
Non-invasive tissue characterization in children and young adults with aortic coarctation—an MRI-based prospective study
Highlight box
Key findings
• Extracellular volume fraction (ECV) was significantly higher in patients with aortic coarctation (CoA) compared to healthy controls.
• The burden of diffuse myocardial fibrosis was associated with the severity of the CoA.
• Patients with mild CoAs but concomitant bicuspid aortic valve (BAV) showed more myocardial fibrosis than those with isolated mild CoA.
• Antihypertensive medication may decrease the degree of myocardial fibrosis and cardiomyocyte hypertrophy.
What is known and what is new?
• ECV is a validated imaging biomarker for myocardial fibrosis.
• We conducted a semiquantitative analysis of the ECV based on the severity of the stenosis and compared isolated CoAs with those who showed the most common concomitant heart defect, the BAV.
• The myocardial intracellular water lifetime, a novel biomarker for cardiomyocyte diameter, may detect cellular hypertrophy in early stages of cardiac remodeling.
What is the implication, and what should change now?
• Patients with severe CoAs and those with concomitant BAV may have a higher lifetime risk for diffuse myocardial fibrosis-related sequelae like diastolic dysfunction and arrhythmias.
• Therefore, such patients should receive intensified clinical monitoring, including periodic magnetic resonance imaging (MRI), electrocardiogram (ECG) and echocardiography and early surgical or interventional therapy, if possible.
• Antihypertensive medication may have a positive effect on myocardial remodeling.
Introduction
Despite advances in managing aortic coarctation (CoA) in pediatric patients, it remains a challenging task, requiring lifelong monitoring (1). Surgical and percutaneous interventions provide acute relief, but progressive stenosis or aortic valve regurgitation often leads to multiple reinterventions (Figure 1). Even after successful obstruction relief, patients may experience diastolic dysfunction and reduced exercise capacity (2,3). This suggests persistent pathophysiological alterations, possibly involving myocardial remodeling, which have proven difficult to investigate non-invasively.
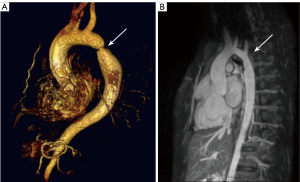
Aortic lesions increase left ventricular (LV) afterload, potentially leading to ventricular hypertrophy and eventual failure (4). LV hypertrophy, though reversible in some cases, substantially elevates the risk of stroke, heart failure, and mortality (5). At the tissue level, LV hypertrophy represents a combination of changes that increase LV wall thickness and mass: initially, pressure overload leads predominantly to cardiomyocyte hypertrophy, but this causes the onset of expansion of the extracellular matrix and accumulation of connective tissue in the interstitial space. While cardiomyocyte hypertrophy is thought to be reversible, the expansion of the extracellular volume may regress to a much lesser extent after normalization of LV afterload (6). Therefore, methods to detect and more precisely phenotype myocardial tissue and determine the contributions from both extracellular volume fraction (ECV) expansion and cardiomyocyte hypertrophy may facilitate earlier detection and intervention and, consequently, preventative therapy for heart failure.
Until now non-invasive methods to phenotype and distinguish cellular hypertrophy and extracellular expansion have been very limited. Studies in animal models have shown that intracellular water lifetime (τic), a novel cardiac magnetic resonance (CMR) biomarker, correlates strongly with cardiomyocyte diameter and can detect cellular hypertrophy in the early stages of cardiac remodeling, even before significant fibrosis occurs (6-8).
In addition to that, cardiac T1 mapping offers a tool, as demonstrated in preliminary studies, detecting significant myocardial remodeling in CoA-patients (9,10). For over a decade, CMR of late gadolinium enhancement (LGE) has been used to detect focal regions of myocardial replacement fibrosis (11). More recently, a CMR technique based on measurements of T1 relaxation times before and after gadolinium administration has been developed to determine the myocardial ECV to assess diffuse fibrosis (12,13). ECV by this technique correlates with myocardial collagen fraction quantified by histopathology in murine hypertension models (14,15) and adults with aortic stenosis (16,17).
While aortic surgery initially improves LV metrics, the long-term durability of these changes requires further investigation. Ultimately, understanding myocardial remodeling in patients with CoA may pave the way for preventive or therapeutic interventions to mitigate or reverse adverse remodeling. While angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are known to reduce cardiac remodeling, especially after myocardial infarction (18) but also in aortic stenosis (19), novel agents targeting fibrotic pathways, such as pirfenidone, could possibly play a therapeutic role in the future (20).
The purpose of this study is to evaluate cardiac remodeling in patients with CoA using non-invasive CMR imaging including native T1, ECV and τic and to compare these findings across patients with low-grade and severe CoAs and those with concomitant bicuspid aortic valve (BAV) to better understand the impact of CoA severity and concomitant BAV on myocardial remodeling. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-497/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This prospective study protocol was approved by the ethics committee of the medical faculty of the Christian Albrechts University in Kiel (Nr. A104/10) and the University of Hamburg (Nr. PV7239). Informed consent was obtained from all individual participants.
Study subjects
Overall, 41 patients from the University Medical Center Schleswig-Holstein and 5 patients from the University Medical Center Hamburg-Eppendorf with CoA were consecutively recruited during follow-up at our institutions (the Children’s Heart Clinic of the University Heart & Vascular Center of the University Medical Center Hamburg-Eppendorf and the Department of Congenital Heart Defects and Pediatric Cardiology of the University Medical Center Schleswig-Holstein) between 2016 and 2022.
Exclusion criteria were other heart defects such as Tetralogy of Fallot or transposition of the great arteries, a moderate to severe valvular stenosis or insufficiency, or generalized connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome, or Loeys-Dietz syndrome. Patients in child- and adulthood up to a maximum age of 45 years were included in the study to largely exclude possible age-related myocardial changes, as Neilan et al. demonstrated in a magnetic resonance imaging (MRI) study that ECV values increase significantly with higher age (21).
The included patients were then grouped into three categories based on various criteria, and the CoA was classified semi-quantitatively.
- Low grade (LG) CoA group designated the “low grade coarctations” and included all patients who:
- Had a maximum flow velocity over the stenosis of <3 m/s (measured by CMR) and;
- Did not receive any blood pressure (BP) medication and;
- Did not receive any re-intervention/-operation until the date of this CMR.
If any of the criteria are not met, the patient was assigned to severe CoA (sCoA) group.
- CoA with BAV includes patients with all grades of CoA who had the most common concomitant heart defect, a BAV, without a moderate to severe valvular stenosis (>25 mmHg systolic pressure gradient, >1 cm2 valve opening area or >3 m/s flow velocity) nor insufficiency (regurgitation fraction >20%).
Comparisons were made with 14 healthy, age-matched individuals. Both patients and healthy individuals with severe claustrophobia, ferromagnetic metal, non-magnetic resonance (MR) compatible aneurysm clip, pacemaker, defibrillator, or other active implant were excluded from participating in this study.
Written informed consent was obtained from all patients, controls, parents, or guardians, as appropriate.
Patients’ clinical characteristics such as age, gender, height, weight, body mass index, body surface area (BSA), heart frequency, systolic and diastolic BP, arterial hypertension medication, age at repair, age at MRI and possible re-interventions were recorded.
CMR acquisition
CMR studies were performed in both centres with the same model of 3.0-Tesla scanner (Achieva 3.0 T, Philips Medical Systems, Netherlands) with a phased-array coil (SENSE™ Cardiac coil, Philips Medical Systems, Netherlands). The maximum flow velocity (Vmax) in the aortic isthmus was determined using two-dimensional phase-contrast flow imaging. The velocity was quantified in the through-plane direction, perpendicular to the imaging slice. The phase-contrast pulse sequence parameter settings were as follows: field of view (FOV), 270 mm × 270 mm; voxel size, 1.64 mm × 1.4 mm × 7 mm; repetition time (TR)/echo time (TE), 4.4/2.7 ms, and a velocity encoding (VENC) range of 200 cm/s. LV volumes and systolic function were measured using a gradient-echo cine sequence in the short-axis plane. Imaging parameters included the following: FOV, 330 mm × 330 mm; matrix size, 176 mm × 190; voxel size, 1.88 mm × 1.74 mm × 6 mm; TR/TE, 3.7/1.8 ms; 25 cardiac phases; number of repetitions: 2; scan duration: 3–6 min. Approximately 15 minutes later, LGE images were acquired with an inversion recovery-prepared 3-dimensional gradient echo sequence (FOV, 300 mm × 178 mm × 80 mm; voxel size, 1.17 mm × 1.27 mm × 10 mm; TR/TE, 3.7/1.83 ms; flip angle, 15°; T1 adjusted to null normal myocardium) with respiratory navigator for detection of diaphragmatic motion. The presence and location of LGE was qualitatively identified within the myocardium by windowing images to null the signal in normal myocardium. T1 measurements for ECV calculation were obtained using a previously described Look-Locker technique (22). This approach was selected because its accuracy and reproducibility have been established. Moreover, compared with a modified Look-Locker inversion recovery approach, this technique has more complete sampling of the T1 recovery curve and is also suited for high heart rates. The latter point was particularly important because a relatively broad range of heart rates was expected, given that both pediatric and adult subjects are referred for CMR studies. An electrocardiogram gated breath-hold Look-Locker sequence with a segmented gradient echo cine acquisition was performed at a single midventricular short-axis slice once before contrast administration and 2–3 times following contrast (5-min post-contrast, 10-min post-contrast, and at the end of the examination). Gadopentetate dimeglumine (Magnevist, Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) was used for contrast, with a bolus dose of 0.2 mmol/kg for patients <20 kg and 0.15 mmol/kg for patients ≥20 kg.
CMR data analysis
Dedicated CMR software (ViewForum, Achieva, Philips Medical Systems, The Netherlands) was used to analyze and postprocess the acquired CMR data.
LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV wall thickness and LV mass were obtained from short-axis cine images by drawing endocardial and epicardial contours at end diastole and end systole. LV stroke volume (SV) was calculated as the difference of LVEDV from the LVESV, and LV ejection fraction (LVEF) was obtained by dividing the LVSV by the LVEDV.
Look-Locker images were segmented along the LV endo- and epicardial borders (Figure 2). The borders were drawn by an assistant doctor in radiology/PhD student in this field and reviewed by a senior cardiac MRI expert with >20 years of experience. Signal intensity versus time curves were generated for six equal sectors of the LV myocardium and the blood pool using commercially available software (QMASS MR, Medis Medical Imaging Systems, Leiden, the Netherlands). Myocardium with LGE was excluded from the region of interest. From these curves, the T1 values were calculated by fitting to an analytical expression for the inversion recovery signal intensity. The myocardial R1 (R1 = 1/T1) was plotted against the blood pool R1. The slope of this relationship defines the partition coefficient for gadolinium (λ). The myocardial ECV was then computed using the following equation: ECV = λ(1 − hematocrit expressed as a fraction).

To quantify cardiomyocyte hypertrophy the τic, a novel cell size-dependent parameter, was determined with a 2-site model for transcytolemmal water exchange by repetitive post-contrast T1 measurements in a subgroup of 22 patients. The average time that a water molecule stays within a cardiomyocyte can be used to detect changes in cell diameter, and thereby characterize cardiomyocyte hypertrophy or atrophy (6). For example, higher values of τic indicate increased myocardial hypertrophy.
Statistical analysis
For statistical analysis and preparation of graphs, including Bland-Altman- and Box-Whisker-plots, the R Statistic package (RRID:SCR_001905, Version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria) and MedCalc (RRID:SCR_015044, Version 14.12.0, Ostend, Belgium) were used.
We powered the study based on preliminary data in healthy adult volunteers (under age 40 years; mean ECV 0.252±0.016). In the absence of previous data on the effect of aortic obstruction in young patients on ECV, we aimed for a minimum effect size of 0.024, which corresponds to the difference in ECV between its mean and the 95th percentile in normal healthy volunteers. This could be deemed a minimal clinically significant difference of ECV. With a standard deviation of 0.016, the analysis of 21 patients gives greater than 90% power to detect this difference with a type 1 error probability of 0.01. In one case that a normal distribution cannot be assumed, a loss of power has to be expected and the number of cases has to be increased to 40 for an error probability of 0.05% (Mann-Whitney U-test).
Patients’ characteristics and clinical data were reported as mean or medians with standard deviation (SD) or interquartile range, respectively, for continuous variables, and as counts and percentages for categorical variables. Ventricular volumes and LV mass were normalized by the body-surface area, estimated by Dubois’ formula. Means between the two groups were compared with the Student’s t-test, or the Wilcoxon-Mann-Whitney test was used as a nonparametric analog if values were not normally distributed. Kruskal-Wallis rank sum test for non-normally distributed and one-way analysis of variance (ANOVA) test for normally distributed cohorts were used to analyze whether there is a significant variance between groups. Post-hoc pairwise t-tests or Wilcoxon-Mann-Whitney tests were performed with adjustment of P values by Holm’s method. Fisher’s exact test was used to test associations between categorical variables. A P value of <0.05 was regarded as significant.
Results
Demographics and clinical characteristics
In total, 46 patients met the criteria for inclusion in the study. The characteristics of the patients and healthy volunteers are summarized in Table 1. The median age, proportion of females, weight, BSA, heart rate, and blood hematocrit were comparable in all groups (Table 1). There was no statistical difference in systolic (P=0.08) and diastolic BP (P=0.06) between the groups. The median age at the time of surgery was 0.3 years (IQR: 0.0–2.5 years) in the LG group, 2.7 years (IQR: 0.1–11.3 years) in the sCoA group and 0.3 years (IQR: 0.1–2.6 years) in the BAV group with no significant difference between the groups, P=0.3. Two patients (14%) in LG group and 4 (25%) in the sCoA group had not yet undergone surgery at the time of the CMR study. One patient with BAV had a balloon dilatation as primary treatment. Most of the patients had a surgical resection with end-to-end anastomosis, 86% of LG group (n=12), 75% of sCoA group (n=12) and 100% of group with BAV (n=16). Seven patients had to undergo a reoperation in the past (part of sCoA and BAV group). Thirteen patients had developed arterial hypertension, which was controlled medically. Mean Vmax in the CoA was 1.86±0.23 m/s in the LG group, 2.61±0.69 m/s in the sCoA group, and 2.03±0.61 m/s in the BAV group (ANOVA P=0.004).
Table 1
| Characteristic | Control (N=14) | LG group (N=14) | sCoA (N=16) | CoA with BAV (N=16) | P value† |
|---|---|---|---|---|---|
| Age at MRI (years) | 16 [13–43] | 23 [17–28] | 17 [12–29] | 20 [15–22] | 0.60 |
| Female | 4 [40] | 9 [64] | 9 [56] | 9 [56] | 0.96 |
| Patient weight (kg) | 55±17 | 70±21 | 59±28 | 62±22 | 0.20 |
| BSA (m2) | 1.57±0.34 | 1.81±0.37 | 1.57±0.52 | 1.66±0.33 | 0.30 |
| Age at surgery (years) | 0.3 [0.0–2.5] | 2.7 [0.1–11.3] | 0.3 [0.1–2.6] | 0.30 | |
| Systolic blood pressure (mmHg) | 129±13 | 125±19 | 117±10 | 0.08 | |
| Diastolic blood pressure (mmHg) | 69±12 | 65±13 | 67±14 | 0.06 | |
| Heart rate (bpm) | 69±19 | 67±7 | 77±17 | 66±18 | 0.30 |
| CoA Vmax (m/s) | 1.86±0.23 | 2.61±0.69 | 2.03±0.61 | 0.002 | |
| Number of CoA surgeries | 0.14 | ||||
| 0 | 2 [14] | 4 [25] | 0 | ||
| 1 | 12 [86] | 7 [44] | 14 [88] | ||
| 2 | 0 | 5 [31] | 2 [12] | ||
| Blood hematocrit (L/L)*100 | 43.6±1.5 | 40.7±3.1 | 36.9±5.4 | 39.8±1.1 | 0.10 |
| T1 native (ms) | 1,213±47 | 1,391±162 | 1,390±127 | <0.001 | |
| ECV (L/L) | 0.26±0.02 | 0.26±0.02 | 0.31±0.04 | 0.31±0.05 | 0.003 |
| Intracellular water lifetime (s) | 0.24±0.01 | 0.28±0.04 | 0.31±0.05 | 0.01 | |
| LV EDV index (mL/m2) | 87±17 | 86±15 | 81±20 | 89±21 | 0.80 |
| LV ESV index (mL/m2) | 33±7 | 27±6 | 28±9 | 37±6 | 0.002 |
| LV ejection fraction (%) | 62±5 | 68±4 | 65±12 | 58±9 | 0.002 |
| LV mass ED index (g/m2) | 63±18 | 57±12 | 55±13 | 55±19 | 0.70 |
| LV mass/EDV (g/mL) | 0.73±0.22 | 0.67±0.10 | 0.69±0.10 | 0.61±0.11 | 0.15 |
| LV cardiomyocyte mass index (g/m2) | 46±14 | 39±8 | 39±11 | 35±12 | 0.30 |
| Cardiac index (mL/min/m2) | 4,133±571 | 4,137±1,187 | 3,572±1,076 | 0.20 | |
| Maximum LV wall thickness (mm) | 12.15±2.13 | 11.35±2.99 | 11.74±1.96 | 0.70 | |
| Beta blocker | 0 | 0 | 6 [38] | 0 | <0.001 |
| ACE inhibitor | 0 | 0 | 1 [6] | 2 [13] | 0.60 |
| ACE + beta-blocker | 0 | 0 | 4 [25] | 0 | 0.02 |
| On BP medication | 0 | 0 | 11 [69] | 2 [13] | <0.001 |
Data are presented as median [interquartile range], n [%], or mean ± standard deviation. †, Kruskal-Wallis rank sum test; Fisher’s exact test. ACE, angiotensin-converting enzyme; BAV, bicuspid aortic valve; BP, blood pressure; BSA, body surface area; CoA, aortic coarctation; ECV, extracellular volume fraction; ED, end-diastolic; EDV, end-diastolic volume; ESV, end-systolic volume; LG, low grade; LV, left ventricular; MRI, magnetic resonance imaging; sCoA, severe coarctation; Vmax, maximum flow velocity.
Myocardial tissue remodeling
ECV with sCoA (0.31±0.04) was significantly higher than with LG CoA (0.26±0.02, P=0.002) and in healthy controls (0.26±0.01, P=0.001). ECV in CoA with BAV (0.31±0.05) was higher compared to LG CoA (P=0.03; Figure 3A) and healthy controls (P=0.03).

Consistent with the results for ECV, native T1 was higher in the sCoA group (T1 =1,391±162 ms), compared to the LG group (T1 =1,213±47 ms; P=0.002) and also higher with BAV (T1 =1,390±127 ms) compared to LG CoA (P=0.002; see Figure 3B). The τic was lowest with LG CoA (0.24±0.03 s) compared to sCoA (0.28±0.04 s; P=0.01) and BAV (0.31±0.05 s; P=0.03; Figure 3C). τic correlated positively with Vmax, but the correlation did not reach statistical significance (r=0.41; P=0.13).
Myocardial ECV in the LG and sCoA groups correlated strongly with the peak blood flow velocity in the aortic coarctation (r=0.8; P<0.001). The correlation of Vmax with ECV was weaker if patients with BAV were included (r=0.46; P=0.01; Figure 4).

LV function and remodeling
The LV ESV index was significantly higher in the group with BAV (37±6 mL/m2) compared to LG (27±6 mL/m2; P=0.001) and sCoA (28±9 mL/m2; P<0.001). The LV mass/EDV was lowest in the group with BAV (0.61±0.11) but not significantly different compared to the LG (0.67±0.1) and sCoA groups (0.69±0.1; P=0.15).
Blood-pressure-lowering therapy
The use of BP medication varied significantly between patient groups (Fisher’s test P<0.001) and was most frequent in the sCoA group (n=11, 69%) compared to the BAV group (n=2, 13%) and LG groups (n=0). Patients on BP medication had undergone surgical repair at a significantly older age [7.3 (interquartile range, 0.1–13.3) vs. 0.1 (interquartile range, 0.0–2.7) years; P=0.02]. When comparing BP values between BP-treated and untreated patients, there were no significant differences observed in systolic BP (123±14 vs. 126±20 mmHg, P=0.60) or diastolic BP (68±13 vs. 65±14 mmHg, P=0.50). Myocardial native T1 (P=0.02) and τic (P=0.03) were significantly lower in patients on BP medication (part of sCoA and BAV group) compared to patients not receiving BP medication (Figure 5A,5B). The ECV was nominally lower in patients receiving BP medication compared to those not being treated (P=0.10; Figure 5C). The effects of BP medication on native T1, ECV and τic were also analyzed with simultaneous adjustment by patient group, to exclude confounding by the latter. This analysis showed a statistically significant effect of BP medication on native T1 (P=0.009) and on τic (P=0.03), indicating a significant reduction. A trend was observed towards lower ECV (P=0.10) with the use of BP medications. No significant effects of BP medication were observed for maximum LV wall thickness (P=0.80), LV EDV index (P=0.30), LV ESV index (P=0.40) and cardiac index (P=0.58).

Discussion
In this study, we evaluated the myocardial tissue and ventricular remodeling in patients with CoAs based on ECV, native T1, τic and global LV parameters.
Patients with CoA had a higher ECV compared to healthy controls. The elevated ECV in patients with CoA indicates a higher burden of myocardial interstitial fibrosis, a known consequence of chronic pressure overload as described in aortic valve stenosis or arterial hypertension (23). The stenosis increases the afterload on the left ventricle, causing hypertrophy and subsequent fibrosis as the myocardium adapts to the increased workload (20). Studies also showed that even after successful resection of the CoA, patients have an impaired artery function with abnormal reactivity and increased stiffness, which also may contribute to the progression of myocardial fibrosis (23). Over time, this fibrotic remodeling can compromise myocardial function and contribute to the clinical manifestations of the disease (24).
Our study also shows that patients with low-grade coarctations have a lower ECV compared to patients with high-grade coarctations. In patients with LG or sCoA there was a strong correlation of ECV with Vmax, indicating that the peak blood flow velocity in the aortic coarctation is indicative of afterload increase. The correlation was weaker when patients with concomitant BAV and a low-grade stenosis (Vmax <3 m/s) were included, suggesting that with a BAV, the peak blood flow velocity in the aortic coarctation only partially captures the LV afterload that may induce ECV expansion and diffuse interstitial fibrosis. Global ventricular remodeling observed with aortic CoA is predominantly hypertrophic, while BAV leads to both hypertrophic and eccentric remodeling can be observed (25). Nevertheless, both sCoA and BAV in this study showed elevated ECV and τic compared to LG CoA. We note that in our study, the LV mass-to-ED-volume ratio was lowest in the BAV group, suggesting less concentric and hypertrophic remodeling than with sCoA. We also found that patients with low-grade stenosis (Vmax <3 m/s) and concomitant BAV had a larger ECV compared to isolated low-grade CoAs (P=0.029). The native T1 times also showed significant differences between patients with concomitant BAV and low-grade stenosis and healthy controls, further supporting the suggestion of significant myocardial tissue remodeling with BAV. Skeffington et al. found that BAV CoA patients have an altered proteome consistent with changes in inflammation, apoptosis and oxidative stress compared to CoA patients of the same age with tricuspid aortic valve. Inflammation and oxidative stress can both be causes of interstitial fibrosis, which would increase ECV and native T1 (26). Nucifora et al. demonstrated that even isolated BAV without any valve deficiency showed higher velocity in the ascending aorta as well as a higher wall shear stress compared to healthy controls, suggesting that there might be an additional burden on the myocardium beyond the CoA (27). Shibagaki et al. showed that these findings could also be explained by kinetic energy and vortex formation, as 4D-flow-MRI showed helical flow in the ascending aorta in a patient with CoA and concomitant BAV (28).
Our results also show that LV hemodynamics are pathologically altered with BAV. The ESV index was significantly higher (P<0.001), potentially indicating some degree of contractility impairment. Supporting this, Bilen et al. demonstrated that patients with isolated BAVs exhibited increased left atrial volumes and reduced E/E' ratio, suggestive of both contractility and relaxation impairments (29).
Understanding the differences in ECV among these groups is crucial for tailoring clinical management strategies. LG patients may benefit from regular monitoring but may not require intensified surveillance and treatment unless their condition changes. In contrast, sCoA patients might need more intensive management, including closer monitoring, potential surgical intervention, especially if a relevant re-coarctation is detectable, and possibly the use of antihypertensive or antifibrotic agents to mitigate the progression of myocardial fibrosis. Despite having low-grade stenoses, patients with concomitant BAV exhibit higher ECV, suggesting a need for comprehensive care addressing both the CoA and the BAV, which could include surgical correction of the stenosis and management of BAV-related complications.
The higher ECV observed in patients with CoA is associated with an increased risk of diastolic dysfunction and arrhythmias (30). Myocardial fibrosis leads to stiffening of the ventricular walls, impairing their ability to relax and fill appropriately during diastole, which can result in symptoms of heart failure, such as shortness of breath and exercise intolerance (31). Additionally, fibrotic tissue can disrupt the normal electrical conduction pathways in the heart, creating areas of electrical heterogeneity that increase the risk of arrhythmias, including ventricular tachyarrhythmias, which can have significant clinical implications, including an increased risk of sudden cardiac death (32). This being said, periodic echocardiographic and electrocardiographic monitoring in follow-up care is indispensable.
The current state of research into antifibrotic therapy for myocardial fibrosis is evolving. Antifibrotic therapies aim to reduce the deposition of extracellular matrix proteins and slow or reverse the fibrotic process. Pharmacological interventions, such as ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists (MRAs), have shown promise in reducing myocardial fibrosis by lowering BP and reducing the workload on the heart. In our cohort, it was observed that patients taking BP medications had significantly lower native T1 values and less myocardial hypertrophy compared to those who did not receive such medications. Future studies could specifically investigate which classes of antihypertensive drugs have the most beneficial effect on myocardial remodeling. Additionally, novel agents targeting fibrotic pathways, such as pirfenidone, TGF-β-, PDGF-, and endothelin 1-inhibitors, are under investigation and have shown promising results in animal models (33). The cardio-protective effects of antihypertensive medications not only improve parameters of LV function but may also be associated with subclinical changes in myocardial remodeling. CMR markers such as ECV, native T1, and τic could serve as sensitive subclinical markers. These markers could help clinicians detect early, subtle changes in myocardial health, providing a surrogate measure to guide the initiation of antihypertensive therapy or enhance follow-up strategies.
Both cardiomyocyte diameter and ECV influence LV mass, but ECV represents a volume fraction, which, with a constant interstitial fibrosis burden, can increase with regression of cardiomyocyte hypertrophy. For example, in patients with aortic stenosis, such an increase of ECV was observed after aortic valve replacement and regression of LV hypertrophy (34). It is therefore useful to assess both cardiomyocyte size and ECV to allow a more complete interpretation of the changes in myocardial tissue structure.
Further research is warranted to explore the mechanisms driving the increased ECV in CoA and to establish the prognostic significance of these findings. Longitudinal studies could assess whether changes in ECV in patients with CoAs de facto correlate with clinical outcomes and whether interventions that reduce these markers can improve these outcomes. Investigating the relationship between ECV and other biomarkers of myocardial health, such as myocardial strain and perfusion, could provide a more comprehensive understanding of myocardial remodeling in CoAs.
The study had the following limitations: the sample size was relatively small, and more extensive studies are needed to confirm these findings. Additionally, the study’s cross-sectional nature precludes conclusions about causality, necessitating longitudinal studies to determine the temporal relationship between CoAs, elevated ECV, and clinical outcomes. In some cases, the ECV could only be calculated using an imputed hematocrit (41%), as some guardians refused blood sampling for their children. A potential confounder that may influence myocardial remodeling is the use of cardiopulmonary bypass (CBP) during the surgical resection. Mechanisms like ischemia-reperfusion injury or mechanical loading alterations may affect the myocardium independently of the underlying pathology and were not specifically addressed in this study. However, it must be noted that when performing a CoA resection with end-to-end anastomosis, CPB is generally not used, except in exceptional cases. Future investigations could explore the impact of the intervention/surgery (e.g., CBP) on MRI-based remodeling parameters. Additionally, it could be investigated how quickly an improvement in ECV or native T1 can be detected following the surgical resolution of the pressure gradient.
Conclusions
This study demonstrates for the first time that patients with CoA exhibit significantly expanded ECV, indicative of increased myocardial fibrosis, especially with high-grade stenoses and concomitant BAV. The results highlight the need for individualized clinical management to address both CoA and associated cardiac abnormalities to mitigate fibrosis and improve patient outcomes. Application of antihypertensive medication was associated with less myocardial fibrosis and cardiomyocyte hypertrophy, demonstrating its therapeutic effectiveness. However, further research is required to explore targeted therapies and their long-term efficacy in this population and in this context, the novel myocardial tissue markers employed in this study may provide important mechanistic insights.
Acknowledgments
We thank Mrs. Traudel Hansen (CMR technologist; Kiel) and Mrs. Alica Krause (study nurse; Hamburg) for their assistance in patient management.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Harald Kaemmerer) for the series “Current Management Aspects of Adult Congenital Heart Disease (ACHD): Part VI” published in Cardiovascular Diagnosis and Therapy. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-497/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-497/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-497/prf
Funding: This study was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-497/coif). The series “Current Management Aspects of Adult Congenital Heart Disease (ACHD): Part VI” was commissioned by the editorial office without any funding or sponsorship. I.V. serves as an unpaid editorial board member of Cardiovascular Diagnosis and Therapy from February 2024 to January 2026. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This prospective study protocol was approved by the ethics committee of the medical faculty of the Christian Albrechts University in Kiel (Nr. A104/10) and the University of Hamburg (Nr. PV7239). Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis intermediate and late follow-up. J Am Coll Cardiol 2010;56:1740-9. [Crossref] [PubMed]
- Kipps AK, McElhinney DB, Kane J, et al. Exercise function of children with congenital aortic stenosis following aortic valvuloplasty during early infancy. Congenit Heart Dis 2009;4:258-64. [Crossref] [PubMed]
- Friedman KG, McElhinney DB, Colan SD, et al. Left ventricular remodeling and improvement in diastolic function after balloon aortic valvuloplasty for congenital aortic stenosis. Circ Cardiovasc Interv 2012;5:549-54. [Crossref] [PubMed]
- Cyran SE, James FW, Daniels S, et al. Comparison of the cardiac output and stroke volume response to upright exercise in children with valvular and subvalvular aortic stenosis. J Am Coll Cardiol 1988;11:651-8. [Crossref] [PubMed]
- Kannel WB. Left ventricular hypertrophy as a risk factor: the Framingham experience. J Hypertens Suppl 1991;9:S3-8; discussion S8-9. [Crossref] [PubMed]
- Coelho-Filho OR, Shah RV, Mitchell R, et al. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation 2013;128:1225-33. [Crossref] [PubMed]
- Ferreira de Souza T, Quinaglia A C. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc Imaging 2018;11:1045-55. [Crossref] [PubMed]
- Coelho-Filho OR, Mitchell RN, Moreno H, et al. MRI based non-invasive detection of cardiomyocyte hypertrophy and cell-volume changes. J Cardiovasc Magn Reson 2012;14:O10. [Crossref]
- Diao KY, Yang ZG, Xu HY, et al. Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson 2016;18:92. [Crossref] [PubMed]
- Karur GR, Aneja A, Stojanovska J, et al. Imaging of Cardiac Fibrosis: An Update, From the AJR Special Series on Imaging of Fibrosis. AJR Am J Roentgenol 2024;222:e2329870. [Crossref] [PubMed]
- Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;43:2260-4. [Crossref] [PubMed]
- Jerosch-Herold M, Sheridan DC, Kushner JD, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2008;295:H1234-42. [Crossref] [PubMed]
- Broberg CS, Chugh SS, Conklin C, et al. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging 2010;3:727-34. [Crossref] [PubMed]
- Coelho-Filho OR, Mongeon FP, Mitchell R, et al. Role of transcytolemmal water-exchange in magnetic resonance measurements of diffuse myocardial fibrosis in hypertensive heart disease. Circ Cardiovasc Imaging 2013;6:134-41. [Crossref] [PubMed]
- Messroghli DR, Nordmeyer S, Dietrich T, et al. Assessment of diffuse myocardial fibrosis in rats using small-animal Look-Locker inversion recovery T1 mapping. Circ Cardiovasc Imaging 2011;4:636-40. [Crossref] [PubMed]
- Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of the diffuse myocardial fibrosis: preliminary validation in humans. Circulation 2010;122:138-44. [Crossref] [PubMed]
- Flett AS, Sado DM, Quarta G, et al. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2012;13:819-26. [Crossref] [PubMed]
- Ma Y, Yuan J, Hu J, et al. ACE inhibitor suppresses cardiac remodeling after myocardial infarction by regulating dendritic cells and AT2 receptor-mediated mechanism in mice. Biomed Pharmacother 2019;114:108660. [Crossref] [PubMed]
- Goh SS, Sia CH, Ngiam NJ, et al. Effect of Renin-Angiotensin Blockers on Left Ventricular Remodeling in Severe Aortic Stenosis. Am J Cardiol 2017;119:1839-45. [Crossref] [PubMed]
- Aimo A, Spitaleri G, Panichella G, et al. Pirfenidone as a novel cardiac protective treatment. Heart Fail Rev 2022;27:525-32. [Crossref] [PubMed]
- Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging 2013;6:672-83. [Crossref] [PubMed]
- Liu J, Drak D, Krishnan A, et al. Left Ventricular Fibrosis and Systolic Hypertension Persist in a Repaired Aortic Coarctation Model. Ann Thorac Surg 2017;104:942-9. [Crossref] [PubMed]
- Heger M, Willfort A, Neunteufl T, et al. Vascular dysfunction after coarctation repair is related to the age at surgery. Int J Cardiol 2005;99:295-9. [Crossref] [PubMed]
- Wedin JO, Vedin O, Rodin S, et al. Patients With Bicuspid Aortic Stenosis Demonstrate Adverse Left Ventricular Remodeling and Impaired Cardiac Function Before Surgery With Increased Risk of Postoperative Heart Failure. Circulation 2022;146:1310-22. [Crossref] [PubMed]
- Kara R, Vergara C. Assessing turbulent effects in ascending aorta in presence of bicuspid aortic valve. Comput Methods Biomech Biomed Engin 2024;27:2349-61. [Crossref] [PubMed]
- Skeffington KL, Bond AR, Bigotti MG, et al. Changes in inflammation and oxidative stress signalling pathways in coarcted aorta triggered by bicuspid aortic valve and growth in young children. Exp Ther Med 2020;20:48. [Crossref] [PubMed]
- Nucifora G, Miller J, Gillebert C, et al. Ascending Aorta and Myocardial Mechanics in Patients with "Clinically Normal" Bicuspid Aortic Valve. Int Heart J 2018;59:741-9. [Crossref] [PubMed]
- Shibagaki Y, Oka H, Nakau K, et al. Intraventricular haemodynamic changes caused by increased left ventricular afterload in re-coarctation of aorta: a case report. Eur Heart J Case Rep 2023;7:ytad514. [Crossref] [PubMed]
- Bilen E, Akçay M, Bayram NA, et al. Aortic elastic properties and left ventricular diastolic function in patients with isolated bicuspid aortic valve. J Heart Valve Dis 2012;21:189-94. [PubMed]
- Dusenbery SM, Jerosch-Herold M, Rickers C, et al. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol 2014;63:1778-85. [Crossref] [PubMed]
- Egbe AC, Miranda WR, Devara J, et al. Effect of Combined Ventricular-Arterial Stiffening on Exercise Hemodynamics in Adults With Repaired Coarctation of Aorta. CJC Open 2021;3:603-8. [Crossref] [PubMed]
- Sohns C, Marrouche NF. Atrial fibrillation and cardiac fibrosis. Eur Heart J 2020;41:1123-31. [Crossref] [PubMed]
- de Jong S, van Veen TA, van Rijen HV, et al. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 2011;57:630-8. [Crossref] [PubMed]
- Treibel TA, Kozor R, Schofield R, et al. Reverse Myocardial Remodeling Following Valve Replacement in Patients With Aortic Stenosis. J Am Coll Cardiol 2018;71:860-71. [Crossref] [PubMed]


