneurysm and diverticulum: combining the use of a new technique, fetal HQ. Front Pediatr 2023;11:1165972. [Crossref] [PubMed]
- DeVore GR, Klas B, Satou G, et al. Twenty-four Segment Transverse Ventricular Fractional Shortening: A New Technique to Evaluate Fetal Cardiac Function. J Ultrasound Med 2018;37:1129-41. [Crossref] [PubMed]
- DeVore GR, Klas B, Satou G, et al. Quantitative evaluation of fetal right and left ventricular fractional area change using speckle-tracking technology. Ultrasound Obstet Gynecol 2019;53:219-28. [Crossref] [PubMed]
- DeVore GR, Klas B, Satou G, et al. Evaluation of Fetal Left Ventricular Size and Function Using Speckle-Tracking and the Simpson Rule. J Ultrasound Med 2019;38:1209-21. [Crossref] [PubMed]
- DeVore GR, Gumina DL, Hobbins JC. Assessment of ventricular contractility in fetuses with an estimated fetal weight less than the tenth centile. Am J Obstet Gynecol 2019;221:498.e1-498.e22. [Crossref] [PubMed]
- DeVore GR, Klas B, Cuneo B, et al. Review of speckle tracking analysis to measure the size, shape, and contractility of the fetal heart in fetuses with congenital heart defects. Echocardiography 2024;41:e15870. [Crossref] [PubMed]
- Mekkaoui C, Porayette P, Jackowski MP, et al. Diffusion MRI tractography of the developing human fetal heart. PLoS One 2013;8:e72795. [Crossref] [PubMed]
- Buckberg GD, Nanda NC, Nguyen C, et al. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J Cardiovasc Dev Dis 2018;5:33. [Crossref] [PubMed]
- Torrent-Guasp F, Buckberg GD, Clemente C, et al. The structure and function of the helical heart and its buttress wrapping. I. The normal macroscopic structure of the heart. Semin Thorac Cardiovasc Surg 2001;13:301-19. [Crossref] [PubMed]
- Goldstein JA, Tweddell JS, Barzilai B, et al. Importance of left ventricular function and systolic ventricular interaction to right ventricular performance during acute right heart ischemia. J Am Coll Cardiol 1992;19:704-11. [Crossref] [PubMed]
- Hoffman D, Sisto D, Frater RW, et al. Left-to-right ventricular interaction with a noncontracting right ventricle. J Thorac Cardiovasc Surg 1994;107:1496-502. [Crossref] [PubMed]
- Buckberg G, Hoffman JI. Right ventricular architecture responsible for mechanical performance: unifying role of ventricular septum. J Thorac Cardiovasc Surg 2014;148:3166-71.e1-4.


