
Development of peripheral biomarker-based prognostic nomograms for short-term and long-term survival in immune checkpoint inhibitor-associated myocarditis
Highlight box
Key findings
• We developed two prognostic model to evaluate the overall survival (OS) of immune checkpoint inhibitor (ICI) myocarditis patients, utilizing critical biomarkers derived from peripheral blood parameters.
What is known and what is new?
• ICI myocarditis is a rare but highly fatal immune related adverse event. Certain cardiac markers and peripheral biomarkers are associated with OS in patients with ICI myocarditis.
• N-terminal pro-brain natriuretic peptide (NTBNP), cardiac troponin-I (cTnI), lactate dehydrogenase-to-albumin ratio (LAR), and systemic inflammatory response index (SIRI) were identified as key biomarkers. Two nomogram prognostic models were developed based on these key factors to assess the 40-day and 1-year OS of patients with ICI myocarditis, which demonstrated great performance and clinical utility
What is the implication, and what should change now?
• Two convenient and efficient survival models were established for clinical use. Further research is essential to identify more reliable assessment methods that can aid in the evaluation of prognosis and facilitate early intervention.
Introduction
The application of immune checkpoint inhibitors (ICIs) has significantly advanced the field of oncology. ICIs stimulate immune cells and enhance the host immune response to eliminate cancer cells (1,2), and are currently used in the treatment of approximately 20 types of cancer, including advanced, metastatic, first-line, and adjuvant settings (1,3). However, the administration of ICIs has the potential to induce immune-related adverse events (irAEs) that may affect various systems, including the cardiovascular system (4-6). ICI-associated myocarditis (ICI myocarditis) is a rare but highly fatal irAEs, with an incidence rate of approximately 0.3–1.7% (7-10) and a mortality rate of around 50% (11-14).
While diagnostic methods for this disease continue to evolve (15,16), the methods available for prognosis evaluation remain limited, particularly the lack of a clinically applicable prognostic model. This presents a significant challenge for both oncologists and cardiologists. Some cardiac magnetic resonance (CMR) parameters are related to the prognosis of myocarditis (17,18), but they are not convenient for widespread use. Echocardiography and electrocardiography (ECG) can aid in prognostic assessment (19,20); however, not all patients exhibit specific findings (10,21). The levels of cardiac biomarkers are associated with the prognosis of patients with ICI myocarditis; however, not all cardiac biomarkers exhibit abnormal elevations (22). Additionally, the levels of cardiac markers may be influenced by other cardiovascular diseases (23). Therefore, further research is needed to identify more valuable indicators.
In recent years, some emerging combination biomarkers have received widespread attention. Increase in neutrophil-to-lymphocyte ratio (NLR) is identified as a potential predictor of poor prognosis (24). Zhuang et al. (25) found that platelet-to-lymphocyte ratio (PLR), aspartate transferase-to-albumin ratio (AAR), and lactate dehydrogenase-to-albumin ratio (LAR) were associated with short-term mortality in patients with myocarditis. Higher neutrophil-to-eosinophil ratio (NER) is also correlated with a higher mortality rate from immune-related cardiotoxicity (26). Additionally, systemic inflammatory index (SII) and systemic inflammatory response index (SIRI) have been implicated in the prognosis of patients undergoing immunotherapy and the occurrence of immune-related adverse events, as well as the outcomes of cardiovascular diseases (27-30). Moreover, monocyte-to-lymphocyte ratio (MLR) is also related to the prognosis of patients with myocarditis or other cardiovascular diseases (31,32).
Therefore, in this study, we further investigated the associations between combination biomarkers, conventional markers, and cardiac markers in peripheral blood and the overall survival (OS) of patients with ICI myocarditis, all of which are readily accessible indicators. Additionally, we identified critical risk factors and developed two survival models for 40-day and 1-year OS in patients with ICI myocarditis, which will assist clinicians in more effectively identifying high-risk patients and implementing timely interventions. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-556/rc).
Methods
Study population
This was a single-center retrospective study. Inclusion criteria: patients who received immunotherapy and diagnosed with ICI myocarditis at the Fourth Hospital of Hebei Medical University between January 2019 and June 2024 were consecutively enrolled in this study. All patients were diagnosed with solid tumors and received at least one cycle of immunotherapy. Exclusion criteria: patients who were lost to follow-up or had incomplete data were excluded. The diagnosis of ICI myocarditis was made using clinical criteria (major and minor) according to the 2022 European Society of Cardiology (ESC) Guidelines on cardio-oncology (16): cardiac troponin elevation with CMR findings diagnostic of acute myocarditis or at least two minor criteria including clinical syndrome, ECG changes, decline in left ventricular systolic function and other irAEs. This study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2024KS048) and complied with the Declaration of Helsinki (as revised in 2013). Since this is a retrospective study that only analyzes existing clinical data and does not involve additional interventions or the collection of personal information from patients, informed consent has been waived.
Study protocol and data collection
Baseline clinical characteristics of the patients and laboratory tests at the onset of myocarditis were collected. Data on cardiac markers and echocardiographic parameters prior to ICI administration and at the onset of myocarditis were also collected. In addition to standard laboratory tests, we investigated the association between a series of combination biomarkers and the prognosis of patients with ICI myocarditis. Peripheral blood data, including absolute neutrophil count (NE), absolute eosinophil count (EO), absolute monocyte count (MO), absolute lymphocyte count (LY), platelet count (PLT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and albumin (ALB), were utilized for the calculation of these combination biomarkers based on previous literature (24-27,31): SII = NE × PLT/LY, SIRI = NE × MO/LY, NLR = NE/LY, NER = NE/EO, MLR = MO/LY, AAR = AST/ALB, and LAR = LDH/ALB. OS time was defined as the duration from the initial diagnosis of myocarditis to the occurrence of all-cause mortality. The primary endpoints for follow-up were all-cause mortality assessed at 40 days and 1 year. The cutoff date for follow-up was September 10, 2024.
Statistical analysis
Continuous variables were reported as mean ± standard deviation or median and interquartile range and were compared using the t-test or Mann-Whitney U test. Categorical variables were presented as numbers and percentages, and comparisons were performed using the Chi-squared test. The Cox proportional hazards regression model was employed to investigate the association between peripheral blood biomarkers and 40-day or 1-year OS. Then the aforementioned risk factors were subjected to least absolute shrinkage and selection operator (LASSO) regression analysis to identify critical biomarkers. Two nomogram prediction models for 40-day and 1-year OS of ICI myocarditis were developed separately based on Cox regression, including biomarkers screened by LASSO regression. A test of the proportional hazards assumption was conducted on the Cox model, and the hypothesis was validated. The predictive performance of the two models were assessed using the concordance index (C-index) and the area under the curve (AUC) of the receiver operating characteristic (ROC) analysis. Brier scores were also calculated to reflect the accuracy of the model. The calibration curves were derived based on Cox regression analysis utilizing bootstrap methods to evaluate the consistency of the models. The decision curve analysis (DCA) was performed to evaluate the clinical utility of the two nomograms. The Kaplan-Meier (K-M) method was employed to compare the 40-day and 1-year OS of high-risk and low-risk patients stratified by the two models respectively. Statistical analysis was performed using SPSS 27 and R software (version 4.4.1), with the significance level set at P<0.05 based on a two-sided test.
Results
Clinical characteristics of patients with ICI myocarditis
Among the 8,133 cancer patients treated with ICIs at our institution, 95 were diagnosed with ICI myocarditis. Following the exclusion of cases with incomplete data and those lost to follow-up, a total of 90 patients were ultimately included in this study (Figure 1A), and their survival and clinical data were subsequently analyzed. All cases were clinically diagnosed by two cardiovascular specialists based on the patients’ clinical symptoms, cardiac biomarkers, ECG, echocardiography, and CMR results. Acute coronary syndrome was excluded through coronary angiography or coronary computed tomography angiography. However, considering the patient’s own wishes, the risk of the procedure and the disease condition, we did not perform an endomyocardial biopsy (EMB). In the 40-day follow-up after ICI myocarditis, 24 (26.7%) patients succumbed. During the one-year follow-up period, a total of 40 (44.4%) patients expired. By the end of the follow-up, a total of 45 (50%) patients died (Figure 1A). The most prevalent tumor types in the cohort were esophageal carcinoma, lung cancer, and gastric cancer, with the majority of patients receiving anti-programmed cell death protein 1 (PD-1) therapy (Table 1, Figure S1). Other concomitant irAEs were presented in 43 (47.8%) patients, among which myositis was the most frequently observed (Figure 1B). Patients with ICI myocarditis exhibited a significant elevation in cardiac biomarkers and a reduction in left ventricular ejection fraction (LVEF). Among them, all patients had elevated cardiac troponin-I (cTnI) levels, but not every individual demonstrated increases in creatine kinase isoenzyme-MB (CK-MB) and N-terminal pro-brain natriuretic peptide (NTBNP), as well as reduced LVEF (Figure 1C-1F). Upon diagnosis of ICI myocarditis, ICI therapy was halted, and patients received corticosteroid treatment at varying doses tailored to their condition. In severe cases, supplementary treatments like immunosuppressive medications, blood purification, or immunoglobulin infusion were administered. The clinical characteristics of patients in survival group and non-survival group are presented in Table 1.
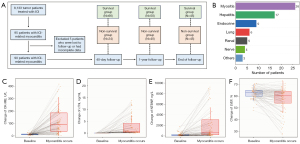
Table 1
| Characteristics | All patients (n=90) | Survival group (n=45) | Non-survival group (n=45) | P value |
|---|---|---|---|---|
| Age (years) | 64.48±10.93 | 64.42±11.06 | 64.53±10.92 | 0.96 |
| BMI (kg/m2) | 23.21±3.67 | 24.01±3.81 | 22.41±3.37 | 0.04 |
| Male | 62 (68.9) | 30 (66.7) | 32 (71.1) | 0.65 |
| Smoking | 36 (40.0) | 16 (35.6) | 20 (44.4) | 0.39 |
| Drinking | 17 (18.9) | 9 (20.0) | 8 (17.8) | 0.79 |
| CAD | 12 (13.3) | 5 (11.1) | 7 (15.6) | 0.54 |
| Hypertension | 23 (25.6) | 13 (28.9) | 10 (22.2) | 0.47 |
| Diabetes | 12 (13.3) | 6 (13.3) | 6 (13.3) | >0.99 |
| Tumor type | 0.61 | |||
| Lung cancer | 23 (25.6) | 10 (22.2) | 13 (28.9) | |
| Esophageal carcinoma | 24 (26.7) | 15 (33.3) | 9 (20.0) | |
| Gastric cancer | 15 (16.7) | 6 (13.3) | 9 (20.0) | |
| liver cancer | 7 (7.8) | 4 (8.9) | 3 (6.7) | |
| Other tumors | 21 (23.3) | 10 (22.2) | 11 (24.4) | |
| Tumor stage | 0.02 | |||
| < III | 47 (52.2) | 29 (64.4) | 18 (40.0) | |
| IV | 43 (47.8) | 16 (35.6) | 27 (60.0) | |
| Therapy mode | ||||
| Combined chemotherapy | 78 (86.7) | 37 (82.2) | 41 (91.1) | 0.22 |
| Combined targeted-therapy | 18 (20.0) | 10 (22.2) | 8 (17.8) | 0.60 |
| Combined radiotherapy | 24 (26.7) | 10 (22.2) | 14 (31.1) | 0.34 |
| Type of ICIs | >0.99 | |||
| Anti-PD-1 | 80 (88.9) | 40 (88.9) | 40 (88.9) | |
| Others | 10 (11.1) | 5 (11.1) | 5 (11.1) | |
| Medication cycles | 2 [1, 3] | 2 [1, 3] | 2 [1, 3.25] | 0.68 |
| Time to myocarditis (days) | 42 [26, 88] | 32 [26, 84] | 46 [25, 116] | 0.40 |
| Concomitant irAEs | 43 (47.8) | 21 (46.7) | 22 (48.9) | 0.83 |
| LVEF at myocarditis onset | 60.5 [54, 64] | 62 [58, 65] | 58 [50, 63] | 0.008 |
| Steroid dosage† | 0.22 | |||
| Low-dosage steroid | 46 (51.1) | 21 (46.7) | 25 (55.6) | |
| High-dosage steroid | 21 (23.3) | 14 (31.1) | 7 (15.6) | |
| Other treatments for ICI myocarditis | 15 (16.7) | 12 (26.7) | 3 (6.7) | 0.01 |
Data are presented as mean ± standard deviation, median [interquartile range], or n (%). †, in our study population, oral or intravenous steroid therapy ≤80 mg/day is regarded as the low-dose group, while any higher dose or high-dose pulse treatment is regarded as the high-dose group. BMI, body mass index; CAD, coronary heart disease; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; LVEF, left ventricular ejection fraction; PD-1, programmed cell death protein 1.
Development and assessment of the nomogram for the 40-day OS of ICI myocarditis
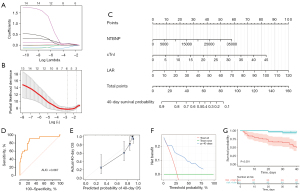
In this study, we aimed to establish a predictive model for the 40-day OS of ICI myocarditis based on peripheral blood biomarkers. Univariate Cox regression analysis showed that 14 factors, including cardiac markers, conventional indicators, and combined biomarkers, were associated with the OS of patients with ICI myocarditis (Table 2). Then the 14 risk factors were subjected to LASSO-Cox regression analysis to identify critical biomarkers (Figure 2A). Tenfold cross-validation was performed and NE, ALB, cTnI, NTBNP, NER, LAR were selected at the criterion of λ by minimum values, while cTnI, NTBNP and LAR were still significantly correlated with OS at the optimum value of the parameter λ by 1 – standard error (s.e.) (Figure 2B). Considering the sample size and the complexity of the model, we ultimately selected cTnI, NTBNP, and LAR as key biomarkers and developed a nomogram predictive model for the 40-day OS in patients with ICI myocarditis (Figure 2C). ROC curve was plotted to evaluate the model’s performance in predicting 40-day survival rates, with an AUC of 0.867 (95% CI: 0.774−0.960) (Figure 2D). The C-index for the nomogram model was 0.824 and was 0.812 after bootstrapping validation, indicating that the model has excellent discriminative ability. The calibration curve illustrates a great concordance between the model’s predictions and the actual observations (Figure 2E). The Brier score for this model is 0.12, indicating good accuracy. DCA indicates that when the threshold probability ranges from 10% to 80%, the application of the model yields more benefits (Figure 2F). Subsequently, we employed this model to stratify patients into high-risk and low-risk groups. The K-M analysis revealed that the high-risk group exhibited a significantly poorer 40-day OS (Figure 2G). In addition to the peripheral blood parameters, we also analyzed the relationship between the clinical features and their 40-day OS, of which only LVEF was related to the prognosis of the patients (Table S1).
Table 2
| Biomarkers | 40-day OS | 1-year OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| NE | 1.266 (1.133, 1.415) | <0.001 | 1.199 (1.079, 1.332) | <0.001 | |
| LY | 0.387 (0.111, 1.343) | 0.14 | 0.4485 (0.196, 1.201) | 0.12 | |
| MO | 1.969 (0.940, 4.162) | 0.07 | 2.392 (1.298, 4.406) | 0.005 | |
| EO | 0.001 (0.001, 1.098) | 0.052 | 0.249 (0.010, 6.182) | 0.40 | |
| PLT | 1.001 (0.996, 1.005) | 0.71 | 1.000 (0.997, 1.004) | 0.87 | |
| ALT | 1.003 (1.003, 1.005) | <0.001 | 1.002 (1.001, 1.004) | 0.007 | |
| AST | 1.003 (1.001, 1.004) | <0.001 | 1.002 (1.001, 1.004) | 0.001 | |
| ALB | 0.862 (0.793, 0.936) | <0.001 | 0.920 (0.863, 0.980) | 0.01 | |
| CK-MB | 1.001 (1.000, 1.002) | 0.15 | 1.000 (0.999, 1.001) | 0.51 | |
| LDH | 1.001 (1.001, 1.001) | <0.001 | 1.001 (1.001, 1.001) | <0.001 | |
| cTnI | 1.122 (1.071, 1.176) | <0.001 | 1.113 (1.067, 1.162) | <0.001 | |
| NTBNP | 1.000 (1.000, 1.000) | <0.001 | 1.000 (1.000, 1.000) | <0.001 | |
| SII | 1.000 (1.000, 1.000) | 0.005 | 1.000 (1.000, 1.000) | 0.004 | |
| SIRI | 1.037 (1.008, 1.068) | 0.01 | 1.043 (1.017, 1.069) | <0.001 | |
| NLR | 1.033 (1.010, 1.057) | 0.005 | 1.033 (1.012, 1.055) | 0.002 | |
| PLR | 1.001 (0.999, 1.003) | 0.20 | 1.001 (0.999, 1.003) | 0.20 | |
| NER | 1.001 (1.001, 1.002) | 0.002 | 1.001 (1.000, 1.002) | 0.04 | |
| MLR | 1.506 (1.041, 2.177) | 0.03 | 1.637 (1.205, 2.223) | 0.002 | |
| AAR | 1.111 (1.060, 1.164) | <0.001 | 1.092 (1.044, 1.143) | <0.001 | |
| LAR | 1.037 (1.023, 1.052) | <0.001 | 1.034 (1.020, 1.048) | <0.001 | |
AAR, aspartate transferase-to-albumin ratio; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CK-MB, creatine kinase isoenzyme; cTnI, cardiac troponin-I; EO, absolute eosinophil count; HR, hazard ratio; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; LAR, lactic dehydrogenase-to-albumin ratio; LY, absolute lymphocyte count; MLR, monocyte-to-lymphocyte ratio; MO, absolute monocyte count; NE, absolute neutrophil count; NER, neutrophil-to-eosinophil ratio; NLR, neutrophil-to-lymphocyte ratio; NTBNP, N-terminal pro-brain natriuretic peptide; OS, overall survival; PLR, platelet-to-lymphocyte ratio; PLT, platelet count; SII, systemic immune-inflammation index; SIRI, system inflammation response index.
Development and assessment of the nomogram for the 1-year OS of ICI myocarditis
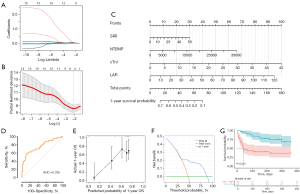
We employed a similar methodology to develop a predictive model for the 1-year OS of ICI myocarditis based on peripheral blood biomarkers. Univariate Cox regression analysis showed that 15 factors were associated with the OS of patients with ICI myocarditis (Table 2), which were then subjected to LASSO-Cox regression analysis to identify critical biomarkers (Figure 3A). Tenfold cross-validation was performed and SIRI, cTnI, NTBNP, NER, LAR were selected at the criterion of λ by minimum values (Figure 3B). We then developed a nomogram prognostic model based on SIRI, cTnI, NTBNP, and LAR for the 1-year OS in patients with ICI myocarditis (Figure 3C). ROC curve was plotted to evaluate the model’s performance in predicting 40-day survival rates, with an AUC of 0.765 (95% CI: 0.664−0.866) (Figure 3D). The C-index for the nomogram model was 0.742 and was 0.728 after bootstrapping validation. The calibration curve shows a great concordance between the model’s predictions and the actual observations (Figure 3E). The Brier score for this model is 0.18, indicating good accuracy. DCA reveals that when the threshold probability ranges from 35% to 87%, the application of the model added more benefits (Figure 3F). When we utilized this model to stratify patients into high-risk and low-risk group, the K-M analysis demonstrated that the high-risk group exhibited a significantly lower 1-year OS (Figure 3G). Besides, the relationship between clinical characteristics and the 1-year OS was also assessed, and only LVEF was related to the prognosis of the patients (Table S1).
Dynamic nomogram and web-based app
To promote the broad use of the models, we developed dynamic nomograms for both models and made them available on our websites: https://hebmugzk.shinyapps.io/DynNomapp40DAY/ (Figure 4A) and https://hebmugzk.shinyapps.io/DynNomapp1YEAR/ (Figure 4B). Clinicians can modify the values of different parameters according to the patient’s clinical information to calculate the predicted survival rate. As this is a single-center retrospective study, we did not have access to data for external validation of the model. To address this, we developed a web-based app and made it available on https://hebmuguan.shinyapps.io/APP-for-OS-model/ (Figure 5). Researchers from other institutions can use their own data as an external validation dataset, following the usage instructions provided on the site. They can upload a .csv file and then click the corresponding buttons to perform model validation for 40-day and 1-year survival rates, and generate calibration curves and DCA curves.


Discussion
In this retrospective study, we separately identified key biomarkers from peripheral blood associated with the 40-day and 1-year OS of patients with ICI myocarditis, and subsequently developed two nomogram prognostic models for 40-day and 1-year OS respectively. To the best of our knowledge, this is the first study to develop visual and efficient nomogram models for predicting the OS of patients with ICI myocarditis, aiming to help clinicians in evaluating for both short-term and long-term survival rates and facilitating timely interventions.
ICI myocarditis poses a significantly higher risk compared to other irAEs and has attracted significant attention from both oncologists and cardiovascular specialists (5). Among patients with varying degrees of disease severity, mortality represents the most adverse final outcome for patients diagnosed with ICI myocarditis who exhibit a poor prognosis. However, research investigating prognostic factors associated with OS in patients with ICI myocarditis remain limited (25,26,33). Although certain cardiac and peripheral blood biomarkers can be utilized to assess the OS of ICI myocarditis, a convenient and effective predictive model remains absent, hindering clinicians’ ability to readily apply these indicators. Therefore, we separately analyzed the influencing factors for 40-day and 1-year OS and developed two nomogram models for short-term and long-term prognosis based on readily accessible peripheral blood biomarkers. In our cohort, the mortality rate for myocarditis patients at 40 days was 26%, while the 1-year mortality rate reached 44%. This is similar to the short-term mortality rate reported by Zhuang et al. (25), but slightly lower than the 1-year mortality rates documented by Itzhaki Ben Zadok et al. (14). These subtle differences may be attributed to variations in tumor type, disease severity, and other factors among patients from different cohorts. These findings reflect a high short-term mortality rate among patients with myocarditis, which continues to rise over time. This poor OS can be attributed not only to myocarditis itself and its associated cardiovascular complications, but also to the interruption of effective anti-cancer treatment (34).
Among a wide array of cardiac markers, conventional indicators, and combined biomarkers, cTnI, NTBNP, and LAR were finally identified as critical indicators significantly associated with 40-day survival rates, and were used to construct the subsequent prediction model. Cardiac troponin, particularly cTnI, has been used for the diagnosis of ICI myocarditis (16). Previous studies have also shown that elevated troponin levels are correlated with major adverse cardiovascular events (MACEs) and poor survival rates in patients with myocarditis (10,22,33,35). Compared to them, our study highlights the role of cTnI, rather than cardiac troponin T (cTnT), which is consistent with the study by Zhuang et al. (25), suggesting the role of cTnI in evaluating the short-term mortality rate of patients with myocarditis. cTnI is considered to have a higher specificity, while cTnT has a higher sensitivity (16,22,36). An elevation in cardiac troponin levels reflects severe myocardial injury and may lead to cardiac dysfunction, resulting in poor prognosis and even death (37,38). NTBNP is a well-established biomarker for heart failure diagnosis, but not all patients with myocarditis have significantly elevated NTBNP levels, similar to the findings of Mahmood et al. (10). While Zhuang et al. did not establish a correlation between BNP and short-term OS, our study (25), based on a larger sample size, found a relationship between NTBNP and OS, which further indicates that patients with ICI myocarditis and concomitant cardiac dysfunction have a worse survival rate. LDH is extensively distributed in cardiac tissue, and elevated LDH levels may indicate myocardial damage and inflammation (39,40). ALB not only reflects the nutritional status of cancer patients, but also plays an important role in inflammation and immune responses (41,42). Consequently, an elevated LAR level may comprehensively reflect the patient’s inflammatory status, nutritional condition, and myocardial injury, thereby influencing their prognosis. A predictive model for the 40-day OS in patients with ICI myocarditis was developed based on cTnI, NTBNP, and LAR. The combined use of these indicators also suggests that, among various factors, the short-term risk of death in ICI myocarditis patients may be more closely related to the extent of myocardial injury and the patient’s overall condition, particularly their tolerance to heart function damage and inflammatory response. However, this view requires further in-depth research into the pathophysiological mechanisms and more clinical findings to confirm. The model showed good discriminatory ability, with an AUC of 0.867 (95% CI: 0.774–0.960) and a C index of 0.824. DCA result suggests that the application of the model provides greater benefits when the threshold probability ranges from 10% to 80%, indicating its substantial clinical applicability.
As for the long-term prognosis of patients with ICI myocarditis, cTnI, NTBNP, LAR, and SIRI were identified as key factors associated with OS. It is worth noting that cTnI, NTBNP and LAR are significantly associated with both the 40-day and 1-year survival rates, which also indicates that the extent of myocardial injury and cardiac function significantly influence both short-term and long-term patient prognosis. Additionally, LDH levels also reflect the degree of tumor burden to some extent (43), thereby enabling LAR to reflect both myocardial injury and tumor status in patients, which subsequently influences long-term survival rates (25). SIRI is composed of neutrophils, monocytes, and lymphocytes, and has been reported to be associated with mortality in myocardial infarction patients as well as with coronary atherosclerosis (44). Additionally, in cancer patients treated with ICIs, higher levels of SIRI are linked to poorer OS and progression-free survival (45). However, its association with prognosis in patients with ICI myocarditis has not been fully explored. In tumor tissues, infiltrating neutrophils and monocytes may promote tumor growth and counteract immune responses by enhancing angiogenesis and releasing cytokines, while lymphocytes can suppress tumor occurrence and recurrence through cytotoxic effects and cytokine release, playing a crucial role in cell-mediated anti-cancer responses (45). Previous research has demonstrated that the infiltration, activation, and reciprocal regulation of neutrophils, lymphocytes, and monocytes-macrophages are intricately associated with the pathogenesis of ICI myocarditis (46,47). Therefore, SIRI may comprehensively reflect the inflammatory infiltration state of myocarditis and the immune and inflammatory status in cancer patients with ICI myocarditis, which could be associated with long-term prognosis (30). Further investigation into the underlying mechanisms is warranted. A predictive model for the 1-year OS in patients with ICI myocarditis was developed based on cTnI, NTBNP, LAR and SIRI. This suggests that the long-term mortality risk in patients with ICI myocarditis is influenced not only by myocardial damage and its physiological consequences, but also by the patient’s prolonged tumor burden, tumor progression, and inherent immune-inflammatory status, reflecting a complex interplay of multiple factors. The model showed good discriminatory ability, with an AUC of 0.765 (95% CI: 0.664−0.866) and a C index of 0.742. DCA result suggests that the application of the model provides greater benefits when the threshold probability ranges from 35% to 87%, indicating its substantial clinical applicability.
In summary, we have developed two prognostic models that can be used to evaluate the 40-day and 1-year OS of patients with ICI myocarditis, with key predictive factors comprising easily accessible peripheral blood markers. This advancement will assist clinicians in identifying high-risk patients with poor prognosis and facilitating timely interventions. Our study also has some limitations. First, this was a single-center retrospective study, which inevitably has a selection bias and limits the generalizability of the findings. Owing to the low incidence rate of the disease and the limited sample size, we were unable to perform external validation of our model and instead conducted internal validation, which may introduce bias in the assessment of the model’s performance. However, we have also created a dynamic nomogram and an app for external validation of the model, both of which are made available on our website. This will facilitate the widespread application and validation of the model. Secondly, considering the patient’s wishes, the disease status, and the associated procedural risks, we did not perform an EMB. Third, during the retrospective follow-up, the causes of death for many patients were unclear, so we were unable to further distinguish between cardiovascular death and non-cardiovascular deaths such as tumor progression, and perform corresponding subgroup analyses. Finally, considering the patients’ preferences and physical tolerance, not all patients in our cohort underwent CMR, and as a result, we did not include parameters from CMR in our model. Nevertheless, we established a predictive model based on easily accessible biomarkers from peripheral blood, which demonstrates good accuracy and practical value. Further multicenter prospective research is needed, including a detailed analysis of various examination and laboratory indicators, such as CMR and echocardiographic parameters, troponin T, and a range of inflammatory factors. A more complete follow-up of cases is also crucial in order to develop a more convenient and reliable survival model for ICI-related myocarditis.
Conclusions
In this study, we investigated a series of peripheral blood biomarkers that have prognostic value for short-term and long-term survival rates in patients with ICI myocarditis based on their clinical data, particularly cTnI, NTBNP, LAR and SIRI. Based on these key factors, we further developed two nomogram prognostic models to assess the 40-day and 1-year OS of patients with ICI myocarditis, which demonstrated great performance and clinical utility during the initial evaluation and validation process. These findings will enable clinicians to more effectively utilize readily available peripheral blood biomarkers for the convenient and efficient identification of high-risk patients with poor prognoses, thereby facilitating early intervention.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-556/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-556/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-556/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-556/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2024KS048) and complied with the Declaration of Helsinki (as revised in 2013). Since this is a retrospective study that only analyzes existing clinical data and does not involve additional interventions or the collection of personal information from patients, informed consent has been waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan S, Day D, Nicholls SJ, et al. Immune Checkpoint Inhibitor Therapy in Oncology: Current Uses and Future Directions: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2022;4:579-97. [Crossref] [PubMed]
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov 2022;21:509-28. [Crossref] [PubMed]
- Zhang L, Reynolds KL, Lyon AR, et al. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol 2021;3:35-47. [Crossref] [PubMed]
- Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. [Crossref] [PubMed]
- Frascaro F, Bianchi N, Sanguettoli F, et al. Immune Checkpoint Inhibitors-Associated Myocarditis: Diagnosis, Treatment and Current Status on Rechallenge. J Clin Med 2023;12:7737. [Crossref] [PubMed]
- Waliany S, Neal JW, Reddy S, et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol 2021;3:137-9. [Crossref] [PubMed]
- Dolladille C, Akroun J, Morice PM, et al. Cardiovascular immunotoxicities associated with immune checkpoint inhibitors: a safety meta-analysis. Eur Heart J 2021;42:4964-77. [Crossref] [PubMed]
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24-34. [Crossref] [PubMed]
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755-64. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579-89. [Crossref] [PubMed]
- Moslehi JJ, Salem JE, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [Crossref] [PubMed]
- Itzhaki Ben Zadok O, Levi A, Divakaran S, et al. Severe vs Nonsevere Immune Checkpoint Inhibitor-Induced Myocarditis: Contemporary 1-Year Outcomes. JACC CardioOncol 2023;5:732-44. [Crossref] [PubMed]
- Herrmann J, Lenihan D, Armenian S, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022;43:280-99. [Crossref] [PubMed]
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229-361. [Crossref] [PubMed]
- Cadour F, Cautela J, Rapacchi S, et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor-induced Myocarditis. Radiology 2022;303:512-21. [Crossref] [PubMed]
- Thavendiranathan P, Zhang L, Zafar A, et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients With Immune Checkpoint Inhibitor-Associated Myocarditis. J Am Coll Cardiol 2021;77:1503-16. [Crossref] [PubMed]
- Awadalla M, Mahmood SS, Groarke JD, et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol 2020;75:467-78. [Crossref] [PubMed]
- Zlotoff DA, Hassan MZO, Zafar A, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer 2021;9:e002007. [Crossref] [PubMed]
- Palaskas N, Lopez-Mattei J, Durand JB, et al. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc 2020;9:e013757. [Crossref] [PubMed]
- Lehmann LH, Heckmann MB, Bailly G, et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation 2023;148:473-86. [Crossref] [PubMed]
- Lehmann LH, Cautela J, Palaskas N, et al. Clinical Strategy for the Diagnosis and Treatment of Immune Checkpoint Inhibitor-Associated Myocarditis: A Narrative Review. JAMA Cardiol 2021;6:1329-37. [Crossref] [PubMed]
- Drobni ZD, Zafar A, Zubiri L, et al. Decreased Absolute Lymphocyte Count and Increased Neutrophil/Lymphocyte Ratio With Immune Checkpoint Inhibitor-Associated Myocarditis. J Am Heart Assoc 2020;9:e018306. [Crossref] [PubMed]
- Zhuang Y, An Q, Wang F, et al. The role of circulating biomarkers in predicting the 30-day mortality of immune checkpoint inhibitors-related myocarditis: a retrospective cohort study. Intern Emerg Med 2024;19:377-89. [Crossref] [PubMed]
- Liang L, Cui C, Lv D, et al. Inflammatory biomarkers in assessing severity and prognosis of immune checkpoint inhibitor-associated cardiotoxicity. ESC Heart Fail 2023;10:1907-18. [Crossref] [PubMed]
- Dziedzic EA, Gąsior JS, Tuzimek A, et al. Investigation of the Associations of Novel Inflammatory Biomarkers-Systemic Inflammatory Index (SII) and Systemic Inflammatory Response Index (SIRI)-With the Severity of Coronary Artery Disease and Acute Coronary Syndrome Occurrence. Int J Mol Sci 2022;23:9553. [Crossref] [PubMed]
- De Giorgi U, Procopio G, Giannarelli D, et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clin Cancer Res 2019;25:3839-46. [Crossref] [PubMed]
- Qiu Q, Wu C, Tang W, et al. Development and validation of a risk-prediction model for immune-related adverse events in patients with non-small-cell lung cancer receiving PD-1/PD-L1 inhibitors. J Zhejiang Univ Sci B 2023;24:935-42. [Crossref] [PubMed]
- Zhao M, Duan X, Han X, et al. Sarcopenia and Systemic Inflammation Response Index Predict Response to Systemic Therapy for Hepatocellular Carcinoma and Are Associated With Immune Cells. Front Oncol 2022;12:854096. [Crossref] [PubMed]
- Jiang R, Ruan H, Wu W, et al. Monocyte/lymphocyte ratio as a risk factor of cardiovascular and all-cause mortality in coronary artery disease with low-density lipoprotein cholesterol levels below 1.4 mmol/L: A large longitudinal multicenter study. J Clin Lipidol 2024;18:e986-94. [Crossref] [PubMed]
- Mirna M, Schmutzler L, Topf A, et al. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep 2021;11:18101. [Crossref] [PubMed]
- Puzanov I, Subramanian P, Yatsynovich YV, et al. Clinical characteristics, time course, treatment and outcomes of patients with immune checkpoint inhibitor-associated myocarditis. J Immunother Cancer 2021;9:e002553. [Crossref] [PubMed]
- Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J 2022;43:4458-68. [Crossref] [PubMed]
- Xu L, Xu M, Sun W, et al. Clinical characteristics and prognostic impact of immune checkpoint inhibitor-associated myocarditis in advanced non-small cell lung cancer. Invest New Drugs 2023;41:816-24. [Crossref] [PubMed]
- Delombaerde D, Vervloet D, Franssen C, et al. Clinical implications of isolated troponinemia following immune checkpoint inhibitor therapy. ESMO Open 2021;6:100216. [Crossref] [PubMed]
- Wereski R, Kimenai DM, Taggart C, et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021;144:528-38. [Crossref] [PubMed]
- Lyngbakken MN, Aagaard EN, Kvisvik B, et al. Cardiac Troponin I and T Are Associated with Left Ventricular Function and Structure: Data from the Akershus Cardiac Examination 1950 Study. Clin Chem 2020;66:567-78. [Crossref] [PubMed]
- Lott JA, Stang JM. Serum enzymes and isoenzymes in the diagnosis and differential diagnosis of myocardial ischemia and necrosis. Clin Chem 1980;26:1241-50. [Crossref] [PubMed]
- Vasudevan G, Mercer DW, Varat MA. Lactic dehydrogenase isoenzyme determination in the diagnosis of acute myocardial infarction. Circulation 1978;57:1055-7. [Crossref] [PubMed]
- Eckart A, Struja T, Kutz A, et al. Relationship of Nutritional Status, Inflammation, and Serum Albumin Levels During Acute Illness: A Prospective Study. Am J Med 2020;133:713-722.e7. [Crossref] [PubMed]
- Yan D, Huang Q, Dai C, et al. Lactic Dehydrogenase to Albumin Ratio Is Associated With the Risk of Stroke-Associated Pneumonia in Patients With Acute Ischemic Stroke. Front Nutr 2021;8:743216. [Crossref] [PubMed]
- Claps G, Faouzi S, Quidville V, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol 2022;19:749-62. [Crossref] [PubMed]
- Chen Y, Xie K, Han Y, et al. An Easy-to-Use Nomogram Based on SII and SIRI to Predict in-Hospital Mortality Risk in Elderly Patients with Acute Myocardial Infarction. J Inflamm Res 2023;16:4061-71. [Crossref] [PubMed]
- Gu X, Han X, Shen Y, et al. Prognostic value of systemic inflammation response index in cancer patients treated with PD-1/PD-L1 immune checkpoint inhibitors: a meta-analysis. Ann Med 2024;56:2413415. [Crossref] [PubMed]
- Ma P, Liu J, Qin J, et al. Expansion of Pathogenic Cardiac Macrophages in Immune Checkpoint Inhibitor Myocarditis. Circulation 2024;149:48-66. [Crossref] [PubMed]
- Wu MM, Yang YC, Cai YX, et al. Anti-CTLA-4 m2a Antibody Exacerbates Cardiac Injury in Experimental Autoimmune Myocarditis Mice By Promoting Ccl5-Neutrophil Infiltration. Adv Sci (Weinh) 2024;11:e2400486. [Crossref] [PubMed]


