
Development and validation of a prognostic model for early mortality risk in patients with fulminant myocarditis
Highlight box
Key findings
• The study identified seven independent predictors of early mortality in fulminant myocarditis (FM): respiratory symptoms, cardiopulmonary resuscitation (CPR), serum creatinine, direct bilirubin, thyroid-stimulating hormone (TSH), lactate, and left ventricular ejection fraction (LVEF). The nomogram model demonstrated strong discriminative accuracy, with areas under the curve of 0.907 (14-day) and 0.880 (28-day) in the training set and 0.853/0.942 in the validation set, alongside a concordance index of 0.889. Kaplan-Meier analysis confirmed significantly lower mortality in low-risk groups, supported by decision curve analysis showing clinical net benefit across threshold probabilities.
What is known and what is new?
• Prior studies established associations between biomarkers (e.g., N-terminal pro-B-type natriuretic peptide, LVEF) and myocarditis outcomes but lacked validated prognostic models for FM.
• This study innovatively integrates clinical, biochemical, and hemodynamic variables via least absolute shrinkage and selection operator Cox regression to construct the first FM-specific mortality risk nomogram. Unlike previous efforts focused on disease identification, this model uniquely prioritizes early mortality prediction, leveraging CPR, lactate, and TSH—variables not previously consolidated into a predictive framework—enhancing clinical utility for risk stratification.
What is the implication, and what should change?
• The model enables early identification of high-risk FM patients, guiding intensified monitoring and tailored interventions (e.g., mechanical circulatory support). Clinicians should adopt this nomogram to optimize resource allocation and improve outcomes. However, external validation in larger, multicenter cohorts is critical to address limitations (small sample size, single-center design). Future research should explore integrating emerging biomarkers and refining the model for broader applicability, ensuring alignment with evolving diagnostic and therapeutic paradigms.
Introduction
Fulminant myocarditis (FM) represents the most severe form of myocarditis, characterized by rapid progression and a high mortality rate during the acute phase. It is marked by extensive cardiac inflammation and dysfunction, often resulting in cardiogenic shock, severe arrhythmias, and sudden death (1). The clinical presentation of FM is often nonspecific, with early symptoms such as fever, fatigue, and chest pain, which can be easily mistaken for other common infectious diseases, contributing to a high rate of clinical misdiagnosis (2). However, FM can deteriorate rapidly, leading to death from cardiac failure or other complications. Studies indicate that more than 70% of patients with FM can achieve recovery of normal left ventricular function following the acute phase (3). Therefore, the early identification of patients who are at high-risk and the implementation of timely interventions are crucial for reducing mortality. Currently, the absence of a standardized model for predicting early mortality risk in patients having FM presents challenges for precise clinical risk assessment (4).
With advancements in big data technology and machine learning algorithms, the development of predictive models using multidimensional clinical data has become increasingly feasible. Previous research has demonstrated that biomarkers in patients with myocarditis, including N-terminal pro-B-type natriuretic peptide (NT-proBNP), myocardial enzyme levels, and imaging characteristics, are significantly associated with patient prognosis (5). However, these studies have primarily focused on association analyses rather than the development of predictive models. By integrating these clinical variables and using advanced data analysis methods such as least absolute shrinkage and selection operator (LASSO) Cox regression for variable selection, effective mortality risk prediction models can be constructed to inform clinical decision-making and enhance early treatment outcomes (6).
The objective of this study is to develop, for the first time, an early mortality risk prediction model for FM using clinical data, identifying key risk factors through multivariate analysis, and improving the predictive accuracy of the model with advanced data analysis techniques. The goal is to support clinicians in early identification of patients who are at high-risk and in implementing more targeted interventions, thereby reducing the mortality rate associated with FM. We present this article in accordance with the TRIPOD+AI reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-583/rc).
Methods
Study population
In this retrospective study, 119 patients diagnosed with FM were included at Central China Fuwai Hospital between January 2018 and December 2023. The average patient age was 38±17 years, with a gender distribution of 59 males (49.6%) and 60 females (50.4%).
The inclusion criteria required patients to meet the clinical diagnostic standards for FM, characterized by acute onset, rapid progression to heart failure, cardiogenic shock, or severe arrhythmias (7). Diagnosis was confirmed using myocardial enzyme spectrum analysis, electrocardiography, echocardiography, coronary angiography [including coronary computed tomography angiography (CTA) or invasive coronary angiography] to rule out coronary artery disease, and additional imaging studies, with treatment involving vasoactive drugs, blood transfusion, or mechanical circulatory support (MCS).
Exclusion criteria were as follows: (I) a previous diagnosis of chronic myocarditis, significant coronary artery disease confirmed by coronary angiography (CTA or invasive coronary angiography), ischemic heart disease, or other severe organic heart diseases (such as rheumatic heart disease, congenital heart disease, valvular heart disease, or pheochromocytoma). (II) Autoimmune diseases or systemic inflammatory disorders affecting the heart. Severe systemic infections, sepsis, or other conditions mimicking myocarditis symptoms. (III) A hospitalization period of less than 24 hours or incomplete clinical data.
All 119 eligible patients received standardized treatment and were monitored until either death or the end of the study follow-up period. The primary endpoint was all-cause mortality.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the People’s Hospital of Zhengzhou University, Heart Center of Henan Provincial People’s Hospital/Central China Fuwai Hospital (No. 201808). Written informed consent was obtained from all participants.
Data collection
Clinical data were extracted from the hospital’s electronic medical record system. The collected variables included age, sex, respiratory symptoms, gastrointestinal symptoms, fever, chest tightness or dyspnea, chest pain, neurological symptoms, medical history, smoking status, alcohol consumption, white blood cell count, neutrophil count, lymphocyte count, hemoglobin, platelet count, C-reactive protein, procalcitonin (PCT), blood urea nitrogen, creatinine, uric acid, blood glucose, electrolytes, D-dimer, fibrinogen, myocardial enzymes, alanine transaminase, aspartate transaminase, total bilirubin, direct bilirubin, thyroid function tests, troponin I, brain natriuretic peptide, lactate, left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter, left atrial diameter, interventricular septum thickness, left ventricular posterior wall thickness, and pericardial effusion. Additional information included instances of cardiopulmonary resuscitation (CPR), ventricular tachycardia or ventricular fibrillation (VT/VF), and third-degree atrioventricular (AV) block. All baseline characteristics, including LVEF, were collected during the first assessment after hospital admission.
To ensure data accuracy, two experienced cardiologists independently verified all data entries. Data were then randomly allocated into a training set (70%) and a validation set (30%). The training set was used for model construction, while the validation set served for internal validation.
Statistical analysis
Data analysis was conducted using R software (version 4.3.3). Continuous variables following a normal distribution were presented as mean ± standard deviation and were compared using the t-test. For variables that did not follow a normal distribution, values were expressed as median (P25, P75) and analyzed through the rank-sum test. Categorical data were displayed as counts (percentages) and analyzed using either the Chi-squared test or Fisher’s exact test. Missing data were input with the “missRanger” package.
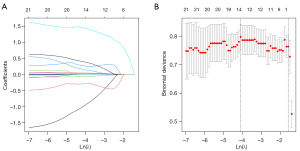
Data were divided randomly into a training set (70%) and a validation set (30%). In the training set, univariate Cox regression was first applied to identify predictive factors (P<0.05), followed by LASSO Cox regression for further variable selection. LASSO regression is a regularization technique that helps prevent overfitting and addresses multicollinearity by adding a penalty term to the regression model. This penalty term shrinks some coefficients towards zero, effectively selecting only the most relevant variables. In the LASSO plot (Figure 1), the X-axis represents the log of the penalty parameter (λ), and the Y-axis shows the coefficient values of the variables. As λ increases, more coefficients shrink to zero. The optimal λ is identified through cross-validation, typically at the point where the model has the lowest prediction error. The variables with non-zero coefficients at this point are selected as key predictors. Multivariate Cox regression was subsequently used to determine independent predictive factors (P<0.05). Based on these independent predictors, a nomogram was constructed, with calibration assessed through bootstrap validation and a calibration curve.
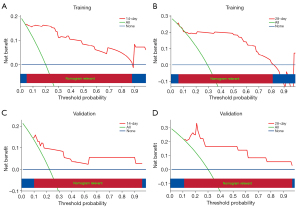
The discriminative ability of the model was assessed by calculating the area under the curve (AUC) and the concordance index (C-index) via receiver operating characteristic (ROC) curves. A decision curve analysis (DCA) was also conducted to assess the model’s clinical utility. The X-axis in the DCA represents the threshold probability, which reflects the predicted risk of early mortality at which a clinician would consider initiating an intervention for a patient with FM. The Y-axis represents the net benefit, which balances the trade-off between true positives (correctly identifying high-risk patients) and false positives (unnecessary interventions in low-risk patients). The “treat-all” line assumes that all FM patients receive aggressive interventions regardless of their predicted risk, which may lead to overtreatment and unnecessary resource utilization. Conversely, the “treat-none” line assumes that no patient receives any intervention, potentially missing high-risk cases who could benefit from timely treatment. A predictive model is considered clinically useful if its net benefit curve lies above both the “treat-all” and “treat-none” lines. The wider the range where the model’s curve exceeds these lines, the greater its clinical value in guiding decision-making for FM management.
The nomogram model was validated using the validation set, with risk stratification and survival analysis conducted through the “survminer” package. A P value of <0.05 was set as the threshold for statistical significance.
Results
Baseline characteristics
The 119 cases of FM were randomly allocated into a training set (n=83) and a validation set (n=36) in a 7:3 ratio. Baseline characteristics showed no differences between the two groups (P>0.05, Table 1).
Table 1
| Variables | Total (n=119) | Training (n=83) | Validation (n=36) | z/χ2/t | P value |
|---|---|---|---|---|---|
| Outcome | χ2=0.001 | 0.97 | |||
| Survival | 89 (74.8) | 62 (74.7) | 27 (75.0) | ||
| Death | 30 (25.2) | 21 (25.3) | 9 (25.0) | ||
| Time (days) | 16.00 (11.50, 25.50) | 16.00 (12.00, 25.50) | 16.50 (11.00, 25.25) | z=0.052 | 0.96 |
| Gender | χ2=0.115 | 0.74 | |||
| Male | 59 (49.6) | 42 (50.6) | 17 (47.2) | ||
| Female | 60 (50.4) | 41 (49.4) | 19 (52.8) | ||
| Age (years) | 38.71±6.80 | 38.39±17.37 | 39.44±15.62 | t=−0.328 | 0.74 |
| Early symptoms | |||||
| Respiratory symptoms | χ2=0.179 | 0.67 | |||
| No | 56 (47.1) | 38 (45.8) | 18 (50.0) | ||
| Yes | 63 (52.9) | 45 (54.2) | 18 (50.0) | ||
| Gastrointestinal symptoms | χ2=0.048 | 0.82 | |||
| No | 61 (51.3) | 42 (50.6) | 19 (52.8) | ||
| Yes | 58 (48.7) | 41 (49.4) | 17 (47.2) | ||
| Fever | χ2=1.726 | 0.19 | |||
| No | 47 (39.5) | 36 (43.4) | 11 (30.6) | ||
| Yes | 72 (60.5) | 47 (56.6) | 25 (69.4) | ||
| Chest tightness or dyspnea | χ2=0.030 | 0.86 | |||
| No | 31 (26.1) | 22 (26.5) | 9 (25.0) | ||
| Yes | 88 (73.9) | 61 (73.5) | 27 (75.0) | ||
| Chest pain | χ2=1.421 | 0.23 | |||
| No | 88 (73.9) | 64 (77.1) | 24 (66.7) | ||
| Yes | 31 (26.1) | 19 (22.9) | 12 (33.3) | ||
| Neurological symptoms | χ2=1.892 | 0.17 | |||
| No | 77 (64.7) | 57 (68.7) | 20 (55.6) | ||
| Yes | 42 (35.3) | 26 (31.3) | 16 (44.4) | ||
| Syncope | χ2=3.459 | 0.06 | |||
| No | 95 (79.8) | 70 (84.3) | 25 (69.4) | ||
| Yes | 24 (20.2) | 13 (15.7) | 11 (30.6) | ||
| Medical history | |||||
| Smoke | χ2=1.065 | 0.30 | |||
| No | 96 (80.7) | 69 (83.1) | 27 (75.0) | ||
| Yes | 23 (19.3) | 14 (16.9) | 9 (25.0) | ||
| Alcohol consumption | χ2=0.095 | 0.75 | |||
| No | 101 (84.9) | 71 (85.5) | 30 (83.3) | ||
| Yes | 18 (15.1) | 12 (14.5) | 6 (16.7) | ||
| Diabetes | Fisher | >0.99 | |||
| No | 115 (96.6) | 80 (96.4) | 35 (97.2) | ||
| Yes | 4 (3.4) | 3 (3.6) | 1 (2.8) | ||
| Hypertension | χ2=0.007 | 0.94 | |||
| No | 102 (85.7) | 71 (85.5) | 31 (86.1) | ||
| Yes | 17 (14.3) | 12 (14.5) | 5 (13.9) | ||
| Malignant arrhythmia | |||||
| VT/VF | χ2=0.667 | 0.41 | |||
| No | 66 (55.5) | 44 (53.0) | 22 (61.1) | ||
| Yes | 53 (44.5) | 39 (47.0) | 14 (38.9) | ||
| Third-degree AV block | χ2=0.001 | 0.97 | |||
| No | 89 (74.8) | 62 (74.7) | 27 (75.0) | ||
| Yes | 30 (25.2) | 21 (25.3) | 9 (25.0) | ||
| Vital signs | |||||
| SBP (mmHg) | 99.91±18.28 | 98.07±19.66 | 104.14±13.94 | t=−1.913 | 0.06 |
| DBP (mmHg) | 62.00 (55.50, 73.00) | 62.00 (53.00, 73.00) | 66.50 (57.50, 72.50) | t=−1.334 | 0.18 |
| Heart rate (bpm) | 100.12±26.57 | 98.34±27.37 | 104.22±24.49 | t=−1.161 | 0.25 |
| Laboratory examinations | |||||
| WBC (×109/L) | 12.31 (8.47, 16.27) | 12.92 (9.07, 17.08) | 10.44 (7.37, 15.24) | z=1.539 | 0.12 |
| Neutrophil count (×109/L) | 10.32 (6.62, 13.92) | 10.94 (7.03, 14.95) | 8.95 (6.12, 13.29) | z=1.501 | 0.13 |
| Lymphocyte count (×109/L) | 1.10 (0.75, 1.56) | 1.03 (0.75, 1.62) | 1.12 (0.78, 1.48) | z=0.084 | 0.93 |
| Hemoglobin (g/L) | 124.29±21.61 | 123.00±21.81 | 127.25±21.13 | t=−0.998 | 0.32 |
| Platelets (×109/L) | 200.85±82.09 | 192.13±80.49 | 220.94±83.33 | t=−1.750 | 0.09 |
| C-reactive protein (mg/L) | 23.95 (9.62, 66.74) | 30.85 (9.73, 81.05) | 22.98 (8.20, 54.51) | z=0.853 | 0.39 |
| PCT (μg/L) | 0.71 (0.14, 4.63) | 1.25 (0.20, 6.14) | 0.32 (0.06, 1.68) | z=2.447 | 0.01 |
| BUN (mmol/L) | 7.20 (4.95, 10.65) | 7.00 (4.90, 10.65) | 7.65 (5.15, 10.07) | z=0.113 | 0.91 |
| Serum creatinine (μmol/L) | 79.00 (55.50, 128.00) | 81.00 (54.00, 140.50) | 77.50 (59.50, 100.75) | z=0.955 | 0.34 |
| Serum uric acid (μmol/L) | 402.00 (267.50, 551.50) | 403.00 (271.00, 617.50) | 328.50 (257.25, 481.00) | z=1.345 | 0.18 |
| Blood glucose (mmol/L) | 8.64 (7.14, 10.88) | 8.60 (6.99, 10.86) | 8.94 (7.21, 11.03) | z=−0.414 | 0.68 |
| Serum potassium (mmol/L) | 4.17 (3.76, 4.63) | 4.17 (3.81, 4.64) | 4.24 (3.67, 4.58) | z=0.755 | 0.45 |
| Serum sodium (mmol/L) | 136.00 (133.00, 139.50) | 137.00 (135.00, 140.50) | 134.50 (132.00, 137.00) | z=2.621 | 0.009 |
| Serum chloride (mmol/L) | 99.58±5.56 | 99.83±5.44 | 98.98±5.87 | t=0.741 | 0.46 |
| Serum calcium (mmol/L) | 2.00 (1.85, 2.12) | 2.00 (1.88, 2.12) | 2.00 (1.81, 2.12) | z=0.049 | 0.96 |
| D-dimer (mg/L) | 3.13 (1.12, 8.00) | 3.50 (1.06, 9.00) | 2.47 (1.50, 6.05) | z=0.460 | 0.65 |
| Fibrinogen (g/L) | 2.95 (2.01, 4.14) | 2.75 (1.95, 4.04) | 3.20 (2.08, 4.16) | z=−0.793 | 0.43 |
| LDH (U/L) | 716.00 (530.50, 1,597.50) | 854.00 (563.50, 1,864.00) | 625.50 (482.25, 1,150.00) | z=1.788 | 0.07 |
| CK (U/L) | 794.00 (337.50, 1,747.50) | 794.00 (415.50, 1,869.50) | 762.00 (294.50, 1,327.25) | z=1.010 | 0.31 |
| CK-MB (U/L) | 89.00 (44.50, 201.00) | 95.00 (52.50, 255.50) | 57.35 (31.75, 153.00) | z=1.831 | 0.07 |
| ALT (U/L) | 139.00 (48.25, 778.30) | 153.00 (51.25, 788.15) | 90.50 (47.12, 649.45) | z=0.981 | 0.32 |
| AST (U/L) | 286.00 (69.85, 833.90) | 449.00 (81.50, 1039.85) | 142.00 (57.25, 682.42) | z=1.874 | 0.06 |
| Total bilirubin (μmol/L) | 12.90 (9.30, 20.40) | 13.80 (9.75, 23.15) | 12.50 (7.42, 19.05) | z=1.137 | 0.26 |
| Direct bilirubin (μmol/L) | 6.60 (4.20, 11.05) | 6.60 (4.45, 11.95) | 6.55 (3.85, 9.30) | z=0.796 | 0.43 |
| Free triiodothyronine (pmol/L) | 2.52 (2.17, 3.07) | 2.52 (2.16, 3.01) | 2.70 (2.20, 3.19) | z=−0.934 | 0.35 |
| Tree thyroxine (pmol/L) | 15.65 (13.69, 18.18) | 15.64 (13.57, 18.18) | 15.96 (13.71, 18.05) | z=−0.422 | 0.67 |
| TSH (mIU/L) | 0.61 (0.36, 1.19) | 0.61 (0.36, 1.18) | 0.74 (0.38, 1.37) | z=−0.541 | 0.59 |
| Cardiac troponin I (ng/mL) | 11.10 (3.16, 20.95) | 11.70 (3.77, 21.20) | 9.34 (2.09, 17.57) | z=0.875 | 0.38 |
| BNP (pg/mL) | 1,030.00 (512.50, 1,960.00) | 1,090.00 (597.50, 1,990.00) | 868.00 (474.25, 1,467.50) | z=1.238 | 0.22 |
| Lactic acid (mmol/L) | 2.82 (1.85, 4.62) | 3.00 (1.95, 5.24) | 2.64 (1.73, 4.18) | z=1.296 | 0.2 |
| Echocardiography | |||||
| LVEF (%) | 27.00 (20.00, 35.00) | 26.00 (19.00, 35.00) | 28.00 (21.00, 35.25) | z=−1.126 | 0.26 |
| LVEDD (mm) | 47.00 (43.00, 51.00) | 47.00 (44.00, 51.00) | 47.00 (42.50, 51.25) | z=0.298 | 0.77 |
| LAD (mm) | 31.38±7.12 | 32.02±7.54 | 29.89±5.87 | t=1.666 | 0.1 |
| IVST (mm) | 9.00 (9.00, 10.00) | 9.00 (9.00, 10.00) | 9.00 (8.75, 10.00) | z=0.451 | 0.65 |
| LV PWT (mm) | 9.00 (8.00, 10.00) | 9.00 (8.00, 10.00) | 9.00 (8.00, 9.25) | z=0.581 | 0.56 |
| Pericardial effusion | χ2=1.019 | 0.31 | |||
| No | 78 (65.5) | 52 (62.7) | 26 (72.2) | ||
| Yes | 41 (34.5) | 31 (37.3) | 10 (27.8) | ||
| CPR | χ2=0.265 | 0.61 | |||
| No | 82 (68.9) | 56 (67.5) | 26 (72.2) | ||
| Yes | 37 (31.1) | 27 (32.5) | 10 (27.8) |
Data are presented as n (%) for categorical variables and median (P25, P75) or mean ± standard deviation for continuous variables. P values are based on Chi-squared test for categorical variables and t-test for continuous variables. ALT, alanine aminotransferase; AST, aspartate aminotransferase; AV, atrioventricular; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, creatine kinase-MB; CPR, cardiopulmonary resuscitation; DBP, diastolic blood pressure; IVST, interventricular septal thickness; LAD, left atrial diameter; LDH, lactate dehydrogenase; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LV PWT, left ventricular posterior wall thickness; PCT, procalcitonin; SBP, systolic blood pressure; TSH, thyroid-stimulating hormone; VT/VF, ventricular tachycardia or ventricular fibrillation; WBC, white blood cell count.
Univariate Cox regression screening
Univariate Cox regression analysis in the training set identified potential predictors of mortality outcomes, including respiratory symptoms (P=0.03), chest tightness or dyspnea (P=0.003), neurological symptoms (P=0.03), smoking (P=0.01), systolic blood pressure (P=0.03), PCT (P=0.044), creatinine (P<0.001), uric acid (P=0.02), potassium (P=0.02), and sodium (P=0.01) (Table 2).
Table 2
| Variables | Estimate | Standard error | z | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 0.000 | Reference | |||
| Female | −0.143 | 0.451 | −0.318 | 0.866 (0.358, 2.097) | 0.75 |
| Age | 0.013 | 0.012 | 1.069 | 1.013 (0.990, 1.036) | 0.29 |
| Early symptoms | |||||
| Respiratory symptoms | |||||
| No | 0.000 | Reference | |||
| Yes | −1.062 | 0.474 | −2.240 | 0.346 (0.137, 0.876) | 0.03 |
| Gastrointestinal symptoms | |||||
| No | 0.000 | Reference | |||
| Yes | 0.144 | 0.438 | 0.329 | 1.155 (0.490, 2.723) | 0.74 |
| Fever | |||||
| No | 0.000 | Reference | |||
| Yes | −0.249 | 0.450 | −0.555 | 0.779 (0.323, 1.881) | 0.58 |
| Chest tightness or dyspnea | |||||
| No | 0.000 | Reference | |||
| Yes | −1.432 | 0.489 | −2.930 | 0.239 (0.092, 0.622) | 0.003 |
| Chest pain | |||||
| No | 0.000 | Reference | |||
| Yes | −0.823 | 0.630 | −1.306 | 0.439 (0.128, 1.510) | 0.19 |
| Neurological symptoms | |||||
| No | 0.000 | Reference | |||
| Yes | 0.966 | 0.449 | 2.154 | 2.628 (1.091, 6.330) | 0.03 |
| Syncope | |||||
| No | 0.000 | Reference | |||
| Yes | −0.052 | 0.627 | −0.084 | 0.949 (0.278, 3.240) | 0.93 |
| Medical history | |||||
| Smoke | |||||
| No | 0.000 | Reference | |||
| Yes | 1.215 | 0.470 | 2.585 | 3.371 (1.341, 8.469) | 0.01 |
| Alcohol consumption | |||||
| No | 0.000 | Reference | |||
| Yes | 0.735 | 0.517 | 1.421 | 2.086 (0.757, 5.751) | 0.16 |
| Diabetes | |||||
| No | 0.000 | Reference | |||
| Yes | 0.538 | 1.031 | 0.522 | 1.713 (0.227, 12.920) | 0.60 |
| Hypertension | |||||
| No | 0.000 | Reference | |||
| Yes | 0.462 | 0.528 | 0.875 | 1.587 (0.564, 4.467) | 0.38 |
| Malignant arrhythmia | 0.326 | 0.439 | 0.742 | 1.385 (0.586, 3.273) | 0.46 |
| VT/VF | |||||
| No | 0.000 | Reference | |||
| Yes | 0.670 | 0.472 | 1.419 | 1.954 (0.774, 4.932) | 0.16 |
| Third-degree AV block | |||||
| No | 0.000 | Reference | |||
| Yes | −0.238 | 0.561 | −0.425 | 0.788 (0.263, 2.366) | 0.67 |
| Vital signs | |||||
| SBP | −0.029 | 0.013 | −2.245 | 0.972 (0.948, 0.996) | 0.03 |
| DBP | −0.012 | 0.014 | −0.850 | 0.988 (0.962, 1.015) | 0.4 |
| Heart rate | −0.000 | 0.008 | −0.042 | 1.000 (0.983, 1.016) | 0.97 |
| Laboratory examinations | |||||
| WBC | 0.005 | 0.030 | 0.153 | 1.005 (0.947, 1.065) | 0.88 |
| Neutrophil count | −0.002 | 0.033 | −0.068 | 0.998 (0.935, 1.065) | 0.95 |
| Hemoglobin | 0.002 | 0.011 | 0.189 | 1.002 (0.981, 1.023) | 0.85 |
| Platelets | −0.001 | 0.003 | −0.408 | 0.999 (0.993, 1.004) | 0.68 |
| Lymphocyte count | 0.013 | 0.215 | 0.061 | 1.013 (0.665, 1.543) | 0.95 |
| C-reactive protein | −0.005 | 0.004 | −1.281 | 0.995 (0.988, 1.003) | 0.20 |
| PCT | 0.014 | 0.007 | 2.016 | 1.014 (1.000, 1.027) | 0.044 |
| BUN | 0.052 | 0.027 | 1.896 | 1.053 (0.998, 1.112) | 0.06 |
| Serum creatinine | 0.003 | 0.001 | 3.534 | 1.003 (1.001, 1.005) | <0.001 |
| Serum uric acid | 0.002 | 0.001 | 2.396 | 1.002 (1.000, 1.003) | 0.02 |
| Blood glucose | 0.069 | 0.040 | 1.708 | 1.071 (0.990, 1.160) | 0.09 |
| Serum potassium | 0.522 | 0.218 | 2.394 | 1.686 (1.099, 2.585) | 0.02 |
| Serum sodium | 0.092 | 0.036 | 2.568 | 1.096 (1.022, 1.175) | 0.01 |
| Serum chloride | 0.011 | 0.042 | 0.275 | 1.012 (0.932, 1.098) | 0.78 |
| Serum calcium | −1.425 | 1.245 | −1.144 | 0.241 (0.021, 2.763) | 0.25 |
| D-dimer | 0.020 | 0.011 | 1.912 | 1.020 (0.999, 1.042) | 0.06 |
| Fibrinogen | −0.431 | 0.177 | −2.439 | 0.650 (0.460, 0.919) | 0.02 |
| LDH | 0.000 | 0.000 | 2.603 | 1.000 (1.000, 1.000) | 0.009 |
| CK | 0.000 | 0.000 | 0.235 | 1.000 (1.000, 1.000) | 0.81 |
| CK-MB | 0.001 | 0.000 | 2.780 | 1.001 (1.000, 1.002) | 0.005 |
| ALT | 0.000 | 0.000 | 2.839 | 1.000 (1.000, 1.000) | 0.005 |
| AST | 0.000 | 0.000 | 3.525 | 1.000 (1.000, 1.000) | <0.001 |
| Total bilirubin | 0.019 | 0.008 | 2.448 | 1.019 (1.004, 1.034) | 0.014 |
| Direct bilirubin | 0.033 | 0.011 | 2.946 | 1.033 (1.011, 1.056) | 0.003 |
| Free triiodothyronine | 0.045 | 0.066 | 0.683 | 1.046 (0.920, 1.190) | 0.49 |
| Tree thyroxine | 0.016 | 0.012 | 1.340 | 1.016 (0.993, 1.040) | 0.18 |
| TSH | 0.329 | 0.149 | 2.201 | 1.389 (1.037, 1.862) | 0.03 |
| Cardiac troponin I | 0.036 | 0.021 | 1.734 | 1.036 (0.995, 1.079) | 0.08 |
| BNP | 0.000 | 0.000 | 0.941 | 1.000 (1.000, 1.000) | 0.35 |
| Lactic acid | 0.083 | 0.026 | 3.239 | 1.087 (1.033, 1.143) | 0.001 |
| Echocardiography | |||||
| LVEF | −0.064 | 0.025 | −2.619 | 0.938 (0.894, 0.984) | 0.009 |
| LVD | 0.007 | 0.030 | 0.248 | 1.008 (0.950, 1.069) | 0.80 |
| LA | 0.012 | 0.029 | 0.418 | 1.012 (0.956, 1.072) | 0.68 |
| IVST | −0.092 | 0.184 | −0.503 | 0.912 (0.636, 1.307) | 0.62 |
| LV PWT | 0.053 | 0.209 | 0.255 | 1.055 (0.700, 1.590) | 0.80 |
| Pericardial effusion | |||||
| No | 0.000 | Reference | |||
| Yes | 0.326 | 0.439 | 0.742 | 1.385 (0.586, 3.273) | 0.46 |
| CPR | |||||
| No | 0.000 | Reference | |||
| Yes | 2.035 | 0.515 | 3.953 | 7.651 (2.790, 20.986) | <0.001 |
| Dopamine | |||||
| No | 0.000 | Reference | |||
| Yes | −0.021 | 0.747 | −0.028 | 0.979 (0.227, 4.231) | 0.98 |
| Norepinephrine | |||||
| No | 0.000 | Reference | |||
| Yes | 18.255 | 5,093.993 | 0.004 | 84,712,395.863 (0.000, Inf) | >0.99 |
| Mechanical ventilation | |||||
| No | 0.000 | Reference | |||
| Yes | 20.569 | 5,584.800 | 0.004 | 856,733,512.594 (0.000, Inf) | >0.99 |
| Temporary pacing | |||||
| No | 0.000 | Reference | |||
| Yes | 0.006 | 0.561 | 0.011 | 1.006 (0.335, 3.019) | >0.99 |
| Blood transfusion | |||||
| No | 0.000 | Reference | |||
| Yes | −0.053 | 0.441 | −0.120 | 0.948 (0.399, 2.251) | 0.90 |
| MCS | |||||
| IABP | 0.000 | Reference | |||
| VA-ECMO | 1.682 | 1.123 | 1.498 | 5.376 (0.595, 48.580) | 0.13 |
| IABP + VA-ECMO | 2.289 | 1.032 | 2.218 | 9.861 (1.305, 74.488) | 0.03 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AV, atrioventricular; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CI, confidence interval; CK, creatine kinase; CK-MB, creatine kinase-MB; CPR, cardiopulmonary resuscitation; DBP, diastolic blood pressure; HR, hazard ratio; IABP, intra-aortic balloon pump; Inf, infinity; IVST, interventricular septal thickness; LAD, left atrial diameter; LDH, lactate dehydrogenase; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LV PWT, left ventricular posterior wall thickness; MCS, mechanical circulatory support; PCT, procalcitonin; SBP, systolic blood pressure; TSH, thyroid-stimulating hormone; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; VT/VF, ventricular tachycardia or ventricular fibrillation; WBC, white blood cell count.
Variable selection using LASSO
To address multicollinearity, variables with P<0.1 from the univariate Cox regression analysis were included in the LASSO Cox regression. Non-zero coefficient variables were identified, which included respiratory symptoms, chest tightness or dyspnea, smoking, CPR, PCT, creatinine, potassium, lactate dehydrogenase, creatine kinase isoenzyme, alanine aminotransferase, direct bilirubin, thyroid-stimulating hormone (TSH), lactate, and LVEF using the lambda value at the minimum deviation (λ=0.017) (Figure 1).
Multivariate Cox regression
The non-zero coefficients identified by LASSO were incorporated into a multivariate Cox regression analysis using the stepwise method to determine independent predictors, which included respiratory symptoms, CPR, creatinine, direct bilirubin, TSH, lactate, and LVEF (Table 3). A nomogram was then constructed based on these independent predictors.
Table 3
| Variables | Estimate | Standard error | z | HR (95% CI) | P value |
|---|---|---|---|---|---|
| Respiratory symptoms | |||||
| No | 0 | Reference | |||
| Yes | −1.721 | 0.587 | −2.93 | 0.179 (0.057, 0.566) | 0.003 |
| CPR | |||||
| No | 0 | Reference | |||
| Yes | 1.685 | 0.539 | 3.126 | 5.392 (1.875, 15.504) | 0.002 |
| Serum creatinine | 0.003 | 0.001 | 2.608 | 1.003 (1.001, 1.005) | 0.009 |
| Direct bilirubin | 0.047 | 0.016 | 2.864 | 1.048 (1.015, 1.082) | 0.004 |
| TSH | 0.586 | 0.205 | 2.863 | 1.796 (1.203, 2.682) | 0.004 |
| Lactate | 0.079 | 0.035 | 2.297 | 1.083 (1.012, 1.159) | 0.02 |
| LVEF | −0.077 | 0.026 | −3.018 | 0.926 (0.880, 0.973) | 0.003 |
CI, confidence interval; CPR, cardiopulmonary resuscitation; HR, hazard ratio; LVEF, left ventricular ejection fraction; TSH, thyroid-stimulating hormone.
Nomogram
A nomogram was constructed based on the selected variables (Figure 2). In the figure, the first row displays the scores assigned to each variable. For each variable, a vertical line is drawn upward to determine its corresponding score. The total score is obtained by summing the individual scores across all variables. This total score is then located on the total score line, and a vertical line is drawn downward to intersect with the prediction line, indicating the predicted probability of the outcome occurrence. For example, a patient with no respiratory symptoms receives 37 points, CPR performed receives 36 points, a creatinine level of 65 µmol/L corresponds to 4 points, a direct bilirubin level of 6.6 µmol/L corresponds to 7 points, TSH of 0.46 mIU/L corresponds to 6 points, a lactate level of 10.7 mmol/L corresponds to 18 points, and an LVEF of 21% corresponds to 66 points. The total score is 37+36+4+7+6+18+66=178. The predicted total survival rate at 14 days is approximately 50%, and at 28 days, the total survival rate is approximately 28%.

Validation
Calibration curve
Bootstrap validation was performed on the constructed model in both the training and validation sets. The results revealed that the predicted 14- and 28-day overall survival (OS) of the model closely matched the actual OS, indicating good calibration (Figure 3).

Time-dependent ROC curve
The time-dependent ROC analysis demonstrated that the AUC for the training set was 0.907 [95% confidence interval (CI): 0.809, 1.000] on day 14 and 0.880 (95% CI: 0.762, 0.999) on day 28 (Figure 4A). In the validation set, the AUC values were 0.853 (95% CI: 0.707, 0.999) and 0.942 (95% CI: 0.840, 1.000) on days 14 and 28, respectively (Figure 4B). The overall C-index for the model was 0.889 (95% CI: 0.802, 0.976) in the training set and 0.809 (95% CI: 0.668, 0.950) in the validation set (Figure 4C), indicating strong predictive performance.

Risk stratification Kaplan-Meier survival curve validation
In this study, nomogram scores for all participants were calculated using the nomogram model. In the training set, with mortality as the endpoint, the optimal cutoff value for the nomogram score was determined to be 167.05, using the surv_cutpoint function from the R package “survminer”. Patients were categorized into low-risk and high-risk groups based on this cutoff. The analysis revealed that the cumulative incidence of death was significantly lower in the low-risk group compared to the high-risk group across the entire population, the training set, and the validation set (P<0.001, P<0.001, P=0.003, Figure 5).

Clinical decision curve
In the training set, the constructed prediction model demonstrated net benefits for predictions at the 14- and 28-day time points within risk threshold probability ranges of 0.04–0.88 and 0.05–0.81, respectively. The net benefit derived from interventions guided by the model was greater than that achieved by applying interventions to all patients or by withholding interventions altogether. Similarly, in the validation set, the model provided net benefits within risk threshold probability ranges of 0.1–0.97 for the 14-day prediction and 0.11–0.98 for the 28-day prediction. The net benefit from model-based interventions also surpassed that from universal intervention or no intervention, indicating good applicability of the model (Figure 6).
Discussion
The acute phase of FM is associated with a high mortality rate and rapid progression, often necessitating the use of vasopressors, positive inotropic agents, or MCS. Currently, there are limited methods for the early identification of patients at high risk of mortality. This study analyzed clinical data from 119 patients with FM diagnosed at Fuwai Hospital over nearly 6 years to develop an early mortality risk prediction model based on clinical variables. The calculation of individual nomogram scores enables the stratification of patients into distinct risk categories, facilitating the timely identification of those at higher risk and supporting closer monitoring and early optimization of treatment strategies to reduce mortality.
The study identified respiratory symptoms, the need for CPR, and levels of blood creatinine, direct bilirubin, TSH, lactate, and LVEF as significant independent predictors of early mortality in patients with FM. These variables are commonly available in clinical practice, thus enhancing the practical applicability of the risk prediction model.
Respiratory symptoms such as coughing emerged as key predictive factors, particularly in patients with FM caused by viral infections, including those related to coronavirus disease 2019 (COVID-19) (8). Furthermore, patients with FM may develop pulmonary circulation impairment due to the rapid decline in cardiac function, leading to symptoms like coughing. The presence of such symptoms not only worsens the patient’s condition but also signifies a heightened risk of mortality (9). CPR was identified as a significant predictive factor, indicating severe deterioration in the condition of the patient that necessitates urgent intervention. CPR is typically administered in cases of cardiac arrest or severe heart failure, both of which are associated with a poor survival prognosis. Previous study has similarly demonstrated that CPR is strongly correlated with higher mortality rates (10). This study also found that elevated blood creatinine levels were significantly associated with increased mortality risk, consistent with existing research, indicating that renal insufficiency is closely linked to cardiac function status.
Renal impairment can adversely impact the prognosis of patients with acute myocarditis (11). In a study by Hao et al., the incidence of renal insufficiency in patients with FM supported by veno-arterial extracorporeal membrane oxygenation (VA-ECMO) was reported to be 46.5% (12). Patients with renal complications had a higher 30-day mortality rate compared to those without, which aligns with the findings of the current study.
Elevated direct bilirubin levels were similarly associated with a higher risk of mortality. Nakamura et al. conducted a retrospective analysis of 22 patients with FM supported by VA-ECMO, of whom 13 survived (13). The study found significantly higher bilirubin levels in the deceased group compared to the survivors, consistent with the results of this research. This relationship may be attributed to liver dysfunction in patients with FM, where impaired liver function could further increase the strain on cardiac function.
Additionally, Miao et al. reported that low T3 syndrome is associated with a poorer prognosis in patients with FM (14). Those with low T3 syndrome exhibited a higher incidence of ventricular arrhythmias, compromised hemodynamics, reduced cardiac function, and severe renal impairment when compared to patients with normal free T3 levels, leading to an increased 30-day mortality rate.
Thyroid function has been associated with FM, though research specifically examining elevated TSH levels in this condition remains limited. A study has indicated that in pediatric cases of chronic viral myocarditis, thyroid hormone levels correlate with the incidence of malignant arrhythmias, typically characterized by decreased FT3 and FT4 levels along with elevated TSH (15).
Elevated lactate levels are often indicative of tissue hypoxia and metabolic disturbances. Based on studies that have been conducted, lactate levels—measured at baseline, after 6 hours, and as clearance rates—are independent predictors of prognosis in patients with cardiogenic shock supported by VA-ECMO, providing enhanced risk stratification and predictive accuracy (16). Furthermore, a meta-analysis identified high lactate levels as independent predictors of mortality in FM, which is consistent with the findings of this study (17). LVEF is a critical measure of cardiac pumping function, and lower LVEF is closely associated with a poorer prognosis. Baritussio et al. demonstrated that a higher LVEF at diagnosis in patients with FM has a protective effect, with each 1% increase in LVEF reducing the risk by 0.93 times (18). Similarly, another study has supported the role of LVEF as a prognostic marker in FM (19).
Xu et al. conducted a retrospective analysis involving 28 patients with FM and 35 patients without FM, developing an FM risk prediction model that included six predictive factors: mean arterial pressure, blood urea nitrogen, aspartate aminotransferase, troponin I, and ventricular wall motion abnormalities (20). Jiang et al. formulated and validated a straightforward predictive model for the early identification of FM, which included 61 patients with FM and was based on four factors: systolic blood pressure, troponin I, LVEF, and wall motion abnormalities (6). These models focus on the early identification of FM risk, with LVEF serving as a common predictive factor. Other factors, such as blood urea nitrogen and aspartate aminotransferase, reflect liver and kidney function, which is similar to the role of blood creatinine and bilirubin in the present study. Moreover, previous studies have suggested an association between AV block and ventricular arrhythmias with the prognosis of myocarditis (21,22). Therefore, we included third-degree AV block and VT/VF in our analysis. However, these variables were ultimately not retained in the final predictive model. A possible explanation is that ECG variables may exhibit collinearity with other predictors that have stronger prognostic power, such as LVEF. Additionally, LASSO regression inherently favors the selection of a smaller set of independent variables by eliminating those that are highly correlated with others but contribute relatively less to the overall prediction. As a result, third-degree AV block and VT/VF were excluded from the final model. Itoh et al. found that B-type natriuretic peptide (BNP) is significantly elevated in FM, but in our study, BNP did not become a significant predictor of mortality in FM (23). In clinical practice, we have observed that some FM patients had persistently elevated cardiac troponin levels, but relatively normal BNP levels, and these patients eventually had poor outcomes. Yu et al. also observed this phenomenon and suggested that the possible reason is that in severe FM patients, neurohormonal compensatory mechanisms may be impaired, leading to a blunted BNP secretion despite significant myocardial injury (24). This phenomenon may reflect a worse prognosis and insufficient compensatory capacity, which could explain why BNP was not retained as an independent predictor of FM prognosis in the final model.
This study, however, represents the first effort to develop and validate a model specifically for predicting early mortality risk in FM patients. This distinguishes it from the aforementioned studies, which primarily focused on the early identification of the disease rather than mortality risk.
Furthermore, this study is the first attempt to develop a mortality risk prediction model for FM by integrating key clinical predictors and presenting them in a nomogram format. Despite the strong predictive performance of the model in both the training and validation sets, there are some limitations: the small sample size and the lack of external validation. These limitations suggest that further external validation in larger, multicenter cohorts is necessary to confirm the model’s robustness and clinical applicability. Future research should focus on expanding the sample size, addressing potential biases, and incorporating additional predictive factors to improve the model’s accuracy and its applicability across diverse clinical settings.
Conclusions
This study developed an early mortality risk nomogram prediction model based on respiratory symptoms, CPR, creatinine, direct bilirubin, TSH, lactate, and LVEF. Upon validation, the model demonstrated high accuracy, reliability, and clinical applicability, providing a valuable tool for the early identification of high-risk patients in clinical practice and offering significant potential for clinical application.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Footnote
Reporting Checklist: The authors have completed the TRIPOD+AI reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-583/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-583/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-583/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-583/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the People’s Hospital of Zhengzhou University, Heart Center of Henan Provincial People’s Hospital/Central China Fuwai Hospital (No. 201808). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hang W, Chen C, Seubert JM, et al. Fulminant myocarditis: a comprehensive review from etiology to treatments and outcomes. Signal Transduct Target Ther 2020;5:287. [Crossref] [PubMed]
- Sawalha K, Abozenah M, Kadado AJ, et al. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc Revasc Med 2021;23:107-13. [Crossref] [PubMed]
- Ammirati E, Cipriani M, Moro C, et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018;138:1088-99. [Crossref] [PubMed]
- Ammirati E, Cipriani M, Lilliu M, et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017;136:529-45. [Crossref] [PubMed]
- Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev 2022;27:251-61. [Crossref] [PubMed]
- Jiang M, Ke J, Fang MH, et al. Development and Validation of a Prediction Model on Adult Emergency Department Patients for Early Identification of Fulminant Myocarditis. Curr Med Sci 2023;43:961-9. [Crossref] [PubMed]
- Kociol RD, Cooper LT, Fang JC, et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation 2020;141:e69-92. [Crossref] [PubMed]
- Pirzada A, Mokhtar AT, Moeller AD. COVID-19 and Myocarditis: What Do We Know So Far? CJC Open 2020;2:278-85. [Crossref] [PubMed]
- Tschöpe C, Ammirati E, Bozkurt B, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 2021;18:169-93. [Crossref] [PubMed]
- Ohki S, Hosokawa K, Tomioka S, et al. Pediatric Fulminant Myocarditis in Japan: A Retrospective Nationwide Database Study of Hospital Volume, Management Practices, and Mortality. Pediatr Crit Care Med 2021;22:e391-401. [Crossref] [PubMed]
- Nasu T, Ninomiya R, Koeda Y, et al. Impella device in fulminant myocarditis: Japanese Registry for Percutaneous Ventricular Assist Device (J-PVAD) registry analysis on outcomes and adverse events. Eur Heart J Acute Cardiovasc Care 2024;13:275-83. [Crossref] [PubMed]
- Hao T, Chen L, Wu C, et al. Impact of renal complications on outcome in adult patients with acute fulminant myocarditis receiving venoarterial extracorporeal membrane oxygenation: an analysis of nationwide CSECLS database in China. Ann Intensive Care 2023;13:93. [Crossref] [PubMed]
- Nakamura T, Ishida K, Taniguchi Y, et al. Prognosis of patients with fulminant myocarditis managed by peripheral venoarterial extracorporeal membranous oxygenation support: a retrospective single-center study. J Intensive Care 2015;3:5. [Crossref] [PubMed]
- Miao G, Pang S, Zhou Y, et al. Low T3 syndrome is associated with 30-day mortality in adult patients with fulminant myocarditis. Front Endocrinol (Lausanne) 2023;14:1164444. [Crossref] [PubMed]
- Fu MY, Wang QW, Xue Y, et al. Relevant researches on chronic viral myocarditis (CVMC) in children, complicated with arrhythmia and thyroid hormone level. Eur Rev Med Pharmacol Sci 2017;21:3083-7. [PubMed]
- Martínez-Solano J, Sousa-Casasnovas I, Bellón-Cano JM, et al. Lactate levels as a prognostic predict in cardiogenic shock under venoarterial extracorporeal membrane oxygenation support. Rev Esp Cardiol (Engl Ed) 2022;75:595-603. [Crossref] [PubMed]
- Vishram-Nielsen JKK, Foroutan F, Rizwan S, et al. Patients with fulminant myocarditis supported with veno-arterial extracorporeal membrane oxygenation: a systematic review and meta-analysis of short-term mortality and impact of risk factors. Heart Fail Rev 2023;28:347-57. [Crossref] [PubMed]
- Baritussio A, Schiavo A, Basso C, et al. Predictors of relapse, death or heart transplantation in myocarditis before the introduction of immunosuppression: negative prognostic impact of female gender, fulminant onset, lower ejection fraction and serum autoantibodies. Eur J Heart Fail 2022;24:1033-44. [Crossref] [PubMed]
- Xie T, Zang X, Xiong Y, et al. Myoglobin and left ventricular ejection fraction as predictive markers for death in children with fulminant myocarditis. Front Pediatr 2022;10:949628. [Crossref] [PubMed]
- Xu G, Chen F, Zhao W, et al. Establishment and assessment of a nomogram model for predicting the risk of fulminant myocarditis: A STROBE compliant cross-sectional study. Medicine (Baltimore) 2021;100:e25317. [Crossref] [PubMed]
- Ogunbayo GO, Elayi SC, Ha LD, et al. Outcomes of Heart Block in Myocarditis: A Review of 31,760 Patients. Heart Lung Circ 2019;28:272-6. [Crossref] [PubMed]
- Adegbala O, Olagoke O, Akintoye E, et al. Predictors, Burden, and the Impact of Arrhythmia on Patients Admitted for Acute Myocarditis. Am J Cardiol 2019;123:139-44. [Crossref] [PubMed]
- Itoh T, Kobayashi T, Oshikiri Y, et al. Clinical and electrocardiographic characteristics in patients with fulminant myocarditis. J Arrhythm 2022;38:763-71. [Crossref] [PubMed]
- Yu SR, Zhang CY, Xiong WJ, et al. An Hypothesis: Disproportion Between Cardiac Troponin and B-Type Natriuretic Peptide Levels-A High Risk and Poor Prognostic Biomarker in Patients With Fulminant Myocarditis? Heart Lung Circ 2021;30:837-42. [Crossref] [PubMed]


