
Applications of multi-modality imaging in the diagnosis of infective endocarditis: a real-life case based contemporary narrative review
Introduction
Infective endocarditis (IE) is a serious infection with an estimated in-hospital mortality up to 22%, up to 40% at 1 year, and 30-day readmission rate estimated at 13.8% (1,2). Modified Duke criteria have been classically used to diagnose IE (3). These criteria are based on clinical features (i.e., evidence of embolic event or immunologic phenomenon by physical examination), microbiology findings, and imaging findings (3). Despite the importance of Modified Duke criteria, it has limitations, particularly, in the presence of culture negative organisms (4,5) and in cardiac implanted devices, often related to challenging diagnosis with echocardiography (3,6).
In the updated 2023 Duke International Society of Cardiovascular Infectious Disease (ISCVD) criteria, “typical” microorganisms isolated from 2 or more separate blood culture sets (each set consisting of 1 aerobic and 1 anaerobic bottle) count as a major criterion. On the other hand, less commonly or rarely encountered microorganisms associated with IE should be isolated in 3 or more separate blood cultures to fulfil the requirement of a major criterion (7). Intraoperative evidence of IE (i.e., vegetations, abscess, valvular destruction, dehiscence of prosthetic valve) was added as a major criterion when other definitive criteria of IE are not available (7). Additionally, predisposing conditions such as the presence prosthetic material (i.e., transcatheter valve implant/repair, endovascular leads of cardiac implanted devices), additional vascular phenomenon (i.e., cerebral abscess, splenic abscess), and glomerulonephritis caused by immune complex deposition (within the immunologic phenomena category) were added to the list of minor criteria. The updated Duke-ISCVD criteria also now recognize findings on advanced cardiovascular imaging including 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) and cardiac computed tomography (CCT) as major imaging criteria for IE (7).
Conventionally, echocardiography is accepted and recognized as the first-line imaging modality for evaluation of IE. However, with increasing use of cardiac implantable devices and prosthetic valves, the rise of complicated cases of IE has been associated with an increased interest in the applications of multimodality imaging in IE (3). This article aims to highlight the role of multimodality imaging including nuclear imaging and CCT in diagnosis and management of IE through real-life scenarios of complex cases. We present this article in accordance with the Narrative Review reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-605/rc).
Methods
Information used to write this paper was collected from PubMed database between Jan 01, 2024 and Oct 01, 2024. Search term used are presented in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | Jan 01, 2024–Oct 01, 2024 |
| Database searched | PubMed |
| Search terms used | “infective endocarditis”, “multi-modality imaging”, “guidelines”, “echocardiography”, “cardiac computed tomography”, “nuclear imaging”, “18F-FDG PET”, “WBC SPECT” |
| Timeframe | Up to Oct 01, 2024 |
| Inclusion criteria | Included most up-to-date society guidelines, investigational studies (both prospective and retrospective), meta-analyses, and updated reviews |
| Selection process | Authors of this review conducted individual relevant literature search |
18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; WBC SPECT, white blood cell single-photon emission computed tomography.
Role of echocardiography
Echocardiography consisting of transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) is widely utilized as an initial evaluation modality for IE (Table 2) (8). The sensitivity and specificity of TTE in diagnosing IE has been shown to range between 50–90% and 90%, respectively (9). TEE has higher sensitivity in detecting IE compared to TTE in both native valves (96% vs. 70%) and prosthetic valves (92% vs. 50%) (8,10). This sensitivity has significantly improved when using contemporary TEE probes (95.3% vs. 85.7%) due to improvement in two-dimensional (2D) and three-dimensional (3D) imaging over time (11). Furthermore, the imaging quality of contemporary TEE probes has continued to improve thanks to higher frame rates and frequencies (12). Importantly, TEE has also demonstrated appreciably better detectability of perivalvular compilations such as peri-aortic abscesses compared to other abscess locations (sensitivity: 86%) (13). To illustrate the importance of echocardiography and modern TEE, the following case presents a late diagnosis of IE with Cutibacterium acnes after mitral valve (MV) repair. The diagnosis was missed on TTE and ultimately found on TEE, after the patient unfortunately presented with recurrent strokes.
Table 2
| Modality | Appropriate use in IE |
|---|---|
| Echocardiography | Initial evaluation modality |
| TEE should be done if clinical suspicion is high despite negative TTE | |
| Cardiac computed tomography | If there are relative contraindications to TEE |
| To detect prosthetic valve vegetations, abscesses, and pseudoaneurysms | |
| For surgical planning and in identifying extracardiac complications | |
| Defining anatomy before CIED extraction | |
| 18F-FDG PET/CT(A) | Can be utilized when echocardiography is inconclusive and there is high suspicion for IE |
| Can be considered in suspected ascending aortic graft infections, IE related to CIED related, cardiac device pocket infection, and infection of the superficial skin, LVAD infections, pocket and/or driveline infections | |
| Can detect peripheral embolic and metastatic infectious | |
| To diagnose IE in setting of MAC | |
| Leukocyte scintigraphy | Could be considered if PVE is suspected despite inconclusive echocardiography and 18F-FDG PET/CT(A) |
| Particularly useful in settings of uncertain diagnosis of PVE, IE related to CIED, and LVAD related IE | |
| Can detect non-cardiac complications (except ophthalmitis and intracerebral infections) | |
| Is generally preferred over 18F-FDG PET/CT in the setting of recent cardiac surgery (within 3 months) |
18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography; CIED, cardiac implanted electronic devices; IE, infective endocarditis; LVAD, left ventricular assist device; MAC, mitral annular calcification; PVE, prosthetic valve endocarditis; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Case 1
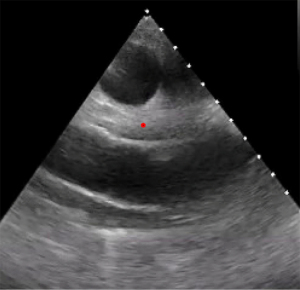
A 52-year-old male with a history of atrial fibrillation and mitral regurgitation status post MV repair 10 years prior presented to clinic after several recent strokes. Over the course of the past 5 months the patient experienced 5 recurrent neurologic episodes including left sided tingling and numbness. With each event, a brain magnetic resonance imaging (MRI) was performed and revealed new acute and subacute cortical infarcts. During that time, the patient’s anticoagulation was transitioned from apixaban to warfarin, followed by enoxaparin by the neurology team. Despite adherence to these medication changes, the patient experienced ongoing strokes. TTE was performed which did not reveal any clear sources of embolization, albeit with sub-optimal image quality (Figure 1A,1B). Three months later a limited TEE was performed and reportedly notable only for presence of a patent foramen ovale (PFO). The patient was referred to our outpatient clinic 6 months later, where a repeat TEE was performed. This revealed two distinct, mobile echodensities along the MV ring, both anteriorly and posteriorly (Figure 2A,2B). He was admitted for further work up, and initial blood cultures as well as serologies for culture negative endocarditis (including Bartonella, Coxiella, Enteric bacteria, and Whipple’s disease) were negative. The patient underwent redo cardiac surgery and mechanical MV replacement (MVR). Polymerase chain reaction testing from the operative sample of the MV yielded Cutibacterium acnes.


Role of CCT
CCT offers excellent anatomical evaluation, specifically in the assessment of peri-annular complications as well as non-cardiac anatomy (14). This is specifically more applicable in patients with relative contraindications to TEE, or patients with intracardiac prosthesis (15). CCT can better visualize prosthetic valve vegetations, abscesses, and pseudoaneurysms (15). It also assists with surgical planning and could identify extracardiac complications (Table 2) (16).
Advancements in CCT technology including the use of dedicated cardiac gated four-dimensional (4D) CCT, have been applied to evaluation of complex cases of IE (14). Endocarditis protocol 4D CCT has the advantage of minimizing motion artifacts and capturing either the entirety or a selected portion of the cardiac cycle, allowing assessment over time (14). 4D CCT enables more accurate anatomical assessment, due to its high spatial resolution. 4D CCT is used to improve the identification of perivalvular extension of infection and assess for complications such as pseudoaneurysm formation. 4D CCT is also used to visualize abnormal leaflet thickening, valve thrombus, and abnormal leaflet motion of prosthetic valves, particularly in scenarios where these findings are not easily appreciated on echocardiography or standard CT imaging (17). The European Society of Cardiology (ESC) guidelines recommend implementing CCT when managing IE, with paravalvular involvement demonstrated by CCT now recognized as a major imaging criterion (8). CCT is also recommended by the American College of Cardiology/American Heart Association as an adjunctive imaging modality for IE management (18).
CCT has overall reported excellent diagnostic performance, achieving 97% sensitivity, 88% specificity, 97% positive predictive value, and 88% negative predictive values for evaluation of appropriate cases of IE (19). This modality is less likely to detect valvular vegetations compared TEE (17) due to lower temporal resolution (20). CCT has 93% sensitivity in detecting prosthetic valve endocarditis (PVE) (17), and when coupled with the traditional evaluation for detecting IE, it has been improved to increase the sensitivity and specificity of detecting PVE further to 100% and 83%, respectively (17). A meta-analysis analyzing 10 studies, encompassing 872 patients, compared CCT to TEE and concluded that CCT had higher sensitivity and specificity for the demonstration of peri-annular complications compared to TEE (93% and 88%, respectively vs. 96% and 70%, respectively, P=0.06) (21). In addition, a change in management strategy was noted in 25% of patients after integrating CCT (22).
CCT has a lesser role in detecting cardiac implanted electronic devices (CIED) (implantable cardioverter defibrillators and pacemakers) related IE, but it can be utilized in conjunction with radionuclide imaging to provide better anatomical localization of areas with nuclear uptake (23). One of the major limitations in this setting is the blooming and beam hardening artifacts caused by the generator in the device pocket (23). CCT also plays a role in planning for potential cardiovascular implantable device extraction when there is suspicion of infective change, since it defines cardiac venous anatomy and could identify other anatomical abnormalities before device extraction (24). CCT was recognized as an important adjuvant imaging modality in the 2023 Duke-ISCVID IE criteria (7).
Despite the strengths of CCT, it mandates dedicated scanning protocols and physicians with expertise in the interpretation of IE and its complications by CCT (14). Since acquired images need to be gated for acquisition (typically during diastole when cardiac motion is minimized), irregular heart rhythm or tachycardia may pose challenges for images quality (14). Although newer scanners have reduced radiation exposure, 4D CCT can still be associated with significant radiation exposure (25). Right sided structures such as the tricuspid valve can also be challenging to interpret due to contrast mixing in the right heart, requiring optimal protocols for right ventricle assessment (15). Due to its dynamic motion and complex anatomy, the MV can also be more difficult to evaluate by CCT, which has been found to have lower specificity in detecting complications relating to the MV (21). Adverse reactions from intravenous (IV) contrast should be taken into consideration when using this modality, which ranges from 3% when using low osmolality agents and this risk goes up to as high as 15% associated with high osmolality agents (25). These reactions could occur immediately or have a subacute onset, causing rash, bronchospasm, angioedema, anaphylaxis or anaphylactoid reaction, vomiting, and injection site discomfort/pain (25). Nephropathy observed 3 days following contrast use has been reported in up to 25% of patients, though this has been predominantly noted in patients with baseline chronic kidney dysfunction (25,26). The following cases illustrate the utility of CCT as an adjunct imaging modality to TEE in the evaluation of PVE following transcatheter aortic valve replacement (TAVR) with paravalvular extension (case 2), and the value of CCT in detecting massive aortic root abscess in a patient with a history of IV drug use (IVDU).
Case 2
A 72-year-old male with a history of rheumatic heart disease, coronary artery disease status post bypass and 35mm bioprosthetic MVR 20 years ago, St. Jude mechanical aortic valve replacement (AVR) in 2004 complicated by several intracranial hemorrhages requiring re-do bioprosthetic AVR, and now status post 26 mm Sapien 3 TAVR in 2022 for prosthetic aortic regurgitation (AR), presented with several weeks of fatigue, intermittent fevers, and weight loss. He was admitted to the hospital for evaluation, where blood cultures grew Streptococcus gordonii. A TTE was performed, notable for a TAVR valve with mildly thickened leaflets, thickened aortic root, trivial AR, and peak/mean gradients of 20/10 mmHg and dimensionless index of 0.43. The bioprosthetic MV showed no clear abnormalities, with peak/mean gradients of 16/7 mmHg at 70 bpm (Figure 3A-3C). A TEE was subsequently performed, revealing an aortic root abscess extending into the aorto-mitral curtain, as well as small echo densities on the prosthetic MV (Figure 4A-4E). A gated CCT revealed several small focal regions of communicating abscesses adjacent to the aortic annulus and aorto-mitral curtain, with moderate thickening and dilation of the aortic root (Figure 5A-5C). His course was complicated by several embolic phenomena, including a lateral ST elevation myocardial infarct, for which he was taken emergently for coronary angiography (Figure 6), undergoing thrombectomy to the left circumflex artery. He also experienced several transient ischemic attacks with acute and subacute infarcts noted in the frontal lobe and cerebellum on brain MRI. He underwent successful fourth redo sternotomy with AVR, aortic root replacement, and MVR.




Case 3
A 32-year-old male with a history of IVDU and two prior AVRs for endocarditis, the most recent being St. Jude Trifecta aortic valve placed one month prior to his presentation, presented to the hospital with concerns of chest and back pain after using his peripherally inserted central catheter line for illicit drug injections. Blood cultures grew Enterobacter hormaechei, Streptococcus viridans, and Candida dubliniensis. A TTE revealed severe prosthetic aortic valve regurgitation due to endocarditis, with an expansile dilated root concerning for aortic root pseudoaneurysm and/or abscess (Figure 7A,7B). As part of the surgical evaluation, the patient was found to have small septic emboli in the occipital lobe by brain MRI, and a mycotic aneurysm by cerebral angiogram with unsuccessful coiling requiring craniotomy for clipping. Pre-operative gated computed tomography angiography (CTA) clearly defined multiple collections around the aortic root with septations, consistent with complex abscess formation (Figure 8). The patient was taken for his third redo AVR with homograft placement after neurology clearance. Intraoperative TEE similarly showed multiple collections with septations around the aortic root, with communication between the left ventricular outflow tract and abscesses (Figure 9). His post-operative course was complicated by complete heart block requiring pacemaker placement, severe right ventricle dysfunction with gradual improvement, and acute deep vein thrombosis. He was successfully discharged on post-operative day 19.



Role of nuclear imaging in IE
Nuclear imaging modalities play a major role in the imaging evaluation of IE, particularly when prosthetic valves and implanted devices are implicated (14). This imaging modality should be utilized, when echocardiography is inconclusive and there is ongoing high clinical suspicion for IE (Table 2) (14). Both 18F-FDG PET/CT(A) and leukocyte scintigraphy are more sensitive than echocardiography in detecting infected implanted cardiac devices. More importantly, they are more effective in early detection of the infection (14). 18F-FDG PET/CT has been incorporated into the 2023 Duke-ISCVID IE criteria as an important adjunct imaging modality (7).
18F-FDG PET/CT has been reported to have high sensitivity and specificity values of 81% and 85%, respectively (27), with positive predictive value and negative predictive value of 93% and 88%, respectively (17).
Leukocyte scintigraphy, in contrast, has reported sensitivity and specificity values of 86% and 97%, respectively (27). It should be noted that a meta-analysis encompassing 27 studies and 1,388 patients concluded that 18F-FDG PET/CT was more sensitive than leukocyte scintigraphy for detecting overall burden of disease including non-cardiac sites of infection (28). It is important to be aware that both tests could yield false positive results in the setting of recent cardiac surgery (within 3 months) or other inflammatory conditions (23).
Leukocyte scintigraphy with single-photon emission CT (SPECT) is highly specific for infection since the granulocytes are recruited to the site of the infection (17). With this modality, the white blood cells (WBC) are labeled to track their accumulation in infectious sites (29). There are two radiotracers available to label the WBC, 111indium-oxine (111In) and 99mTechnetium-hexamethylpropyleneamine oxime (99mTc-HMPAO) (29). This imaging study is particularly useful in the scenario of uncertain diagnosis of PVE, CIED associated IE, and left ventricular assist device (LVAD) related IE (17). It also is also able to detect non-cardiac complications (outside of ophthalmitis and intracerebral infections) (Table 2) (30). The absence of IE on imaging rules out the disease and correlates with favorable clinical outcomes (30). High intensity uptakes, on the other hand, has been associated with worse clinical outcomes and may have prognostic value and assist with guiding management (17). Data related to using radiolabeled leukocyte scintigraphy in diagnosing IE remains limited (31).
18F-FDG PET/CT technique relies on the ability of the 18F-FDG to enter cells through glucose transporters, thus allowing identification of cells with increased metabolic activity (29). Unlike glucose,18F-FDG does not undergo further glycolysis, unlike it accumulates in the cells, reflecting concentrations of glucose in the tissue (25). This modality provides dynamic information on the extent of IE before occurrence of structural damage using a single test (17). In addition, 18F-FDG PET/CT could help diagnose IE in the presence of the mitral annular calcification (MAC) (28), which has in fact been observed in patients with MAC (Table 2) (32). Furthermore, 18F-FDG PET/CT can detect peripheral embolic and metastatic infectious complications, thereby reducing the rate of misdiagnosed IE (15).
The use of 18F-FDG PET/CT in setting of native valve endocarditis (NVE) should be limited to situations when peripheral septic embolism is suspected (33) due to lower sensitivity in detecting NVE (sensitivity and specificity of 31% and 98%, respectively) (34). In the setting of PVE, 18F-FDG PET/CT has a reported sensitivity value range from 73% to 100% and specificity value range from 71% to 100% (17). In patients with PVE in particular, combining 18F-FDG PET with CTA should be considered for better detection of abscess formation (Table 2) (17,35,36). 18F-FDG PET/CT(A) is even more valuable in diagnosing ascending aortic graft infections and when combined with TEE, challenging cases of graft infections are more likely to be detected (Table 2) (37). This could manifest as a heterogenous pattern of 18F-FDG uptake (37). In infection related to CIED, device pocket infection, and infection of the superficial skin, 18F-FDG PET/CT has reported sensitivity values of 80% to 89%, specificity values of 86% to 100% (17) and negative predictive values of 85% to 88% (18). In patients with suspected LVAD infections, 18F-FDG PET/CT has reported excellent diagnostic performance, with sensitivity of 100%, specificity of 80%, positive predictive value of 94%, and negative predictive value of 100% (38). In addition, 18F-FDG PET/CT has reported 95% sensitivity, 91% specificity for pocket infection and associated driveline infection, and 92% sensitivity and 82% specificity, for driveline infection (Table 2) (29).
Leukocyte scintigraphy with SPECT could be considered if PVE is suspected in the context of inconclusive echocardiography and 18F-FDG PET/CT(A) (Table 2) (17). In patients with recent cardiac surgery, leukocyte scintigraphy is generally recommended over 18F-FDG PET/CT due to higher specificity (17). 99mTc-HMPAO-labeled leukocyte scintigraphy has reported 94% sensitivity, 100% specificity, 100% positive predictive value, and 94% positive predictive value for IE related to CIED (39). The sensitivity is reduced in cases of small vegetations (23).
Despite the advantages of 18F-FDG PET/CT, false negative studies could be seen in the setting of diffuse myocardial 18F-FDG uptake, since metabolism of cardiac myocytes relies on the balance between glucose and free fatty acids (29). This could be avoided by optimizing fasting conditions, diet, and blood insulin levels prior to the test (29). False negative results could also be seen if prior antimicrobial therapy was administered before obtaining the imaging, small vegetations size, or elevated blood glucose concentration (33). False positive results have been reported in cases of persistent host reaction against biomaterials or foreign body reactions (23), after recent cardiac procedures (within 3 months), recent emboli, or inadequate patient preparation (33). Other limitations for 18F-FDG PET/CT include limited access, preparation challenges, and the expertise needed for interpretation (33). Limited data exist in the literature regarding the cost-effectiveness of a multi-modality imaging approach in assessing IE. False negative leukocyte scintigraphy could be seen in the setting of prior treatment with antibiotics (17), and in the scenario of certain organisms including Candida species and Enterococcal species which are known to cause biofilms and escape from the host defense mechanisms (23). The following case illustrates the role of leukocyte scintigraphy and 18F-FDG PET/CT in the evaluation of vascular graft infection.
Case 4
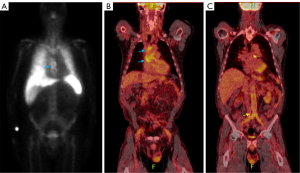
A 62-year-old male with a history of familial aortic aneurysm disease, prior resection of abdominal aortic aneurysm with aorto-bifemoral bypass graft placement, as well as valve-sparing aortic root, ascending, and arch replacement 6 years prior to presentation, presented to a local hospital with symptoms of fevers, chills, and lower extremity swelling. Blood cultures obtained on admission grew methicillin sensitive Staphylococcus aureus (MSSA) and he was started on IV cefazolin and rifampin. Nuclear imaging was performed due to concern for aortic graft infection (Figure 10). A tagged WBC scan was obtained, revealing mildly increased uptake in the ascending aorta, and no uptake in the abdominal graft (Figure 10A). The patient was discharged home on IV antibiotics but represented one week later with malaise and worsening appetite. A TEE revealed a normal aortic valve and echo-dense material around the ascending aortic graft (Figure 11). A gated CT was obtained without contrast due to significant renal dysfunction; within this limitation there was suggestion of mild soft tissue stranding adjacent to the ascending aortic graft (Figure 12A-12C). Considering these findings, 18F-FDG PET/CT was pursued, revealing increased 18F-FDG avidity in the ascending aortic graft, with only low-level uptake in the abdominal graft (Figure 10B,10C). Serial blood cultures remained negative while on IV cefazolin. Given his clinical stability and absence of graft complications, the patient was discharged home on IV antibiotics and surgical intervention was deferred. One month later, a repeat non-contrast gated CT showed minimal improvement of the soft tissue stranding around the ascending graft (Figure 12C) and the patient reported ongoing fatigue and malaise. The clinical and imaging findings were supportive of indolent ascending aorta graft infection. He ultimately underwent successful redo aortic root, ascending aorta, and arch replacement with valved aortic homograft.

All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and in line with the Helsinki Declaration (as revised in 2013). The cases in this work did not refer to the demographics of actual patients. The focus is on the applications of multimodality cardiac imaging. The description is fully anonymized, and is used for illustrative purposes only.
Discussion
Case 1 highlights a missed opportunity for the use of multi-modality imaging in a patient with recurrent embolic events with unclear etiology. Our patient’s serology was unrevealing, and he lacked classic infectious symptoms. A large case series of Cutibacterium acnes endocarditis revealed that this pathogen generally fails to produce a classic infectious clinical picture, usually with no fevers, normal inflammatory markers, and negative or very slow growing blood cultures (40). Despite the absence of a classic clinical syndrome, the presence of a prosthetic MV ring and the continued occurrence of embolic events detected by brain MRI should raise suspicion for possible endocarditis. In fact, identification of peripheral lesions by brain or whole-body imaging has been added as a minor diagnostic criterion for IE in the updated ESC guidelines (3). While sensitivity for detecting PVE on TEE is very high, with a reported range of 86–94% as noted in a large series by Daniel et al. (41), this modality and its interpretation is highly operator dependent. Guidelines have recommended repeating echocardiography within a week of negative or inconclusive results (3); however, this was not performed in our patient’s case until referral to our hospital. Newer guidelines would now advocate for the concomitant use of 18F-FDG PET/CT or cardiac CTA for further evaluation (3), which may have avoided delay in this patient’s diagnosis as well as prevented recurrent stroke recurrence.
The use of cardiac CTA to evaluate both valvular lesions as well as paravalvular complications is nicely highlighted in cases 2 and 3. Patients with suspected TAVR infections, such as the one discussed in case 2, can prove challenging to evaluate by echocardiography alone. In a clinical series of 55 patients with TAVR PVE, only a quarter of patients were found to have vegetations by echocardiography (42), and subtle signs like mild leaflet thickening as initially noted on our patient’s TTE are often the only indicator of possible TAVR involvement. While TEE has generally been shown to be superior in detection of small vegetations compared to CCT, the latter has been found to have higher sensitivity for pseudoaneurysm detection (21); this is illustrated in case 2, where TEE of the patient reveals an aortic root abscess and small MV vegetations, but the impressive degree of infectious extension and involvement of the aorta is best defined by CCT, allowing for better pre-operative planning. In case 3, CCT was pursued to better define the PVE instead of TEE. The patient had severe acute AR with tenuous respiratory status during his clinical course with concern that sedation required for the TEE may cause unfavorable hemodynamic consequences. These two cases highlight the utility of CCT both as an adjunct as well as alternative imaging tool to TEE for the evaluation of PVE in appropriate cases, reflecting the updated ESC guidelines on IE for CCT use (3).
Aortic graft infections remain clinically challenging as patients often present with non-specific systemic symptoms, negative serum microbiology, and echocardiography findings that are generally limited to aortic thickening, if at all visualized. Case 4 highlights the utility of nuclear imaging in these challenging cases and demonstrates important points from the new ESC guideline recommendations. Leukocyte scintigraphy is more widely available across hospital centers (43) and for this reason was the first modality used to evaluate the patient in case 4, prior to transfer to our facility. The case demonstrates how tagged WBC scan has higher specificity (43,44) showing no uptake in the non-infected descending graft but mild uptake in the infected ascending graft. In contrast, 18F-FDG PET is a more sensitive modality particularly when combined with CT (43,44). In case 4, there was clear increased uptake in the infected ascending graft but also low-level uptake in the descending graft. Currently 18F-FDG PET/CT and cardiac CTA are recommended for further evaluation of PVE when echocardiography is inconclusive, with WBC SPECT considered a reasonable alternative if 18F-FDG PET/CT is not available (3). There are no guidelines exploring the utility of combining both nuclear modalities if the diagnosis of infection remains in question, particularly in the early post-operative period where false positive results by 18F-FDG PET/CT have been reported in contrast to WBC SPECT, which may lead to erroneous diagnoses of infection (3,43,45). The use of nuclear imaging for monitoring response to anti-microbial treatment has also been suggested in recent ESC guidelines, but data regarding this is limited (3).
Conclusions
IE is a complex condition with a myriad of presentations. Appropriate use of multimodality imaging helps with the diagnostic evaluation and management of patients with of complicated IE. Clinicians need to be aware of the appropriate indications, strengths, and weaknesses of different imaging modalities to achieve the best patient outcomes.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-605/rc
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-605/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-2024-605/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and in line with the Helsinki Declaration (as revised in 2013). The cases in this work did not refer to the demographics of actual patients. The focus is on the applications of multimodality cardiac imaging. The description is fully anonymized, and is used for illustrative purposes only.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lo Presti S, Elajami TK, Zmaili M, et al. Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis: A contemporary narrative review. World J Cardiol 2021;13:254-70. [Crossref] [PubMed]
- Khayata M, Hackney N, Addoumieh A, et al. Impact of Opioid Epidemic on Infective Endocarditis Outcomes in the United States: From the National Readmission Database. Am J Cardiol 2022;183:137-42. [Crossref] [PubMed]
- Delgado V, Ajmone Marsan N, de Waha S, et al. 2023 ESC Guidelines for the management of endocarditis. Eur Heart J 2023;44:3948-4042. [Crossref] [PubMed]
- Isaza N, Shrestha NK, Gordon S, et al. Contemporary Outcomes of Pulmonary Valve Endocarditis: A 16-Year Single Centre Experience. Heart Lung Circ 2020;29:1799-807. [Crossref] [PubMed]
- Ding F, Shrestha NK, Chetrit M, et al. Clinical and Echocardiographic Characteristics of Bartonella Infective Endocarditis: An 8-Year Single-Centre Experience in the United States. Heart Lung Circ 2022;31:350-7. [Crossref] [PubMed]
- Habib G, Derumeaux G, Avierinos JF, et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol 1999;33:2023-9. [Crossref] [PubMed]
- 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria | Clinical Infectious Diseases | Oxford Academic. Accessed July 9, 2024. Available online: https://academic.oup.com/cid/article/77/4/518/7151107?login=true
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075-128. [Crossref] [PubMed]
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol 2017;69:325-44. [Crossref] [PubMed]
- Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010;11:202-19. [Crossref] [PubMed]
- Montané B, Chahine J, Fiore A, et al. Diagnostic performance of contemporary transesophageal echocardiography with modern imaging for infective endocarditis. Cardiovasc Diagn Ther 2023;13:25-37. [Crossref] [PubMed]
- Bai AD, Steinberg M, Showler A, et al. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. J Am Soc Echocardiogr 2017;30:639-646.e8. [Crossref] [PubMed]
- Evangelista A, Gonzalez-Alujas MT. Echocardiography in infective endocarditis. Heart 2004;90:614-7. [Crossref] [PubMed]
- Mgbojikwe N, Jones SR, Leucker TM, et al. Infective endocarditis: Beyond the usual tests. Cleve Clin J Med 2019;86:559-67. [Crossref] [PubMed]
- Haq IU, Haq I, Griffin B, et al. Imaging to evaluate suspected infective endocarditis. Cleve Clin J Med 2021;88:163-72. [Crossref] [PubMed]
- Hughes D, Linchangco R, Reyaldeen R, et al. Expanding utility of cardiac computed tomography in infective endocarditis: A contemporary review. World J Radiol 2022;14:180-93. [Crossref] [PubMed]
- Gomes A, Glaudemans AWJM, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017;17:e1-e14. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. [Crossref] [PubMed]
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53:436-44. [Crossref] [PubMed]
- Sifaoui I, Oliver L, Tacher V, et al. Diagnostic Performance of Transesophageal Echocardiography and Cardiac Computed Tomography in Infective Endocarditis. J Am Soc Echocardiogr 2020;33:1442-53. [Crossref] [PubMed]
- Jain V, Wang TKM, Bansal A, et al. Diagnostic performance of cardiac computed tomography versus transesophageal echocardiography in infective endocarditis: A contemporary comparative meta-analysis. J Cardiovasc Comput Tomogr 2021;15:313-21. [Crossref] [PubMed]
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014;30:377-87. [Crossref] [PubMed]
- Erba PA, Lancellotti P, Vilacosta I, et al. Recommendations on nuclear and multimodality imaging in IE and CIED infections. Eur J Nucl Med Mol Imaging 2018;45:1795-815. [Crossref] [PubMed]
- Laczay B, Patel D, Grimm R, et al. State-of-the-art narrative review: multimodality imaging in electrophysiology and cardiac device therapies. Cardiovasc Diagn Ther 2021;11:881-95. [Crossref] [PubMed]
- Pasternak JJ, Williamson EE. Clinical pharmacology, uses, and adverse reactions of iodinated contrast agents: a primer for the non-radiologist. Mayo Clin Proc 2012;87:390-402. [Crossref] [PubMed]
- Colen TW, Gunn M, Cook E, et al. Radiologic manifestations of extra-cardiac complications of infective endocarditis. Eur Radiol 2008;18:2433-45. [Crossref] [PubMed]
- Juneau D, Golfam M, Hazra S, et al. Molecular Imaging for the diagnosis of infective endocarditis: A systematic literature review and meta-analysis. Int J Cardiol 2018;253:183-8. [Crossref] [PubMed]
- Cantoni V, Sollini M, Green R, et al. Comprehensive meta-analysis on [18F] FDG PET/CT and radiolabelled leukocyte SPECT–SPECT/CT imaging in infectious endocarditis and cardiovascular implantable electronic device infections. Clin Transl Imaging 2018;6:3-18. [Crossref]
- Mikail N, Hyafil F. Nuclear Imaging in Infective Endocarditis. Pharmaceuticals (Basel) 2021;15:14. [Crossref] [PubMed]
- Borst U, Becker W, Maisch B, et al. Clinical and prognostic effect of a positive granulocyte scan in infective endocarditis. Clin Nucl Med 1993;18:35-9. [Crossref] [PubMed]
- Khayata M, Sanchez Nadales A, Xu B. Contemporary applications of multimodality imaging in infective endocarditis. Expert Rev Cardiovasc Ther 2024;22:27-39. [Crossref] [PubMed]
- Minardi G, Pino PG, Sordi M, et al. Infective endocarditis on mitral annular calcification: a case report. Cases J 2009;2:9072. [Crossref] [PubMed]
- Wong D, Rubinshtein R, Keynan Y. Alternative Cardiac Imaging Modalities to Echocardiography for the Diagnosis of Infective Endocarditis. Am J Cardiol 2016;118:1410-8. [Crossref] [PubMed]
- Wang TKM, Bin Saeedan M, Chan N, et al. Complementary Diagnostic and Prognostic Contributions of Cardiac Computed Tomography for Infective Endocarditis Surgery. Circ Cardiovasc Imaging 2020;13:e011126. [Crossref] [PubMed]
- Gomes A, van Geel PP, Santing M, et al. Imaging infective endocarditis: Adherence to a diagnostic flowchart and direct comparison of imaging techniques. J Nucl Cardiol 2020;27:592-608. [Crossref] [PubMed]
- Ten Hove D, Slart RHJA, Sinha B, et al. (18)F-FDG PET/CT in Infective Endocarditis: Indications and Approaches for Standardization. Curr Cardiol Rep 2021;23:130. [Crossref] [PubMed]
- Xu B, Reyaldeen R. Ascending aortic graft infection - an expanding role for multi-modality cardiac imaging. Int J Cardiol 2021;333:246-8. [Crossref] [PubMed]
- Dell'Aquila AM, Mastrobuoni S, Alles S, et al. Contributory Role of Fluorine 18-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in the Diagnosis and Clinical Management of Infections in Patients Supported With a Continuous-Flow Left Ventricular Assist Device. Ann Thorac Surg 2016;101:87-94; discussion 94. [Crossref] [PubMed]
- Erba PA, Conti U, Lazzeri E, et al. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med 2012;53:1235-43. [Crossref] [PubMed]
- Heinen FJ, Arregle F, van den Brink FS, et al. Clinical Characteristics and Outcomes of Patients With Cutibacterium acnes Endocarditis. JAMA Netw Open 2023;6:e2323112. [Crossref] [PubMed]
- Daniel WG, Mügge A, Grote J, et al. Comparison of transthoracic and transesophageal echocardiography for detection of abnormalities of prosthetic and bioprosthetic valves in the mitral and aortic positions. Am J Cardiol 1993;71:210-5. [Crossref] [PubMed]
- Mangner N, Woitek F, Haussig S, et al. Incidence, Predictors, and Outcome of Patients Developing Infective Endocarditis Following Transfemoral Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;67:2907-8. [Crossref] [PubMed]
- Xu B, Sanaka KO, Haq IU, et al. Role of multimodality imaging in infective endocarditis: Contemporary diagnostic and prognostic considerations. Prog Cardiovasc Dis 2023;S0033-0620(23)00110-X.
- Rouzet F, Chequer R, Benali K, et al. Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med 2014;55:1980-5. [Crossref] [PubMed]
- Liberatore M, Misuraca M, Calandri E, et al. White blood cell scintigraphy in the diagnosis of infection of endovascular prostheses within the first month after implantation. Med Sci Monit 2006;12:MT5-9. [PubMed]


