
Beta-blockers in post-myocardial infarction with preserved ejection fraction: systematic review and meta-analysis
Highlight box
Key findings
• Beta-blockers (BBs) do not provide any additional advantage for patients with preserved ventricular function following a myocardial infarction (MI) in the post-reperfusion era.
What is known and what is new?
• There are differences in the literature regarding the use of BBs in the post-MI scenario.
• Post-MI patients who maintain preserved left ventricular function have no additional benefit in reducing total, cardiac or major adverse cardiac events mortality with the routine use of BBs.
What is the implication, and what should change now?
• The routine use of BBs in post-MI patients with preserved left ventricular ejection fraction should be reconsidered, as there is no clear benefit in terms of mortality or other major outcomes. Current guidelines might need to be updated to reflect the lack of benefit in this subgroup of patients.
Introduction
Myocardial infarction (MI) is still a very common condition and remains one of the main causes of morbidity and mortality worldwide (1,2). Beta-blockers (BBs) have been a cornerstone in the treatment of MI since the era of the Beta-Blocker Heart Attack Trial (BHAT), which demonstrated their potential to reduce mortality.
It is known that BBs play an important role in adrenergic control, promoting a reduction in heart rate, myocardial oxygen demand and myocardial work. Its use in patients with ventricular dysfunction was beneficial in several scenarios, especially after MI. In line with this evidence, the European Society of Cardiology guideline recommends the routine use of BBs in patients with acute coronary syndrome, regardless of ventricular function, with recommendation class IIa and level of evidence B (3). Similarly, the American Heart Association/American College of Cardiology Foundation (AHA/ACCF) guideline (4) recommends long-term treatment (3 years) with adrenergic BBs after MI for patients with preserved left ventricular ejection fraction (LVEF) (class I), preferably started early after the ischemic event. After 3 years, the AHA/ACCF maintains chronic therapy with BBs as a recommendation for this group of patients (class IIa).
However, the evidence supporting their long-term use in patients with preserved LVEF is limited, particularly in the post-reperfusion era. It is known that individuals with moderate to severe ventricular dysfunction benefit from the use of BBs, but observational studies suggest that the clinical gain is not similar in patients with preserved LVEF (5-7).
Systematic reviews on this subject have analyzed the use of BBs in patients with mild ventricular dysfunction along with those with preserved LVEF (8-10). However, these patients behave differently and should be interpreted separately. The aim of this study was to carry out a systematic review with meta-analysis on the impact of prolonged BB use on reducing mortality only in patients with preserved LVEF after MI. We present this article in accordance with the PRISMA reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-368/rc) (11,12).
Methods
Search strategy and selection criteria
The predefined research protocol was registered in PROSPERO under the ID CRD42024554630. A systematic search was conducted in Embase, the Cochrane Central Register of Controlled Trials, and PubMed for studies published in English up to September 1, 2024, using the succeeding medical subject terms: ‘myocardial infarction’, ‘preserved ejection fraction’, and ‘beta-blockers’. Furthermore, the references from the included studies and systematic reviews were assessed to identify any additional studies. The full electronic search strategy is detailed in Appendix 1.
The variables of interest that will be analyzed are the following: mortality from all causes, mortality from cardiac causes, major adverse cardiac events (MACEs), reinfarction, hospitalization for HF, and stroke. The variables of interest analyzed in this study were clearly defined as follows: mortality from all causes: death due to any reason, as reported in the included studies, irrespective of the underlying etiology; mortality from cardiac causes: death attributed specifically to cardiovascular conditions, including MI, heart failure (HF), or arrhythmias; MACE: a composite outcome that includes non-fatal MI, cardiovascular death, and hospitalization for unstable angina or HF, as defined by the individual studies; reinfarction: recurrence of MI, confirmed by clinical symptoms, electrocardiographic changes, and biomarker elevation, as per the criteria reported in the included studies; hospitalization for HF: admission to the hospital for clinical deterioration due to HF, confirmed by signs and symptoms such as dyspnea, fatigue, peripheral edema, or radiological evidence of pulmonary congestion; stroke: an acute neurological deficit caused by vascular injury, either ischemic or hemorrhagic, confirmed by clinical diagnosis and/or imaging.
These outcomes were chosen based on their clinical relevance and availability in the included studies. Standardized definitions were applied wherever possible, and variability in reporting was addressed in the analysis.
We included studies that met the following eligibility criteria: (I) randomized controlled trials (RCTs) or cohort studies; (II) comparing beta-adrenergic blocking agents with placebo; (III) in patients with preserved HF (pHF) post-MI; and (IV) reporting at least one of the clinical outcomes of interest. We excluded studies with (I) overlapping patient populations; (II) without a placebo control group; or (III) with a crossover design.
Randomized trials or cohort studies of post-MI HF were included only if they reported dedicated outcomes in the pHF population. Cardiovascular outcome trials are typically powered for a composite of MACEs, lacking enough power to evaluate statistical significance of secondary, yet clinically relevant endpoints. Therefore, we sought to perform a systematic review and meta-analysis of these endpoints.
We extracted data for: (I) death from any cause; (II) death from cardiovascular causes; (III) MI; (IV) stroke; and (V) hospitalization for HF. These outcomes were compared using pooled hazard ratios (HRs) to preserve time-to-event data from individual studies. Importantly, we sought to evaluate the efficacy of BBs relative to placebo in subgroups of post-MI with preserved ejection fraction.
Furthermore, information on clinical comorbidities and baseline characteristics was systematically collected from the individual studies. This included patient demographics (age, sex), cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia), and relevant medical histories, such as prior MI and chronic obstructive pulmonary disease (COPD). Data on interventions, including the use of BBs and the comparator (e.g., placebo or absence of BBs), were also extracted, focusing on the type of BB, dose ranges, and duration of therapy where reported.
These variables were gathered to ensure a comprehensive understanding of the study populations and to facilitate subsequent subgroup and sensitivity analyses. This detailed extraction allowed us to assess the influence of baseline characteristics on clinical outcomes and to evaluate potential sources of heterogeneity among the included studies.
Data analysis
Two authors (L.C.C.F. and M.V.M.) independently extracted baseline characteristics reported in Table 1 and outcomes data using prespecified criteria for search, data extraction, and quality assessment. Disagreements were resolved by consensus among three authors (R.A.F.G., L.C.C.F., and M.V.M.). Treatment effects for binary endpoints were compared using pooled HR or odds ratios (ORs) with 95% confidence intervals (CIs). As described, mortality and MI outcomes were analyzed with HR to preserve time-to-event data. Weighted mean differences were used to pool continuous outcomes. To evaluate heterogeneity, we used Cochran’s Q test and the I2 statistic. Following established guidelines (12), I2 values were interpreted as follows: low heterogeneity for I2<25%, modest for 25–50%, and large for >50%. A P value <0.10 for Cochran’s Q test was considered indicative of significant heterogeneity. These thresholds were used to determine the degree of variability across studies and guide the choice of a random-effects or fixed-effects model. We used a fixed-effects model for endpoints with I2<25% (low heterogeneity), and a random-effects model for endpoints with higher heterogeneity (I2>25%). In pooled outcomes with high heterogeneity, DerSimonian and Laird random-effects model was used. Review Manager online (Nordic Cochrane center, The Cochrane Collaboration, Copenhagen, Denmark) was used for statistical analysis. We used the Cochrane Collaboration’s tool for assessing risk of bias in non-randomized studies of interventions (ROBINS-I) or risk-of-bias tool for randomized trials (RoB2). Each trial received a score of high, some concerns or low risk of bias in domains of trial design, conduct, and reported result.
Table 1
| Studies | Total patient | Patients | Age (years) | Male sex | Hypertension | Diabetes | Cigarette smoker | Dyslipidemia | COPD/asthma | LVEF (%) | Descriptions | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | Non-BB | BB | |||||||||||
| Chen et al. [2021] (13) | 2,397 | 2,060 | 337 | 57.3 (11.5) | 60.4 (11.9)** | 1,668 (81.0) | 277 (82.2) | 1,246 (60.5) | 180 (53.4)** | 660 (32.0) | 89 (26.4)** | NA | NA | NA | NA | NA | NA | 57.7 (4.1) | 58 (3.8) | NA | |||||||||
| Choo et al. [2014] (14) | 3,019 | 2,424 | 595 | 60.9 (12.1) | 63.1 (12.8)** | 1,799 (74.2) | 412 (69.2)** | 1,219 (50.3) | 290 (48.7) | 986 (40.7) | 241 (40.5) | 1,089 (44.9) | 239 (40.2) | NA | NA | NA | NA | 60.4 (6.8) | 60.2 (6.8) | NA | |||||||||
| Ishak et al. [2023] (15) | 43,618 | 34,253 | 9,365 | 64 [56–71] | 65 [57–74] | 25,658 (74.9) | 6,829 (72.9) | 13,152 (38.4) | 3,530 (37.7) | 4,601 (13.4) | 1,108 (11.8) | 10,820 (31.6) | 2,494 (26.6) | NA | NA | 957 (2.8) | 367 (3.9) | NA | NA | NA | |||||||||
| Joo et al. [2021] (6) | 12,200 | 10,251 | 1,949 | 63.2 (12.5) | 65,6 (12.9)** | 7,655 (74.7) | 1,414 (72.6)** | 5,230 (51.0) | 926 (47.5)** | 2,902 (28.3) | 509 (26.1)* | 4,123 (40.2) | 726 (37.2)** | NA | NA | NA | NA | 52.2 (10.8) | 52.5 (12.1) | Carvedilol, bisoprolol, nebivolol | |||||||||
| El Nasasra et al. [2021] (16) | 7,392 | 6,007 | 1,385 | 60.8 (12.1) | 62.2 (13.0)** | 4,757 (79.2) | 1,084 (78.3) | 3,352 (55.8) | 739 (49.8)** | 1,850 (30.8) | 367 (26.5)** | 2,486 (41.4) | 577 (41.7) | 3,940 (65.6) | 842 (60.8)** | 216 (3.6) | 130 (9.4)** | NA | NA | NA | |||||||||
| Raposeiras-Roubín et al. [2015] (17) | 3,236 | 2,277 | 959 | 63.8 (12.0) | 67.8 (11.5) | 1,692 (74.3) | 656 (68.4) | 1,236 (54.3) | 542 (56.5) | 540 (23.7) | 266 (27.7) | 669 (29.4) | 243 (25.3) | NA | NA | 77 (3.4) | 247 (25.8) | NA | NA | NA | |||||||||
| Siu et al. [2010] (18) | 208 | 154 | 54 | 62 (1.0) | 65 (1.0)* | 111 (72.1) | 44 (81.5) | 90 (58.4) | 27 (50.0) | 58 (37.7) | 19 (35.2) | 50 (32.5) | 24 (44.4) | 133 (86.4) | 40 (74.1)** | NA | NA | 55 (0.3) | 54 (0.5)* | Metoprolol, carvedilol, bisoprolol, atenolol | |||||||||
| Song et al. [2021] (19) | 6,874 | 5,810 | 1,064 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||||||||
| Wen et al. [2022] (20) | 2,519 | 2,049 | 470 | 62 [52–70] | 64 [55–73]** | 1,624 (79.3) | 378 (80.4) | 1,147 (56) | 249 (53.0) | 643 (31.4) | 138 (29.4) | 1,337 (65.3) | 316 (67.2) | 424 (20.7) | 80 (17.0)* | NA | NA | 58 [55–62] | 60 [56–63]** | NA | |||||||||
| Yndigegn et al. [2024] (21) | 5,020 | 2,508 | 2,512 | 65 [57–73] | 65 [57–73] | 1,945 (77.6) | 1,944 (77.4) | 1,155/2,507 (46.1) | 1,163/2,509 (46.4) | 346/2,506 (13.8) | 354/2,509 (14.1) | 478/2,466 (19.4) | 530/2,483 (21.3) | NA | NA | NA | NA | NA | NA | Metoprolol, bisoprolol | |||||||||
| Silvain et al. [2024] (22) | 3,698 | 1,852 | 1,846 | 63.5 (10.9) | 63.5 (11.2) | 1,531 (82.7) | 1,530 (82.9) | 805 (43.5) | 786 (42.6) | 375 (20.2) | 372 (20.2) | 342 (18.5) | 385 (20.9) | 994 (53.7) | 948 (51.4) | NA | NA | 60 [52–60] | 60 [52–60] | Bisoprolol, acebutolol, atenolol, nebivolol, metoprolol, carvedilol, celiprolol, sotalol, celectol, betaxolol, propranolol, nadolol | |||||||||
Data are presented as n (%), mean (SD), or median [interquartile range]. *, P<0.10; **, P<0.05. BB, beta-blocker; COPD, chronic obstructive pulmonary disease; LVEF, preserved left ventricular function; NA, not available; SD, standard deviation.
We performed predefined subgroup analyzes to explore potential sources of heterogeneity and to assess the robustness of our findings. Subgroups were stratified based on key variables, including age, sex, baseline comorbidities (e.g., hypertension, diabetes mellitus, and dyslipidemia), and study design (cohort vs. RCTs). These subgroup analyses were conducted to evaluate whether these variables influenced the primary outcomes, such as all-cause mortality, reinfarction, and hospitalization for HF.
For sensitivity analysis, we used two approaches to evaluate the robustness of our results:
- Leave-one-out sensitivity analysis: this method systematically excluded one study at a time from the meta-analysis to determine the influence of individual studies on the pooled effect estimates. This approach was applied to assess the stability of the results and identify potential outliers.
- Stratified sensitivity analysis: we stratified studies based on key methodological factors, such as the quality of included studies (e.g., low vs. moderate risk of bias) and the use of propensity score matching in observational studies, to evaluate the consistency of the findings across different study subsets.
For sensitivity analysis, studies classified as having a high risk of bias were excluded. This approach was implemented to assess the robustness of the results when considering only studies with low or moderate risk of bias. These analyses ensured that our conclusions were not disproportionately influenced by a single study or methodological bias, enhancing the robustness and interpretability of the results.
The leave-one-out sensitivity analysis and stratified analyses were conducted using standard meta-analysis software (Review Manager, Cochrane Collaboration). Heterogeneity was assessed for each subgroup and sensitivity analysis using the I2 statistic and Cochran’s Q test, with adjustments to the model where necessary.
Role of the funding source
This study did not receive any external funding. All authors had unrestricted access to the study’s data, and the decision to submit the manuscript for publication was ultimately made by the corresponding author.
Results
Study selection and baseline characteristics
As illustrated in Figure 1, 156 studies were identified, of which 113 were excluded based on review of the title or abstract. 43 were completely revised studies, based on the inclusion criteria. After the final evaluation, 11 manuscripts remained eligible for inclusion (6,13-22) in this meta-analysis. A total of 85,607 patients were included, of which 65,790 (76.8%) were using BBs after MI with preserved ejection fraction. The average age ranged from 57 to 66 years, and 61,444 patients were male (71.8%). Individual study characteristics are reported in Table 1.
Due to the non-randomized nature of most studies, we report baseline characteristics stratified by BB use or not (Table 1). There was a greater occurrence of young people, males, high blood pressure, diabetes mellitus and dyslipidemia in the group that was exposed to BBs. The control group had a higher incidence of patients with COPD.
Risk of bias
We used the Cochrane Collaboration’s tool to assess the ROBINS-I. Of the nine observational studies selected, four were classified as having a low overall risk of bias, while the remaining five were classified as having a moderate risk (Figures 2,3). The risk of bias assessment for the two randomized clinical trials included was conducted using the RoB2 tool, also developed by Cochrane (Figure 4), with both trials classified as having a low risk of bias.



Pooled analysis of all studies
The results are organized into key clinical outcomes, including all-cause mortality, cardiac mortality, MACE, reinfarction, hospitalization for HF, and stroke incidence, an overview of the results of each article can be seen in Table 2. Each outcome is presented with corresponding figures and statistical analyses for clarity.
Table 2
| Studies | Mortality from all causes | Mortality from cardiac causes | MACEs | Reinfarction | Hospitalization for HF | Stroke |
|---|---|---|---|---|---|---|
| Chen et al. [2021] (13) | 0.44 (0.20, 0.96) | 0.28 (0.08, 0.97) | 0.84 (0.49, 1.43) | NA | NA | NA |
| Choo et al. [2014] (14) | 0.58 (0.38, 0.88) | 0.38 (0.22, 0.66) | NA | 1.10 (0.45, 2.69) | 0.05 (0.85, 1.30) | 0.59 (0.26, 1.34) |
| Ishak et al. [2023] (15) | 1.00 (0.92, 1.09) | 0.98 (0.84, 1.15) | NA | 1.00 (0.91, 1.09) | NA | 1.02 (0.89, 1.17) |
| Joo et al. [2021] (6) | 1.27 (0.83, 1.94) | 1.38 (0.85, 2.25) | 1.16 (0.91, 1.48) | 1.23 (0.72, 2.10) | 0.90 (0.54, 1.50) | 2.15 (0.96, 4.82) |
| El Nasasra et al. [2021] (16) | 0.71 (0.45, 1.12) | NA | NA | NA | NA | NA |
| Raposeiras-Roubín et al. [2015] (17) | 0.64 (0.48, 0.86) | NA | NA | NA | NA | NA |
| Siu et al. [2010] (18) | 0.35 (0.15, 0.081) | 0.30 (0.09, 1.02) | NA | NA | NA | NA |
| Song et al. [2021] (19) | 0.85 (0.55, 1.32) | NA | 0.77 (0.54, 1.09) | 0.84 (0.48, 1.47) | 0.86 (0.49, 1.50) | NA |
| Wen et al. [2022] (20) | 1.17 (0.70, 1.95) | 1.36 (0.48, 2.32) | 1.03 (0.80, 1.33) | 1.11 (0.92, 1.10) | 1.63 (0.98, 2.71) | NA |
| Yndigegn et al. [2024] (21) | 0.94 (0.71, 1.24) | 1.15 (0.72, 1.84) | NA | 0.96 (0.74, 1.24) | 0.91 (0.50, 1.66) | NA |
| Silvain et al. [2024] (22) | NA | NA | 0.87 (0.73, 1.03) | NA | NA | NA |
Data are presented as HR (95% CI). CI, confidence interval; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiac event; NA, not available.
The sensitivity analysis included only studies rated as low risk of bias, as assessed using the Cochrane Collaboration’s ROBINS-I and RoB2. We performed a pre-specified subgroup analysis grouping studies that reported HRa for mortality, taking into account several confounding factors. There was no significant difference in the groups regarding sex, age, history of hypertension, or diabetes mellitus.
All-cause mortality
All-cause mortality was lower among BB users compared to non-users in the overall follow-up (HR =0.81; 95% CI: 0.67–0.98; P=0.03), as shown in Figure 5A. However, this analysis exhibited high heterogeneity (I2=67%, P=0.001), indicating considerable variability among the included studies. In the subgroup analysis that evaluated only studies using propensity score matching (Figure 5B), a non-significant trend toward reduced all-cause mortality was observed among BB users (HR =0.83; 95% CI: 0.63–1.09; P=0.18), with heterogeneity remaining high (I2=75%, P=0.001). These findings suggest that while BBs may provide a modest reduction in mortality, this effect is less pronounced and less consistent in analyses adjusted by propensity score.
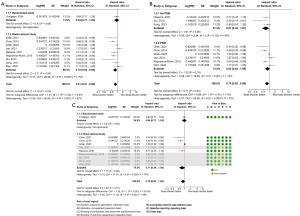
Furthermore, a detailed evaluation of the data revealed that studies with a higher risk of bias seem to have contributed to favoring BBs. In the sensitivity analysis, which excluded high-risk studies and accounted for confounding variables, the all-cause mortality outcome lost its clinical significance (HR =0.79; 95% CI: 0.62–1.02; P=0.07), as demonstrated in Figure 5C.
Cardiac mortality
Our data showed no statistically significant difference between patients who received BB therapy compared to those who were not on BB for cardiac mortality (Figure 6A,6B). In the sensitivity analysis of mortality from cardiovascular causes, BB therapy did not reach statistical significance (Figure 6C).

MACE
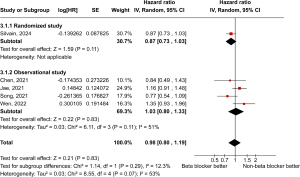
Regarding MACE, our data showed no statistically significant difference between patients who received BB therapy compared to those who were not on BB (Figure 7).
Reinfarction
Our analysis demonstrated no significant differences in the incidence of reinfarction between individuals who used BBs and those who did not, as highlighted in Figure 8.

Hospitalization for HF
Our analysis demonstrated no significant differences in the incidence of hospitalization for HF between individuals who used BBs and those who did not, as highlighted in Figure 9.

Stroke
Our analysis demonstrated no significant differences in the incidence of stroke between individuals who used BBs and those who did not, as highlighted in Figure 10. Although the heterogeneity due to reinfarction and hospitalization for HF was low, the substantial heterogeneity in the incidence of stroke (I2=60%) suggests caution in interpreting this result.

Discussion
This systematic review and meta-analysis revealed that the long-term use of BBs in patients with preserved LVEF after MI does not significantly reduce all-cause mortality, cardiovascular mortality, or MACE. Additionally, no significant differences were observed in the rates of reinfarction, hospitalization for HF, or stroke. These findings challenge the routine use of BBs in this population and suggest a more individualized approach to post-MI management.
Our work shows that the benefit in patients who persist with preserved ventricular function after MI is limited. Since these patients usually do not have significant fibrosis and do not develop significant arrhythmias, long-term use of yet another medication with limited effect can trigger side effects and impair medication adherence to medications that actually change clinical outcomes.
The meta-analysis of the studies presented showed no significant difference in MACE, overall mortality, or cardiovascular mortality. Also, no difference was observed in the occurrence of hospitalization for HF, MI, or stroke. Al-Bawardy et al. (23) demonstrated a reduction in mortality with the use of BBs 1 year after MI through an observational study carried out in Saudi Arabia. Joo et al. (6) also identified this trend in Korea, but both studies used patients with mid-range LVEF after MI.
Kim et al. (24) did not find a reduction in all-cause mortality with the use of BBs for 1 or more years post-MI (OR =0.8; 95% CI: 0.559–1.145). Hu et al. (25), also showed that BBs were not associated with reduced MACE, cardiac death, MI, and HF, however, the use of BBs reduced all-cause mortality in the studied population (OR =0.74; 95% CI: 0.59–0.94). However, data from these studies may be compromised by the inclusion of patients with mild ventricular dysfunction in their analyses.
It is important to highlight that in the post myocardial reperfusion era, we only detected few randomized clinical trial, published in April and August 2024 (21,22), which was aimed at answering whether the long-term use of BBs in patients with preserved ventricular function would change relevant clinical outcomes. However, some observational studies identified in this systematic review performed propensity-matched score with findings similar to those found in meta-analysis.
Hu et al. demonstrated that BB therapy can reduce the risk of all-cause death in patients without HF or left ventricular systolic dysfunction after acute coronary syndrome (25). However, it should be noted that this meta-analysis included observational studies, making it possible for confounding factors to be present. Furthermore, it included patients with reduced and slightly reduced LVEF, making the comparison of their results to ours unreliable, as our objective was based on the evaluation of only patients with preserved LVEF.
The present work is similar to the meta-analysis written by Kim and collaborators, who carried out a meta-analysis of 5 observational studies on the use of BBs in 219,092 patients after acute MI without clinical signs of HF (24). However, two of the studies included by Kim et al. do not specify the ejection fraction of the included patients. In a similar way to the present article, in the work of Kim et al., there was no reduction in mortality from all causes, 1 year after the ischemic event, in patients using BBs compared to the group without BBs.
In line with the results of the present work, Maqsood et al. performed a meta-analysis of randomized clinical trials and observational studies, with a total of 12 studies included, investigating the long-term use of BBs in patients after acute MI with ST-segment elevation and preserved LVEF undergoing coronary intervention percutaneous injection, in a total sample of 32,108 patients (8). This study concluded that the long-term use of BBs was associated with a significant reduction in all-cause mortality, but was not significantly associated with a lower incidence of MACE. Furthermore, due to the limited number of randomized clinical trials included by Maqsood et al., which presented neutrality regarding outcomes in long-term use of BBs, their results must be carefully analyzed (8).
The ABYSS study (22), a French multicenter randomized noninferiority trial, investigated the effects of discontinuing vs. continuing BB therapy in 3,698 patients with a history of uncomplicated MI and a LVEF of at least 40%. The results showed that discontinuing BBs failed to achieve noninferiority compared to continuing therapy. These findings suggest that discontinuation of BBs could worsen outcomes by increasing the likelihood of cardiovascular events in this population, emphasizing the potential risks associated with stopping treatment without clear indications.
There are several ongoing randomized clinical studies that could contribute to these discussions regarding the continued use of BBs in asymptomatic patients with preserved ventricular function after a MI. The DANBLOCK (26) (Danish trial of BB treatment after MI without reduced ejection fraction) recruited approximately 5,700 individuals and is expected to reach 950 primary endpoints until 2025. The BETAMI (27), a Norwegian study, followed up for at least 6 months and the REBOOT trial (28) will provide more evidence to guide the prescription of β-blockers until 2025.
Among the limiting factors of the present work, only two articles specified the BB used by patients and no study reported whether patients reached the maximum dose of this medication. Furthermore, it is noteworthy that the majority of patients were male and aged in their 60s, with the population of women and elderly people being underrepresented.
Conclusions
Long-term BB use in patients with preserved left ventricular function after MI did not decrease all-cause mortality, cardiovascular mortality, or MACE. There was also no identified reduction in hospitalizations for HF, MI, or stroke in the average follow-up of 3 years.
Acknowledgments
We extend our gratitude to the Cardiac Arrhythmia Division-PROCAPE/University of Pernambuco for their support and guidance during this study. We also thank our colleagues and collaborators for their valuable contributions to the development and review of this manuscript.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-368/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-368/prf
Funding: This study was financed in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-368/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Woodruff RC, Tong X, Khan SS, et al. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010-2022. Am J Prev Med 2024;66:582-9. [Crossref] [PubMed]
- Salari N, Morddarvanjoghi F, Abdolmaleki A, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord 2023;23:206. [Crossref] [PubMed]
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720-826. Erratum in: Eur Heart J 2024;45:1145. [Crossref] [PubMed]
- Writing Committee Members. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2023;82:833-955. Erratum in: J Am Coll Cardiol 2023;82:1808 Erratum in: J Am Coll Cardiol 2024;83:1716. [Crossref] [PubMed]
- Konishi H, Miyauchi K, Kasai T, et al. Long-term effect of β-blocker in ST-segment elevation myocardial infarction in patients with preserved left ventricular systolic function: a propensity analysis. Heart Vessels 2016;31:441-8. [Crossref] [PubMed]
- Joo SJ, Kim SY, Choi JH, et al. Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother 2021;7:475-82. [Crossref] [PubMed]
- Ferreira JA, Baptista RM, Monteiro SR, et al. Usefulness of universal beta-blocker therapy in patients after ST-elevation myocardial infarction. Medicine (Baltimore) 2021;100:e23987. [Crossref] [PubMed]
- Maqsood MH, Alam M, Atar D, et al. Efficacy of Long-Term Oral Beta-Blocker Therapy in Patients Who Underwent Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction With Preserved Left Ventricular Ejection Fraction: A Systematic Review and Meta-analysis. J Cardiovasc Pharmacol 2021;77:87-93. [Crossref] [PubMed]
- Misumida N, Harjai K, Kernis S, et al. Does Oral Beta-Blocker Therapy Improve Long-Term Survival in ST-Segment Elevation Myocardial Infarction With Preserved Systolic Function? A Meta-Analysis. J Cardiovasc Pharmacol Ther 2016;21:280-5. [Crossref] [PubMed]
- Safi S, Sethi NJ, Korang SK, et al. Beta-blockers in patients without heart failure after myocardial infarction. Cochrane Database Syst Rev 2021;11:CD012565. [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Chen RZ, Liu C, Zhou P, et al. Prognostic impacts of β-blockers in acute coronary syndrome patients without heart failure treated by percutaneous coronary intervention. Pharmacol Res 2021;169:105614. [Crossref] [PubMed]
- Choo EH, Chang K, Ahn Y, et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart 2014;100:492-9. [Crossref] [PubMed]
- Ishak D, Aktaa S, Lindhagen L, et al. Association of beta-blockers beyond 1 year after myocardial infarction and cardiovascular outcomes. Heart 2023;109:1159-65. [Crossref] [PubMed]
- El Nasasra A, Beigel R, Klempfner R, et al. Comparison of outcomes with or without beta-blocker therapy after acute myocardial infarction in patients without heart failure or left ventricular systolic dysfunction (from the Acute Coronary Syndromes Israeli Survey [ACSIS]). Am J Cardiol 2021;143:1-6. [Crossref] [PubMed]
- Raposeiras-Roubín S, Abu-Assi E, Redondo-Diéguez A, et al. Prognostic Benefit of Beta-blockers After Acute Coronary Syndrome With Preserved Systolic Function. Still Relevant Today? Rev Esp Cardiol (Engl Ed) 2015;68:585-91. [Crossref] [PubMed]
- Siu CW, Pong V, Jim MH, et al. Beta-blocker in post-myocardial infarct survivors with preserved left ventricular systolic function. Pacing Clin Electrophysiol 2010;33:675-80. [Crossref] [PubMed]
- Song PS, Kim M, Seong SW, et al. Heart failure with mid-range ejection fraction and the effect of β-blockers after acute myocardial infarction. Heart Vessels 2021;36:1848-55. [Crossref] [PubMed]
- Wen XS, Luo R, Liu J, et al. Short-term/long-term prognosis with or without beta-blockers in patients without heart failure and with preserved ejection fraction after acute myocardial infarction: a multicenter retrospective cohort study. BMC Cardiovasc Disord 2022;22:193. [Crossref] [PubMed]
- Yndigegn T, Lindahl B, Mars K, et al. Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction. N Engl J Med 2024;390:1372-81. [Crossref] [PubMed]
- Silvain J, Cayla G, Ferrari E, et al. Beta-Blocker Interruption or Continuation after Myocardial Infarction. N Engl J Med 2024;391:1277-86. [Crossref] [PubMed]
- Al-Bawardy R, Alqarawi W, Al Suwaidi J, et al. The Effect of Beta-Blocker Post-Myocardial Infarction With Ejection Fraction >40% Pooled Analysis From Seven Arabian Gulf Acute Coronary Syndrome Registries. Angiology 2025;76:476-86. [PubMed]
- Kim Y, Byun S, Kim HY, et al. Long-Term Beta-Blocker Therapy After Myocardial Infarction Without Heart Failure in the Reperfusion Era-Systemic Review and Meta-analysis. J Cardiovasc Pharmacol 2022;79:650-4. [Crossref] [PubMed]
- Hu MJ, Wang XN, Tan JS, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients without heart failure or left ventricular systolic dysfunction after acute coronary syndrome: An updated systematic review and meta-analysis. Arch Cardiovasc Dis 2022;115:637-46. [Crossref] [PubMed]
- Kristensen AMD, Munkhaugen J, Halvorsen S, et al. The Danish-Norwegian randomized trial on beta-blocker therapy after myocardial infarction: Design, rationale, and baseline characteristics. Eur Heart J Cardiovasc Pharmacother 2024;10:175-83. [Crossref] [PubMed]
- Munkhaugen J, Ruddox V, Halvorsen S, et al. BEtablocker Treatment After acute Myocardial Infarction in revascularized patients without reduced left ventricular ejection fraction (BETAMI): Rationale and design of a prospective, randomized, open, blinded end point study. Am Heart J 2019;208:37-46. [Crossref] [PubMed]
- Rossello X, Raposeiras-Roubin S, Latini R, et al. Rationale and design of the pragmatic clinical trial tREatment with Beta-blockers after myOcardial infarction withOut reduced ejection fracTion (REBOOT). Eur Heart J Cardiovasc Pharmacother 2022;8:291-301. [Crossref] [PubMed]



