
Initial outcomes of novel guideline-directed pharmacotherapy for systemic right heart failure in adults with congenital heart disease
Highlight box
Key findings
• This study demonstrates a positive impact on New York Heart Association (NYHA) functional class and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in adult with congenital heart disease (ACHD) patients with systemic right ventricle (sRV) when treated with angiotensin receptor-neprilysin inhibitors (ARNi) and sodium-glucose co-transporter 2 inhibitors (SGLT2is).
What is known and what is new?
• Many patients with sRV develop heart failure at a young age, yet evidence for effective treatment in this group remains limited. Given the groundbreaking results of guideline-directed heart failure therapy in left heart failure, we investigated whether ARNi and SGLT2i therapy might also benefit patients with sRV failure. Here, we provide new data on the tolerability and effectiveness of this novel combination of medications for treating heart failure in sRV patients.
What is the implication, and what should change now?
• We are currently optimistic that ARNi and SGLT2i therapy represents a promising treatment option for systemic right ventricular heart failure, particularly given the absence of severe side effects in our study. However, due to the small cohort size, further research through prospective studies is crucial to include comprehensive examination tests, MRI data, and right heart catheterization results to validate and expand upon our findings.
Introduction
Despite the growing population of adult with congenital heart disease (ACHD) patients, most studies on the efficacy of heart failure therapy are based on small sample sizes and do not provide definitive evidence on the benefits of medical therapy for adults with systemic right ventricle (sRV) dysfunction (1).
Patients with sRV failure can be categorized into three groups. Each group, despite all having sRV, exhibits significant differences in hemodynamics and structural or valvular abnormalities (2).
The first group consists of individuals who were born with transposition of the great arteries (TGA) and underwent atrial switch operations (Senning or Mustard) during childhood. These procedures aim to achieve hemodynamic correction by rerouting oxygenated blood from the pulmonary veins to the tricuspid valve and right ventricle, and deoxygenated blood from systemic veins to the mitral valve and left ventricle. Despite this correction, the great arteries remain discordantly connected to the ventricles. Consequently, the morphologically and embryologically right ventricle is tasked with handling systemic circulation, resulting in an unnaturally high pre- and afterload (Figure 1A).
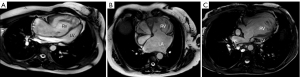
The second group of patients with sRV includes those with congenitally corrected transposition of the great arteries (cc-TGA) who did not undergo double-switch operations (atrial and arterial) during childhood. Instead, they received only corrections for associated anomalies, such as ventricular septal defect closure or pacemaker implantation for complete atrioventricular block, or no intervention at all. In these patients, the morphologic right ventricle is positioned in the subaortic location, typically on the left side of the heart (Figure 1B).
The third group includes patients with a congenitally single ventricle that has the morphologic characteristics of the right ventricle (e.g., hypoplastic left heart syndrome). These patients have undergone a multistage surgical correction leading to the establishment of a total cavopulmonary connection (or Fontan-like circulation) (Figure 1C).
Life expectancy in patients with a right ventricle performing systemic function is reduced compared to those with a systemic left ventricle (3-5). Unlike the left ventricle, the right ventricle has a unique fibromuscular architecture, shape, and function, with differences in coronary artery supply, tricuspid valve abnormalities, conduction system issues, and varied adaptation to pressure or volume overload as shown by Brida et al. (6). This leads to late complications and a poor prognosis, including heart failure, arrhythmias, and premature death in this patient group (6-8).
Management of heart failure in ACHD patients, especially those with a morphologically right systemic ventricle, still lacks a robust evidence base for definitive recommendations. This is despite significant advances in the treatment of chronic left heart failure with reduced ejection fraction (EF) in recent years and a substantial body of evidence supporting the efficacy of the new four-drug combination of angiotensin receptor-neprilysin inhibitors (ARNi), sodium-glucose co-transporter 2 inhibitors (SGLT2is), mineralocorticoid receptor antagonist (MRA), and beta-blockers (BBs) (9-11).
This retrospective study aims to assess whether the recent heart failure therapy regimen, including ARNi and SGLT2i—strongly recommended (Class IA) for left-sided heart failure in recent European and American guidelines—improves functional status and biomarker levels in a cohort of patients with a sRV. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-452/rc).
Methods
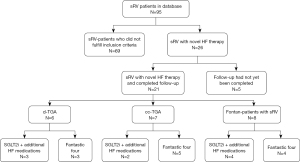
The primary cohort of patients was selected through a review of the medical information system database, which contains the data of all in-patients and out-patients treated at the University Heart and Vascular Center Hamburg. Since 2021, 95 adult patients with sRV have been identified for potential inclusion. Of those, 26 were treated with the new guideline-directed heart failure therapy and fulfilled the inclusion criteria. For five patients, heart failure therapy was started after February 2024, and therefore, the follow-up had not yet been completed by the end of data entry. Thus, a total of 21 patients were analyzed in the end (Figure 2). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). According to the Hamburg Hospital Law (HmbKHG), § 12, all patients who are treated at the University Heart and Vascular Center Hamburg automatically agree with using their health data anonymously for research when they consent to treatment at any hospital in Hamburg. Because our study was retrospective and pseudonymized, it falls within the scope of this law.
Inclusion/exclusion criteria
Inclusion criteria: adult patients aged 18 years or older with a sRV, including those with Fontan circulation, cc-TGA, or d-transposition of the great arteries (d-TGA) after atrial switch procedure, who exhibit signs of heart failure [reduced right ventricle (RV) function, elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, or clinical symptoms], with normal renal function (defined based on serum creatinine 0.5–1.1 mg/dL for women and 0.6–1.2 mg/dL for men) and normal blood pressure.
Exclusion criteria: patients with a systemic left ventricle or ambiguous ventricle, low blood pressure (systolic blood pressure <100 mmHg), renal insufficiency, or normal RV function in combination with normal NT-proBNP levels.
Treatment and follow-up
In this single-center study, we conducted a retrospective analysis of 21 patients with varied anatomy (univentricular heart, cc-TGA, or d-TGA after atrial switch procedure) and sRV during the time period from October 2021 to September 2024 who were treated at the University Heart and Vascular Center Hamburg. All variables of interest were extracted from the electronic health records of our hospital. For an estimation of statistical power, we set the goal of achieving a target NT-proBNP of less than 1,000 pg/mL (12). Patients in our clinic with sRV and signs of heart failure typically had a median NT-proBNP level of 1,800 ng/dL. We therefore targeted an 800 ng/dL reduction (−45%) of NT-proBNP. The coefficient of variation for serial NT-proBNP measurements was estimated to be on the order of 40% (13), meaning for a mean NT-proBNP level of 1,800 ng/dL, the standard deviation is on the order of 720 ng/dL. To achieve 90% power and detect a change of 800 ng/dL at the 0.01 level of confidence would require approximately 16 patients.
Treatment regimens
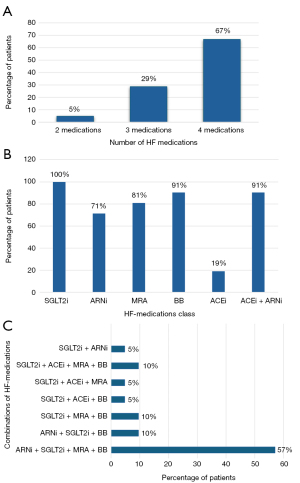
Twelve patients received the full “fantastic four” therapy, which included ARNi (sacubitril/valsartan) at the highest tolerated dose, SGLT2i (dapagliflozin or empagliflozin) at 10 mg/day, BB (bisoprolol, metoprolol, or carvedilol) at a variable dose, and MRA (spironolactone or eplerenone) at 25 mg/day as a starting dose at the beginning of the therapy with potential increase to 50 mg/day.
Three patients were treated with ARNi (sacubitril/valsartan), SGLT2i (dapagliflozin or empagliflozin), and BB (bisoprolol, metoprolol, or carvedilol).
Six patients, who exhibited preserved RV function but clinical signs of heart failure, did not receive ARNi. Instead, they were treated with SGLT2i (dapagliflozin or empagliflozin) in combination with angiotensin-converting enzyme inhibitors (ACEis) (ramipril or enalapril), and/or BBs (bisoprolol, metoprolol, or carvedilol), and/or MRAs (spironolactone or eplerenone) (Figure 3A-3C).
To evaluate the therapeutic effects, we assessed various parameters, including blood biomarkers (NT-proBNP, creatinine, and potassium), echocardiography measures (RV-EF), vital signs (blood pressure, oxygen saturation, and heart rate), and heart failure symptoms (NYHA class). To evaluate the safety and tolerability, we assessed side effects such as urinary tract infections, hypertension, hyperkalemia, an increase of creatinine level, and discontinuation of therapy.
Treatment initiation
Treatment with ARNi and SGLT2i, either in combination or as monotherapy with SGLT2i, was commenced based on a clinical assessment of NYHA class, RV-EF by echocardiography, and blood serum biomarkers [NT-proBNP, creatinine, glomerular filtration rate (GFR)] in either an in-patient or out-patient setting. ARNi and SGLT2i combination therapy was started in patients with reduced RV function, while SGLT2i alone was administered to those with normal RV function but elevated NT-proBNP levels and/or clinical signs of heart failure. No changes were made to the rest of the therapy after the new drugs were added. The median follow-up period after treatment initiation was 15 months.
Additional treatments
Thirteen patients received diuretics. Two patients were given specialized medical treatments: one received levetiracetam and mirtazapine for epilepsy, and one was prescribed sildenafil as a rescue strategy in a failing Fontan situation; this last patient subsequently underwent heart transplantation.
Limitations
As there is currently no guideline recommendation for heart failure therapy in sRV, patients in our group were treated with different combinations of the mentioned drugs, depending on the individual patient and the assessment of the responsible physician. These different regimens reflect the real-life clinical situation.
In our retrospective observational study, we had no control group.
Echocardiography
2D echocardiography was performed using Philips ultrasound systems, and analysis was conducted with TomTec-Arena (TTA) Ultrasound Workspace (Philips Medical Systems, Andover, MA, USA). Estimating the function of the sRV remains challenging due to its complex morphology and remodeling processes, which are influenced by increased preload and afterload. This difficulty is further exacerbated in adults with multiple surgeries, who often present a limited acoustic window. The RV-EF was categorized before treatment initiation and at follow-up as follows: >50% (normal), 40–50% (mildly reduced), 30–39% (moderately reduced), and <30% (severely reduced). Different echocardiographic methods, including the one-plane Simpson method, fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), and visual assessment, were used to provide a standardized basis for all evaluations.
Statistical analysis
Statistical analysis was conducted using R software (version 4.4.0, R Foundation for Statistical Computing, Vienna, Austria, 2024). Data are presented as mean ± standard deviation for normally distributed variables, and as median and interquartile range (IQR) for non-normally distributed variables. Comparisons between two groups were made using Student’s t-test for parametric data and the Wilcoxon-Mann-Whitney test for non-parametric data. Before applying Student’s t-test or analysis with a linear mixed-effect (LME) model, NT-proBNP values were logarithmically transformed so that they had an approximately normal distribution. Normality was assumed based on non-rejection of the null-hypothesis by the Shapiro-Wilk test. Fisher’s exact test was employed to assess associations between categorical variables. Analysis of variance (ANOVA) was used to compare multiple groups, with P values adjusted for multiple comparisons using Holm’s method. The strength of correlations between variables was evaluated with Pearson’s correlation coefficient.
Log-transformed NT-proBNP levels before and after treatment initiation were analyzed using a LMEs model, implemented with the “lme4” package in R. This model included a random intercept for each patient to account for patient-specific unobserved characteristics leading to within-patient correlations. Fixed effects in the model included time point (pre- and post-treatment initiation), age at medication initiation, sex, and the interaction between sex and time point. Standardized regression coefficients were used as direct measures to assess the sensitivity of the model response to a change in a predictor.
Changes in NYHA class before and after treatment initiation were analysed using McNemar’s test. Additionally, NYHA class was converted into a numerical score, and a paired Wilcoxon-Mann-Whitney test was used to compare pre- and post-treatment scores. All statistical tests were two-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
This retrospective observational study included 21 ACHD patients who had a morphologically right ventricle in a systemic position and exhibited heart failure, classified as NYHA functional class II or worse, or had at least mildly reduced systolic function of the sRV as diagnosed by cardiac ultrasound. These patients were treated at the University Heart and Vascular Center Hamburg.
Of the 21 patients, 8 had a total cavopulmonary connection (Fontan-like circulation) due to a univentricular heart, and 13 had TGA (7 with cc-TGA and 6 with d-TGA after atrial switch operation). The median age of the patients was 37 years (range, 28–52 years). A significant age difference was observed at the time of therapy initiation, with patients having univentricular hearts starting therapy at a median age of 26 years, compared to 51 years for those with TGA.
At the start of guideline-directed therapy, most patients were classified as NYHA functional class II or III (85.7%), while 14.3% were in NYHA functional class I but had significantly reduced sRV-EFs. The median NT-proBNP level was 870 ng/L (IQR, 593–1,774 ng/L). Baseline comparisons showed no significant differences between TGA patients and those with a single ventricle in terms of NYHA functional class, EF, and NT-proBNP levels. Detailed baseline patient characteristics are summarized in Table 1.
Table 1
| Characteristic | Total (N=21) | Single ventricle (N=8) | TGA (N=13) | P value† |
|---|---|---|---|---|
| Age (years) | 37 [28–52] | 26 [21–33] | 51 [38–56] | <0.001 |
| Female | 13 [62] | 3 [38] | 10 [77] | 0.20 |
| Height (cm) | 167±10 | 170±12 | 166±9 | 0.40 |
| Weight (kg) | 71±19 | 70±19 | 72±19 | 0.80 |
| BMI (kg/m2) | 25.3±4.9 | 24.2±4.4 | 26.1±5.3 | 0.40 |
| Systolic BP (mmHg) | 116±13 | 113±10 | 118±14 | 0.40 |
| Diastolic BP (mmHg) | 69±11 | 69±12 | 68±10 | 0.80 |
| Heart rate (bpm) | 69±10 | 71±8 | 68±11 | 0.50 |
| Blood O2 Sat. (%) | 96.0 [90.0–98.0] | 89.0 [88.0–90.5] | 98.0 [96.0–98.0] | <0.001 |
| Diagnosis | <0.001 | |||
| ccTGA | 7 [33] | 0 | 7 [54] | |
| d-TGA, p.o. atrial switch | 6 [29] | 0 | 6 [46] | |
| Univentricular heart p.o. TCPC | 8 [38] | 8 [100] | 0 | |
| Previous coronary catheterization | 12 [57] | 8 [100] | 4 [31] | 0.005 |
| NYHA before | 0.056 | |||
| 1 | 3 [14.3] | 3 [38] | 0 [0] | |
| 2 | 7 [33.3] | 1 [13] | 6 [46] | |
| 3 | 11 [52.4] | 4 [50] | 7 [54] | |
| Ejection fraction (TTE) | 0.046 | |||
| ≥50% | 4 [19] | 4 [50] | 0 [0] | |
| 40–49% | 3 [14] | 1 [13] | 2 [15] | |
| 30–39% | 7 [33] | 2 [25] | 5 [38] | |
| <30% | 7 [33] | 1 [13] | 6 [46] | |
| NT-proBNP (ng/L) | 870 [593–1,774] | 741 [292–955] | 1,665 [654–1,938] | 0.08 |
| Creatinin (mg/dL) | 0.83 [0.70–0.92] | 0.79 [0.68–0.89] | 0.83 [0.73–0.95] | 0.50 |
| Potassium (mmol/L) | 4.10 [3.70–4.50] | 3.95 [3.65–4.25] | 4.20 [4.00–4.50] | 0.15 |
| Pacemaker | 11 [52] | 2 [25] | 9 [69] | 0.08 |
| Therapy duration (years) | 1.24±0.65 | 0.96±0.42 | 1.41±0.72 | 0.08 |
Data are presented as median [interquartile range], n [%] or mean ± standard deviation. †, welch two sample t-test; Fisher’s exact test; Wilcoxon rank sum test. BMI, body mass index; BP, blood pressure; cc-TGA, congenitally corrected transposition of the great arteries; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; O2 Sat., oxygen saturation; p.o., post operation; TCPC, total cavopulmonary connection; TEE, transthoracic echocardiography; TGA, transposition of the great arteries; TTE, transthoracic echocardiography.
Patients’ follow-up
We compared clinical status, echocardiography findings, and laboratory parameters before the addition of SGLT2i and ARNi to standard heart failure therapy with those at the last follow-up during combined therapy. The mean follow-up period was 15 months. Complete data before and after treatment were available for 21 patients.
Safety and tolerability
No significant side effects, such as urinary tract infections or hyperkalemia, were observed during the therapy period. However, there was a statistically significant increase in creatinine levels, from 0.83 (range, 0.70–0.92) to 0.88 (range, 0.76–1.0) mg/dL. Despite this increase, creatinine levels remained within normal ranges.
Serum biomarkers
The addition of SGLT2i and ARNi to standard heart failure therapy resulted in a significant reduction in serum NT-proBNP levels, decreasing from 870 (range, 593–1,774) to 373 (range, 189–743) ng/L (P<0.001, paired t-test). NT-proBNP levels were positively correlated with age both before treatment (r=0.48; P=0.03) and at the last follow-up (r=0.49; P=0.02). While there was no significant difference in NT-proBNP levels between male and female patients before treatment, males experienced a greater reduction in NT-proBNP levels compared to females after therapy (P<0.001, Wilcoxon test) (Figure 4). The LME model predicts a mean baseline NT-proBNP in males is 761 pg/mL. Under treatment, the NT-proBNP level is reduced by a factor of 0.25 (~75% reduction; P<0.001) in male patients, but in females the effect is significantly smaller (P=0.009) and corresponds to a factor of 0.25×2.58 to 0.645, i.e., a 35% reduction (Table 2).
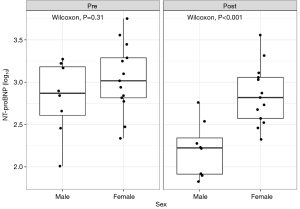
Table 2
| Predictors | NT-proBNP (log) | ||
|---|---|---|---|
| Estimates | 95% CI | P value | |
| (Intercept) | 761.32 | 414.91–1,396.94 | <0.001 |
| Age (years) | 1.03 | 1.00–1.05 | 0.04 |
| Female | 1.33 | 0.61–2.91 | 0.47 |
| Under therapy | 0.25 | 0.14–0.43 | <0.001 |
| Female: under therapy | 2.58 | 1.29–5.18 | 0.009 |
| Random effects | |||
| σ2 | 0.29 | ||
| τ00ID | 0.38 | ||
| ICC | 0.57 | ||
| NID | 21 | ||
| Observations | 42 | ||
| Marginal R2/conditional R2 | 0.466/0.769 | ||
Conditional R2 is the amount of explained variance for the entire model. In this case, both the fixed and random effects explain about 76.9% of the variance of the outcome. The marginal R2 explains how much of this variance is attributed to the fixed effects alone (46.6%). In the table of regression coefficients estimates for the predictors, as shown, can be interpreted as multiplicative factors for NT-proBNP in pg/mL on account of the fact that the model was for the logarithm of NT-proBNP and the coefficient estimates were transformed with the exponential function. The mean baseline NT-proBNP in males is 761.32 pg/mL. Post-treatment the NT-proBNP level is reduced by a factor of 0.25 (~75% reduction) in male patients, but in females the effect is significantly smaller and corresponds to a factor of 0.25×2.58 to 0.645, i.e., a 35% reduction. σ2, within-group variance; τ00, between-group-variance; ICC, intraclass correlation; NID, number of patients/ID’s; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
NT-proBNP levels were nominally higher in patients with TGA compared to those with single ventricle physiology, both before and after therapy initiation. However, NT-proBNP levels decreased significantly in the single ventricle group (Figure 5). The logarithmically transformed NT-proBNP values appeared to be normally distributed based on the Shapiro-Wilk test (P=0.86).
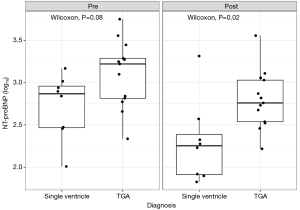
Echocardiography
In the present study, there was no significant effect (P=0.09) of complex heart failure therapy including SGLT2i and ARNi on EF.
Clinical characteristics
A significant positive effect on the NYHA functional class was observed following SGLT2i and ARNi therapy. Before treatment, 52.4% of patients were classified as NYHA functional class III, 33.3% as NYHA class II, and 14.3% as NYHA class I. After therapy, 9% of patients were classified as NYHA class III, 43% as NYHA class II, and 48% as NYHA class I (Figure 6).
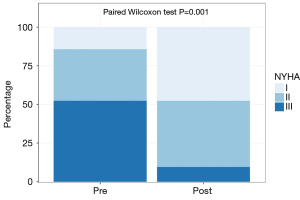
Discussion
Pharmacological therapy for heart failure in patients with sRV is becoming increasingly crucial as this patient population ages (14). There is an ongoing effort to identify effective pharmacological therapies for patients with sRV to mitigate ventricular dysfunction and its complications. Previous research has explored this challenge, such as the study by Dore et al., which found no improvement in cardiorespiratory test parameters or NT-proBNP levels with valsartan treatment after a mean follow-up of 4 months (15). Van der Bom et al. also reported a lack of efficacy of valsartan on RV function, RV size, and peak oxygen consumption (VO2) after 3 years of administration (16). Therrien et al. found no significant improvement in sRV-EF and observed a worsening of exercise tolerance with the use of the ACE inhibitor enalapril (17). In the multi-center retrospective observational study by Ladouceur et al., no significant reduction in mortality or heart failure incidence was observed in patients taking ACEis or angiotensin II receptor blockers (ARBs), despite notable neurohormonal activation (18). Doughan et al. investigated the role of BBs (carvedilol or metoprolol) in patients with TGA and sRV dysfunction. The study found no improvement in sRV function; however, it did show a reduction in right ventricle size over a mean follow-up period of 10 months, as well as an improvement in the patients’ functional class (19). Dos et al. conducted a randomized, double-blind, controlled trial to evaluate the effect of eplerenone. The study found no changes in right ventricle size, mass, or function according to MRI, although baseline values in the study group were near normal (RVEF 55%, NYHA class I) (20).
The results from studies evaluating ACEis, ARBs, MRAs, and BBs did not identify an effective approach for managing heart failure in patients with sRV dysfunction (1,15-20). Consequently, trials have been initiated to explore the efficacy of novel guideline-directed therapies, specifically ARNi and SGLT2i, which have proven effective for treating left-sided heart failure in non-ACHD patients, in the context of sRV (16,21-30).
In our retrospective study, we evaluated a cohort of ACHD patients with sRV who were treated with ARNi and/or SGLT2i. The data obtained in this study complement the results of other studies currently being conducted to investigate these novel therapies. Unlike other studies that have typically focused either on patients with TGA or those with Fontan physiology, our cohort included both TGA and Fontan patients. We observed significant improvements in NT-proBNP levels and NYHA functional class in both groups over a median follow-up period of 15 months. These findings are consistent with recent data from Bright et al. and highlight the need for further research in this emerging area of treatment (30). Bright et al. investigated a mixed cohort of ACHD patients with either systemic right or left ventricles and found improvements in functional and imaging parameters with ARNi and SGLT2i therapy. However, they noted that the sRV exhibited a more heterogeneous response compared to the systemic left ventricle group. They also emphasized the advantages of combination therapy in managing these patients (30). In a study by Karnakoti et al., monotherapy with SGLT2i did not result in significant changes in systemic ventricular function in ACHD patients with either systemic RV or left ventricles (31).
Neijenhuis et al. investigated the effects of ARNi and SGLT2i in patients with sRV failure. Their data suggest that ARNi is well-tolerated and leads to a stable increase in distance covered in the 6-minute walk test, improved sRV function as assessed by echocardiography, and a reduction in NT-proBNP levels. In contrast, therapy with SGLT2i alone (without sacubitril/valsartan) resulted in fewer hospitalizations, a significant decrease in NT-proBNP levels, and improvements in functional class, but did not alter the contractile function of the sRV (25,32).
We did not observe a significant change in systolic RV function, either improvement or deterioration. This contrasts with findings from large studies on left-sided heart failure, such as the PARADIGM-HF trial (33) and the results from Fusco et al. (26), who reported improvements in sRV contractile function following ARNi treatment, along with reductions in NT-proBNP levels and increased distances in the 6-minute walk test. In the DAPA-SERVE trial (27,28), the research group demonstrated a significant improvement in sRV function with the addition of SGLT2i to existing therapy with ACEi or ARNi. However, there were no significant changes in NT-proBNP levels, systolic blood pressure, or distance in the 6-minute walk test. Similarly, our study did not observe a significant effect on sRV function. This could be attributed to the small cohort size and the diverse range of congenital heart defects in our sample. Additionally, variations in cardiac ultrasound assessments by different examiners might introduce bias. Despite this, patients reported improvements in NYHA class, suggesting that factors such as RV compliance and diastolic function may be more influential. The interpretation of sRV diastolic function remains challenging, and improvements noted in sRV function in other studies were assessed using strain or 3D imaging, which was not employed in our study. Furthermore, hemodynamics in patients with a single ventricle differ from those with TGA, and there are hemodynamic variations between d-TGA and cc-TGA (2). Therefore, right heart catheterization follow-up data could be valuable in evaluating effects on end-diastolic pressure in the sRV or wedge pressure.
In our cohort, we treated patients with ARNi and SGLT2i who had either mildly or severely reduced systolic RV function. In contrast, major studies such as the EMPEROR-Preserved trial demonstrated that Empagliflozin significantly reduced the combined risk of cardiovascular death or hospitalization for heart failure in patients with heart failure and preserved EF (34).
Our targeted effect size is not based on data from a previous study in a similar patient collective with TGA and provides only a rough estimate of the necessary sample size. Accordingly, results from this study need to be seen as hypothesis-generating, rather than providing any definite evidence of the therapeutic benefits of the new guideline-directed heart failure pharmacotherapy.
Overall, it appears that our cohort may be too small to draw definitive conclusions about the impact on EF. Nederend et al. observed a consistent improvement in NT-proBNP levels in their cohort of TGA patients after atrial switch and cc-TGA patients. They noted a significant decrease in NT-proBNP levels within the first 3 months of therapy, followed by a stable plateau with no further reductions over a 24-month period (35). It remains uncertain whether the stable decrease in NT-proBNP levels is a permanent phenomenon or if further changes may occur with extended observation. Nonetheless, NT-proBNP is well established as a reliable surrogate marker for heart failure in children, adults, and ACHD (36-38).
Patients in this study did not experience adverse effects that would suggest discontinuation of therapy, such as renal dysfunction or hyperkalemia, which suggests that the treatment appears to be safe for patients with normal renal function. Statistical analyses revealed a correlation between sex and NT-proBNP levels, with a more significant decrease observed in male patients compared to female patients (reduction of 75% vs. 35% with simultaneous adjustment by age) (Figures 4,5, Table 2). However, it is important to note that the male patients predominantly belonged to the single-ventricle group, which might indicate a pathophysiological rather than gender-related phenomenon. Further research is needed to determine whether biological sex affects therapy response, as gender-related medicine is an evolving field.
Conclusions
Our retrospective analysis showed that guideline-directed heart failure pharmacotherapy, including ARNi (sacubitril/valsartan) and SGLT2i (empagliflozin or dapagliflozin), results in a significant reduction in NT-proBNP levels and improvement in NYHA class in patients with a sRV. This therapy appears to be safe and well tolerated in patients with normal renal function. However, further randomized studies with a larger number of patients are necessary to verify these promising results and to identify additional treatment effects (such as changes in EF) or rare side effects. Furthermore, it should be investigated whether these effects are effective in this patient group through SGLT2i alone or in combination therapy.
Acknowledgments
We would like to thank Mrs. Anneke Mueller, Mrs. Nicole Burghardt, and Mrs. Nadine Finkel for patient management.
Footnote
Provenance and Peer Review: The article was commissioned by the Guest Editor (Harald Kaemmerer) for the series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part VI” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-452/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-452/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-452/prf
Funding: C.R. and A.K. are partially supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-452/coif). The series “Current Management Aspects in Adult Congenital Heart Disease (ACHD): Part VI” was commissioned by the editorial office without any funding or sponsorship. Arash Kheradvar is the recipient of multiple research grants from NIH and NSF (NIH R01HL153724, NIH R01HL162687, NSF 2109959, R01HL153724, R56HL173809). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). According to the Hamburg Hospital Law (HmbKHG), § 12, all patients who are treated at the University Heart and Vascular Center Hamburg automatically agree in using their health data anonymously for research when they consent to treatment at any hospital in Hamburg. Because our study was retrospective and pseudonymized, it falls within the scope of this law.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zaragoza-Macias E, Zaidi AN, Dendukuri N, et al. Medical Therapy for Systemic Right Ventricles: A Systematic Review (Part 1) for the 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e801-13. [Crossref] [PubMed]
- Miranda WR, Jain CC, Egbe AC, et al. Hemodynamics in Adults with Systemic Right Ventricles: Differences Between Congenitally Corrected and Complete Transposition of the Great Arteries. Pediatr Cardiol 2025;46:189-97. [Crossref] [PubMed]
- Vejlstrup N, Sørensen K, Mattsson E, et al. Long-Term Outcome of Mustard/Senning Correction for Transposition of the Great Arteries in Sweden and Denmark. Circulation 2015;132:633-8. [Crossref] [PubMed]
- Prieto LR, Hordof AJ, Secic M, et al. Progressive tricuspid valve disease in patients with congenitally corrected transposition of the great arteries. Circulation 1998;98:997-1005. [Crossref] [PubMed]
- Kawahira Y, Uemura H, Yoshikawa Y, et al. Double inlet right ventricle versus other types of double or common inlet ventricle: its clinical characteristics with reference to the Fontan procedure. Eur J Cardiothorac Surg 2001;20:228-32. [Crossref] [PubMed]
- Brida M, Diller GP, Gatzoulis MA. Systemic Right Ventricle in Adults With Congenital Heart Disease: Anatomic and Phenotypic Spectrum and Current Approach to Management. Circulation 2018;137:508-18. [Crossref] [PubMed]
- Bevilacqua F, Pasqualin G, Ferrero P, et al. Overview of Long-Term Outcome in Adults with Systemic Right Ventricle and Transposition of the Great Arteries: A Review. Diagnostics (Basel) 2023;13:2205. [Crossref] [PubMed]
- Lebherz C, Gerhardus M, Lammers AE, et al. Late outcome, therapy and systemic ventricular function in patients with a systemic right ventricle: data of the German National Register for Congenital Heart Defects. Cardiol Young 2022;32:1235-45. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627-39. [Crossref] [PubMed]
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895-e1032. [Crossref] [PubMed]
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017;318:713-20. [Crossref] [PubMed]
- Chami J, Fleming S, Taylor CJ, et al. Point-of-care NT-proBNP monitoring for heart failure: observational feasibility study in primary care. BJGP Open 2022;6:BJGPO.2022.0005.
- Wu MH, Lu CW, Chen HC, et al. Adult Congenital Heart Disease in a Nationwide Population 2000-2014: Epidemiological Trends, Arrhythmia, and Standardized Mortality Ratio. J Am Heart Assoc 2018;7:e007907. [Crossref] [PubMed]
- Dore A, Houde C, Chan KL, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 2005;112:2411-6. [Crossref] [PubMed]
- van der Bom T, Winter MM, Bouma BJ, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation 2013;127:322-30. [Crossref] [PubMed]
- Therrien J, Provost Y, Harrison J, et al. Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study. Int J Cardiol 2008;129:187-92. [Crossref] [PubMed]
- Ladouceur M, Segura de la Cal T, Gaye B, et al. Effect of medical treatment on heart failure incidence in patients with a systemic right ventricle. Heart 2021;107:1384-9. [Crossref] [PubMed]
- Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol XL) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol 2007;99:704-6. [Crossref] [PubMed]
- Dos L, Pujadas S, Estruch M, et al. Eplerenone in systemic right ventricle: double blind randomized clinical trial. The evedes study. Int J Cardiol 2013;168:5167-73. [Crossref] [PubMed]
- Lluri G, Lin J, Reardon L, et al. Early Experience With Sacubitril/Valsartan in Adult Patients With Congenital Heart Disease. World J Pediatr Congenit Heart Surg 2019;10:292-5. [Crossref] [PubMed]
- Appadurai V, Thoreau J, Malpas T, et al. Sacubitril/Valsartan in Adult Congenital Heart Disease Patients With Chronic Heart Failure - A Single Centre Case Series and Call for an International Registry. Heart Lung Circ 2020;29:137-41. [Crossref] [PubMed]
- Zandstra TE, Nederend M, Jongbloed MRM, et al. Sacubitril/valsartan in the treatment of systemic right ventricular failure. Heart 2021;107:1725-30. [Crossref] [PubMed]
- Egorova AD, Nederend M, Tops LF, et al. The first experience with sodium-glucose cotransporter 2 inhibitor for the treatment of systemic right ventricular failure. ESC Heart Fail 2022;9:2007-12. [Crossref] [PubMed]
- Neijenhuis RML, Nederend M, Jongbloed MRM, et al. The potential of sodium-glucose cotransporter 2 inhibitors for the treatment of systemic right ventricular failure in adults with congenital heart disease. Front Cardiovasc Med 2023;10:1093201. [Crossref] [PubMed]
- Fusco F, Scognamiglio G, Merola A, et al. Safety and Efficacy of Sacubitril/Valsartan in Patients With a Failing Systemic Right Ventricle: A Prospective Single-Center Study. Circ Heart Fail 2023;16:e009848. [Crossref] [PubMed]
- Abbate M, Fusco F, Scognamiglio G, et al. Dapaglifozin in adults with a systemic right ventricle:initial results from the DAPA-SERVE trial. European Heart Journal 2023;44:ehad655.1914.
- Fusco F, Scognamiglio G, Abbate M, et al. Dapagliflozin in Patients With a Failing Systemic Right Ventricle: Results From the DAPA-SERVE Trial. JACC Heart Fail 2024;12:789-91. [Crossref] [PubMed]
- Saef J, Sundaravel S, Ortega-Legaspi J, et al. Safety and Treatment Experience With Sodium/glucose Cotransporter-2 Inhibitors in Adult Patients With Congenital Heart Disease. J Card Fail 2023;29:974-5. [Crossref] [PubMed]
- Bright C, Rizvi A, Ezekwueme F, et al. Impact of guideline directed medical therapy on myocardial function in adults with congenital heart disease. Int J Cardiol 2024;414:132413. [Crossref] [PubMed]
- Karnakoti S, Andi K, Miranda WR, et al. Outcomes of Sodium-Glucose Cotransporter 2 Inhibitor Use in Adults With Congenital Heart Disease. CJC Pediatr Congenit Heart Dis 2024;3:115-6. [Crossref] [PubMed]
- Neijenhuis RML, MacDonald ST, Zemrak F, et al. Effect of Sodium-Glucose Cotransporter 2 Inhibitors in Adults With Congenital Heart Disease. J Am Coll Cardiol 2024;83:1403-14. [Crossref] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021;385:1451-61. [Crossref] [PubMed]
- Nederend M, Kiès P, Regeer MV, et al. Tolerability and beneficial effects of sacubitril/valsartan on systemic right ventricular failure. Heart 2023;109:1525-32. [Crossref] [PubMed]
- Schmitt W, Diedrich C, Hamza TH, et al. NT-proBNP for Predicting All-Cause Death and Heart Transplant in Children and Adults with Heart Failure. Pediatr Cardiol 2025;46:694-703. [Crossref] [PubMed]
- Schmitt W, Rühs H, Burghaus R, et al. NT-proBNP Qualifies as a Surrogate for Clinical End Points in Heart Failure. Clin Pharmacol Ther 2021;110:498-507. [Crossref] [PubMed]
- Yumita Y, Xu Z, Diller GP, et al. B-type natriuretic peptide levels predict long-term mortality in a large cohort of adults with congenital heart disease. Eur Heart J 2024;45:2066-75. [Crossref] [PubMed]


