Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: from bench to bedside
Introduction
One of the major developments in cardiac imaging during the past decades has been the non-invasive assessment of myocardial fibrosis by means of late gadolinium enhancement, also known as delayed enhancement (DE) imaging. This has been originally conceived, explored, and documented using cardiac magnetic resonance imaging (CMR). Indeed, a large number of studies have demonstrated an excellent correlation between post-mortem specimens and DE-CMR regarding the presence, extent, and patterns of fibrosis (1-3). DE-CMR has therefore emerged as an alternative to endomyocardial biopsy in various scenarios. Furthermore, in the past decade, robust evidence has been gathered supporting an increasingly relevant prognostic value of the presence and extent of DE in diverse etiologies, ranging from hypertrophic cardiomyopathy (HCM) to dilated cardiomyopathy and ischemic cardiomyopathy (ICM) (4-8).
Cardiac computed tomography (CT) has been originally conceived for the assessment of the coronary tree, and has rapidly positioned as an accurate non-invasive tool for the evaluation coronary atherosclerosis mostly based on an excellent negative predictive value.
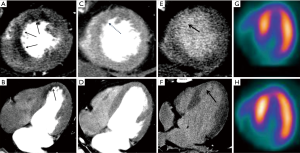
However, over the past decade cardiac CT has evolved to embrace several non-coronary applications, including myocardial perfusion, assessment of valve and ventricular function, structural heart disease, and detection of embolic sources, among other. Gadolinium and iodinated based contrast agents share similar kinetics, thus leading to comparable myocardial characterization with CMR and CT at both first-pass perfusion and DE imaging (Figure 1) (9).
We will therefore review the available evidence of DE imaging for the assessment of myocardial infarction (MI) using delayed-enhancement CT (CTDE), from animal to clinical studies, and from 16-slice CT to dual-energy CT systems (DECT).
Pre-clinical studies and comparison with magnetic resonance
Initially using animal models, a number of studies have validated the role of CT for acute and chronic MI characterization (3,9-12). In the study of Lardo et al., a canine model was used for the acute setting, and a porcine model was used for the chronic MI model. Images were acquired using a 32-slice multidetector CT scanner, after a 150-mL bolus of iodinated contrast was administrated (retrospective gating; 135 kV, 420 mA). Images were acquired every 5 minutes after the first-pass perfusion. In this study, DE images reached the maximum levels at ~5 minutes after injection, and chronic MI volumes correlated well with postmortem myocardial staining (10). The histopathology findings of DE regions obtained from the acute model comprised myocyte necrosis, including contraction band necrosis without nuclear changes, and neutrophils leakage and also migration to the interstitial spaces (10).
In the study of Gerber et al., seven pigs underwent surgical ligation of the left anterior descending (LAD) artery and underwent CTDE 2 to 6 weeks after using 2 mL/kg of iodinated contrast (concentration 400 mgI/mL) (9). CTDE images were acquired every 2 minutes with a 16-slice CT scanner (retrospective gating; 90 keV). In line with the previous study, MI regions showed the highest signal densities at 2 to 6 minutes after injection, with the highest differences compared to the remote myocardium at 6 minutes (9). Among the patients with previous MI also included in this study, a moderately good agreement (kappa 0.61, P<0.001) was observed between CTDE and DE-CMR for the identification of segments with DE regions, being discordant segments more frequent within chronic patients (kappa 0.52, P<0.0001).
Using a porcine model with occlusion of the second diagonal branch and CTDE acquired with a 64-CT scanner, Brodoefel et al. were among the first to report a good correlation with DE-CMR by means of low dose (80 kV) imaging (13). In another minipig chronic infarct model, CTDE (dual source CT in a dual energy mode; one tube at 140 kV/95 mAs and the other at 100 kV/165 mAs) images were obtained 61 days after occlusion of the LAD. In this study, CTDE images using DECT showed a higher sensitivity compared to 100 kV images (14).
More recently, Jang et al. reported their findings using a novel cardiovascular interventional therapeutic CT system that consisted of a hybrid system comprising both invasive coronary angiography and a multidetector (320-slice) CT. Using a Yucatan miniature swine model with a 2-week old MI, the authors demonstrated the optimal differences in CT attenuation values between infarcted areas and normal myocardium at 2–5 minutes after contrast injection (15).
It is worth mentioning that the signal density values (Hounfield units) of DE regions assessed by CTDE are directly related to the attenuation of the X-ray beam by iodine molecules. On the contrary, DE assessed during DE-CMR is associated to gadolinium-induced alterations of proton (water) relaxivity and is therefore an indirect measure of the amount and biodistribution of the contrast agent (16). Accordingly, CTDE might possibly appear as a more scar-specific technique than DE-CMR.
Another possible advantage of CTDE compared with DE-CMR is an improved isotropic spatial resolution, leading to a significant reduction in partial volume effects. Evidence in this regard comes from an animal study including 15 mini-pigs with chronic MI induced by distal LAD balloon occlusion, who underwent CTDE and DE-CMR imaging 191±4 days after MI, and pathology examination. Images were acquired using a 64-slice CT scanner, after a 150 mL bolus of iodinated contrast (retrospective gating; 120 kV; 400 mA). In this study, the optimal image quality occurred at 10 minutes after contrast injection, and an excellent correlation was found between CTDE and both DE-CMR (R2=0.92, P<0.0001) and pathology (R2=0.97, P<0.0001). Interestingly, CTDE outperformed DE-CMR for the assessment of peri-infarct zones, which have been shown potential to identify patients predisposed to ventricular arrhythmias (11,17).
As previously mentioned, contrast kinetics of both iodine and gadolinium are very similar. This has been translated to comparable imaging regarding myocardial contrast wash-in and wash-out (Figure 1). One of the most interesting aspects of the study of Gerber et al. was the inclusion of a direct comparison of gadodiamide and iomeprol kinetics, infused in an isolated rabbit’s heart. In this model, signal densities of both contrast agents were similar over time (wash-in and wash-out) at the infarct core, peripheral rim, and at the remote myocardium (9). In the context of MI, the infarct core suffers a significant reduction in contrast delivery (delayed wash-in), resulting in myocardial hypoenhancement during first-pass imaging (Figure 1). This might be related to epicardial or microvascular obstruction in the acute setting, and to a reduced capillary density in the chronic MI setting. On the contrary, DE-CMR or CTDE during late image acquisition, performed approximately 5-10 minutes after contrast injection, leads to hyperenhanced areas (delayed or late enhancement) that result from an expansion of the extracellular space (Figures 1-3). Such increase in the volume of distribution is related to sarcolemmal membrane disruption in the acute MI setting (~75% of the total myocardial volume is intracellular). On the contrary, it indicates interstitial and replacement fibrosis combined with residual capillaries and dilated vessels, and extensive extracellular matrix deposition and lack of cellular structure in the chronic MI setting (9,18).
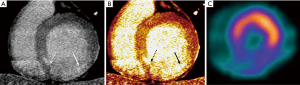
Other studies using animal models showed similar findings, with excellent correlation with both DE-CMR and pathology. Among other, the study of Baks et al. (64-slice CT; 120 kV; 900 mA; retrospective gating) comprised domestic pigs with occlusion of the left circumflex. Images were obtained 15 minutes after administration of 1 gI/kg of iodinated contrast (400 mgI/mL) (3). It is noteworthy that most of the aforementioned animal studies used contrast doses significantly higher than those currently applied in clinical practice.
Documentation of microvascular damage can also be assessed using CTDE (10,19). In the study of Lardo et al., areas of microvascular obstruction (identified as a hypoenhanced core within hyperenhanced areas) were detected in 3 of 7 animals, and more clearly observed early after contrast injection (10). Such areas of microvascular obstruction, also known as “no reflow” areas and frequently associated to reperfusion damage, can be detected as result of capillary blockage despite restoration of epicardial flow, and are determinants of adverse prognosis (20,21). Furthermore, Carlsson et al., in a specifically designed microembolization swine model, found that CTDE (64 mm × 0.625 mm; 120 kV; 650 mAs) performed at 3 to 5 minutes after contrast injection had a comparable ability for the detection of heterogeneous microinfarcts compared to DE-CMR (22).
With regard to acquisition parameters, an animal study that specifically addressed the impact of tube current in CTDE for the evaluation of acute reperfused MI, found that areas of DE and no reflow, as well as image quality, did not differ significantly using different mAs (23). Moreover, CTDE evaluation using prospective ECG gating has demonstrated similar accuracy compared to retrospective ECG acquisitions, with substantially reduced effective radiation doses (19).
Evidence from clinical studies, and prognostic value of DE
After an acute MI, one of the major determinants of event-free survival is the extent of myocardial irreversible damage (24). As expected, most evidence regarding the prognostic value of DE is based on DE-CMR studies. Indeed, numerous studies have consistently placed the presence and extent of DE as an independent predictor of ventricular dysfunction, cardiac events, and death (6,8,20). This has not only been shown among patients with ICM, but also among those with HCM and non-ischemic dilated cardiomyopathy (4,5,7).
In addition, DE transmurality and the fibrotic burden have been shown as effective tools to assess the likelihood of regional functional recovery and survival after revascularization, therefore refreshing the concept of myocardial viability as a therapeutic goal (25-27).
CTDE acquisition protocols are not well established, and various scan parameters have been reported (Table 1). However, most current CTDE examinations are acquired 6 to 8 minutes after iodine administration using low radiation dose protocols, including prospective ECG gating, low kV (usually 80 or 100 kV) setting, and variable (though generally low) tube current. These parameters lead to very low effective radiation doses (Table 1). Regarding image analysis, 5 to 10 mm average multiplanar reconstructions are typically recommended, using a smooth filter and adjusting window setting at the reader’s discretion (Figures 1-3).
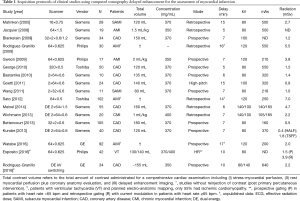
Full table
As previously mentioned, DE-CMR has superior contrast resolution compared to CTDE. This is caused at least in part by the possibility to use specific pulse sequences with the former, that enable nulling of the normal remote myocardium thereby facilitating the discrimination between normal and infarcted myocardium. This can partially explain the suboptimal results of CTDE in the chronic setting, with most studies reporting a high specificity but a low sensitivity. Not even the latest generation CT scanners (using single energy acquisitions) have conclusively solved this limitation, and scarred areas evaluated using single energy CTDE among stable patients are vaguely defined, and usually portrayed as regions where the discrimination between the myocardium and the left ventricular cavity is unclear (28-33). In fact, in a recent study comprising chronic MI patients that used DE-CMR as reference standard, Bettencourt et al. reported that CTDE identified only 9 of the 17 ischemic scars, with an excellent specificity (98%) but a poor sensitivity (53%) (28).
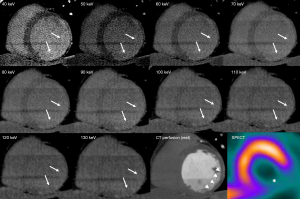
It has been hypothesized that such weakness of CTDE among chronic MI patients might be related to a combination of factors, being the most important the relatively poor contrast resolution compared to DE-CMR, but also to the fact that different physiopathological mechanisms are involved in the extracellular expansion of acute (sarcolemmal membrane rupture, Figure 4) and chronic (interstitial and replacement fibrosis) MI (Figures 1-3) (9,18).
In the acute MI setting, several studies have shown that CTDE appears as an excellent tool for the assessment of infarct size and myocardial viability (Figure 4) (34-39). The study of Mahnken et al. was among the first to evaluate this after primary percutaneous coronary intervention (PCI), with excellent agreement with DE-CMR. The authors included 28 patients who underwent retrospective gated CTDE (16-slice CT, 80 kV, 550 mA) after administration of 120 mL of iodinated contrast (35). The studies of Rodriguez-Granillo et al. (36) and Jacquier et al. (40) have further confirmed the ability of CTDE for the early assessment of myocardial viability. The former included 30 patients who were immediately transferred from the catheterization lab to the CT scanner after primary PCI and underwent CTDE without contrast reinjection (64-slice CT; 120 kV; 550 mA; retrospective gating with dose modulation). The authors reported that the presence of DE was related to a poor microvascular reperfusion, a higher rate of in-hospital morbidity, and lower functional recovery rates at 6 months (36). The study of Jacquier et al. included 19 patients with acute MI and revascularization. CTDE acquisition was performed 5 and 10 minutes after contrast administration (1.5 mL/kg of iodinated contrast). In this study, the extent of DE by CTDE and DE-CMR were highly correlated (r=0.85; P<0.0001), and image quality was significantly better at 5 minutes compared to 10-minute imaging (40).
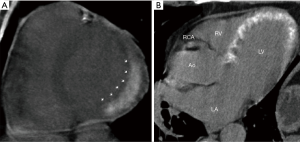
Most clinical CTDE studies had very small sample sizes (Table 1). The largest study included 102 patients with first acute MI who underwent CTDE immediately after primary PCI without iodine reinjection. In this study, after a 2-year follow-up with a 19% rate of major adverse events, the extent of DE was identified as an independent predictor of events after adjustment for TIMI risk score, ejection fraction, and SPECT findings (HR for third tertile 16.1; 95% CI, 1.45–72.4; P=0.022) (37).
Also, a recent study that included 92 patients with acute MI who underwent CTDE without iodine reinjection immediately after primary PCI, demonstrated the presence of a heterogeneous enhancement (concomitant hypo and hyperenhancement) as an independent predictor of microvascular obstruction and ventricular remodeling (Figure 4) (38).
Role of dual energy CT for the assessment of DE
Conventional single energy CT imaging is influenced by technical issues related to the polychromatic nature of X-rays, such as blooming and beam hardening artifacts (BHA). Myocardial perfusion imaging is typically affected by these artifacts, occasionally simulating perfusion defects and thus potentially leading to increments in downstream testing. With the advent of dual energy DECT, some of these technical limitations have been minimized (41). DECT comprises image acquisition at two different tube voltages. Until recently, DECT was not clinically available, being this mainly attributed to technical limitations as well as high radiation doses. This approach offers the possibility to assess the chemical composition of different tissues with regard to their atomic number. With the introduction of dual source CT and single-source kV switching, DECT for cardiovascular applications became viable. Virtual monochromatic imaging (VMI) obtained from DECT has allowed major reductions in the iodinated contrast load, with up to 50% reduction with preserved image quality in coronary angiography and up to 60% reduction in aortic angiography examinations (42,43). Such reductions might widen the scope of patients eligible to undergo contrast-enhanced studies, potentially leading to the inclusion of the previously precluded patients at risk of contrast induced nephropathy. Furthermore, myocardial perfusion using DECT has shown to provide a significant incremental value over CTCA alone for the detection of hemodynamically significant stenosis (44-47).
In the context of CTDE, DECT appears to have great potential to overcome the aforementioned limitations of conventional (single-energy) CTDE for the assessment of myocardial fibrosis in the stable setting (14). Particularly, the higher intravascular attenuation levels attainable using low energy monochromatic imaging show promise to improve the detection of myocardial fibrosis (Figures 1-3).
Using VMI at the lowest energy levels (40–50 keV), the discrimination between scarred and remote (normal) myocardium might be substantially improved, since the presence of iodine within tissues significantly is markedly enhanced among such energy levels (48).
In the first studies using single-source VMI, iterative reconstruction (IR) algorithms were only available for energy levels larger than 60 keV, and this has therefore been a drawback for the technique, in the sense that images at 40–60 keV had significantly higher image noise. Such limitation has been recently solved with the incorporation of IR for all energy levels, leading to 40–50 keV imaging to achieve the highest signal densities (HU) with preserved image quality.
In 55 patients with suspected CAD who underwent adenosine stress, rest, and CTDE (DECT with dual source), Meinel et al. reported a lack of improvement of the accuracy of the comprehensive scan by the addition of CTDE; with 7% of segments with fixed perfusion defects at SPECT being misclassified as reversible (44).
In opposition, animal data using dual energy imaging acquired by dual source CT scanners (one tube with 165 mAs/rotation at 100 kV and the second tube with 140 mAs/rotation at 140 kV) has shown inferior results regarding scar imaging compared to single energy 100 kV CTDE imaging (49).
With regard to reconstruction techniques, image blending with different weighing factors (percentage of low and high kV) using DECT (80/140 kV) has shown encouraging findings towards the visualization of necrosis, with superior results in different studies compared to images obtained at 80 or 140 keV, or to 100 and 140 kV (50,51). Targeted spatial frequency filtration (TSFF), a hybrid reconstruction algorithm comprising HALF and full-scan reconstruction aimed at the stabilization of the attenuation levels and the achievement of high temporal resolution, has been originally developed for dynamic CT myocardial perfusion. In the context of CTDE, TSFF and image averaging over half-scan reconstructions have shown to improve image quality and interobserver reproducibility (52).
In line with our clinical experience with kV switching DECT, Wichmann et al. suggested that the evaluation of dual-energy CTDE (dual source) using color-coded iodine maps provides inferior accuracy compared to linear blending (100/140 kV) (53).
The ability of low monochromatic images to reduce iodinated contrast volume might be of interest in this regard. Indeed, in most studies evaluating CTDE relatively large total contrast volumes were administrated, with highly concentrated iodine (Table 1).
The aforementioned technical advancements might also potentially encourage further research towards the use of dual energy CTDE for a number of clinical applications other than ICM. Indeed, the usefulness of CTDE using single energy has shown promising findings among patients with non-ischemic cardiomyopathies including HCM, myocarditis, and even for the guidance of electro-anatomic mapping for ventricular tachycardia ablation (31,49,54-58). This might be of particular interest among patients with implantable cardioverters (58).
Clinical perspectives and conclusions
During the past decade, DE-CMR has gained an important role in several clinical scenarios, mainly attributed to its lack of ionizing radiation, high contrast resolution, and plethora of clinical evidence supporting the prognostic value of the presence and extent of myocardial DE. However, though highly appealing, a number of limitations or warnings have encouraged the search for an alternative DE imaging modality. One of these are safety issues. Although both CMR and gadolinium agents have been originally deemed innocuous, a number of concerns have been recently issued. Firstly, lymphocyte DNA double-strand breaks, a marker of biological damage and genotoxic effects associated with medical procedures, have been shown to increase during CMR examinations, in levels comparable to low dose (<4 mSv) coronary CTCA. Such DNA damage seems to be reversible, although the clinical significance of this remains unknown (59,60).
In parallel, gadolinium agents, formerly considered practically innocuous, are currently associated (although very rarely) with nephrogenic systemic fibrosis among patients with severe renal dysfunction (61). Moreover, recent studies have demonstrated gadolinium deposition in the skin and certain brain structures (dentate nucleus and globus pallidus) even among patients with normal renal function (62,63). These concerns have to be placed in the context of non-negligible rates of claustrophobia (64,65). Furthermore, DE-CMR has a number of logistic drawbacks compared with CTDE, such as higher costs and longer scanning times (thus also with an impact in cost), and difficulties to scan patients with inability to lie supine for extended periods and those with impaired breath-holding capabilities. Overall, these issues might encourage the role of CTDE as an alternative for DE-CMR in selected populations (66,67).
The possibility to have an alternative for DE-CMR towards the assessment of fibrosis has therefore major clinical implications. Such statement is sustained not only by the aforementioned (though probably overemphasized) concerns regarding DE-CMR safety, but also by the increasingly rates of patients with implantable cardiac devices (cardiac defibrillators, resynchronizing devices, structural heart disease devices) that preclude or worsen CMR image quality.
A number of studies have suggested that CTCA appears to be a cost-effective strategy among patients with suspected CAD, whereas among symptomatic revascularized patients, stress-CMR is more cost-effective than CTCA (68-70). Cost-effectiveness analyses can hardly be extrapolated to different regions or countries, not only given the different diagnostic algorithm strategies used by local cardiologists, but also the substantially different costs of each technique (71). Furthermore, in the context of DE imaging, such comparison is hard to explore for several reasons. DE imaging using CT usually comprises coronary CT angiography/myocardial perfusion plus CTDE. In turn, DE-CMR usually comprises morphological and functional ventricular assessment plus DE imaging. Besides, DE imaging in the context of ICM can range from evaluation of small infarcts the assessment of myocardial viability among patients with severe systolic dysfunction. Nonetheless, CTDE imaging used exclusively for the evaluation of scar imaging would probably be a very inexpensive procedure, given the very low radiation required and hence the minimal tube usage. Also, given the relatively low sensitivity and high specificity, it probably shouldn’t lead to increased downstream testing. Nevertheless, all of these issues remain fairly speculative and warrant further exploration.
It is worth mentioning that CTDE can be performed using a very low radiation doses (with approximate effective radiation doses ranging from 0.5 mSv using prospective ECG gating with 80 kV/160 mAs acquisition, to 2.2 mSv using single-source dual energy imaging, and approximately 4.7 mSv using dual-source dual energy imaging; Table 1) (28,31,44). Furthermore, novel CT developments such as DECT might enable improvements in scar imaging among stable patients with chronic MI, and it is expected to allow a significant reduction in the iodinated contrast load.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Moon JC, Reed E, Sheppard MN, et al. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 2004;43:2260-4. [Crossref] [PubMed]
- Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-2002. [Crossref] [PubMed]
- Baks T, Cademartiri F, Moelker AD, et al. Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J Am Coll Cardiol 2006;48:144-52. [Crossref] [PubMed]
- Chan RH, Maron BJ, Olivotto I, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484-95. [Crossref] [PubMed]
- Kuruvilla S, Adenaw N, Katwal AB, et al. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging 2014;7:250-8. [Crossref] [PubMed]
- Klem I, Shah DJ, White RD, et al. Prognostic value of routine cardiac magnetic resonance assessment of left ventricular ejection fraction and myocardial damage: an international, multicenter study. Circ Cardiovasc Imaging 2011;4:610-9. [Crossref] [PubMed]
- Weng Z, Yao J, Chan RH, et al. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging 2016;9:1392-1402. [Crossref] [PubMed]
- Larose E, Rodes-Cabau J, Pibarot P, et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J Am Coll Cardiol 2010;55:2459-69. [Crossref] [PubMed]
- Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation 2006;113:823-33. [Crossref] [PubMed]
- Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 2006;113:394-404. [Crossref] [PubMed]
- Schuleri KH, Centola M, George RT, et al. Characterization of peri-infarct zone heterogeneity by contrast-enhanced multidetector computed tomography: a comparison with magnetic resonance imaging. J Am Coll Cardiol 2009;53:1699-707. [Crossref] [PubMed]
- Udholm S, Laugesen S, Agger P, et al. Delayed uptake and washout of contrast in non-viable infarcted myocardium shown with dynamic computed tomography. Cardiovasc Diagn Ther 2014;4:350-6. [PubMed]
- Brodoefel H, Klumpp B, Reimann A, et al. Late myocardial enhancement assessed by 64-MSCT in reperfused porcine myocardial infarction: diagnostic accuracy of low-dose CT protocols in comparison with magnetic resonance imaging. Eur Radiol 2007;17:475-83. [Crossref] [PubMed]
- Deseive S, Bauer RW, Lehmann R, et al. Dual-energy computed tomography for the detection of late enhancement in reperfused chronic infarction: a comparison to magnetic resonance imaging and histopathology in a porcine model. Invest Radiol 2011;46:450-6. [Crossref] [PubMed]
- Jang Y, Cho I, Hartaigh BW, et al. Viability assessment after conventional coronary angiography using a novel cardiovascular interventional therapeutic CT system: Comparison with gross morphology in a subacute infarct swine model. J Cardiovasc Comput Tomogr 2015;9:321-8. [Crossref] [PubMed]
- Saeed M, Bremerich J, Wendland MF, et al. Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular MR contrast media in rats. Radiology 1999;213:247-57. [Crossref] [PubMed]
- Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006-14. [Crossref] [PubMed]
- Wang J, Xiang B, Lin HY, et al. Differential MR delayed enhancement patterns of chronic myocardial infarction between extracellular and intravascular contrast media. PLoS One 2015;10:e0121326. [Crossref] [PubMed]
- Chang HJ, George RT, Schuleri KH, et al. Prospective electrocardiogram-gated delayed enhanced multidetector computed tomography accurately quantifies infarct size and reduces radiation exposure. JACC Cardiovasc Imaging 2009;2:412-20. [Crossref] [PubMed]
- Hadamitzky M, Langhans B, Hausleiter J, et al. Prognostic value of late gadolinium enhancement in cardiovascular magnetic resonance imaging after acute ST-elevation myocardial infarction in comparison with single-photon emission tomography using Tc99m-Sestamibi. Eur Heart J Cardiovasc Imaging 2014;15:216-25. [Crossref] [PubMed]
- Hamirani YS, Wong A, Kramer CM, et al. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2014;7:940-52. [Crossref] [PubMed]
- Carlsson M, Saloner D, Martin AJ, et al. Heterogeneous microinfarcts caused by coronary microemboli: evaluation with multidetector CT and MR imaging in a swine model. Radiology 2010;254:718-28. [Crossref] [PubMed]
- Martini C, Maffei E, Palumbo A, et al. Impact of tube current in the quantitative assessment of acute reperfused myocardial infarction with 64-slice delayed-enhancement CT: a porcine model. Radiol Med 2010;115:1003-14. [Crossref] [PubMed]
- Risk stratification and survival after myocardial infarction. New Engl J Med 1983;309:331-6. [Crossref] [PubMed]
- Glaveckaite S, Valeviciene N, Palionis D, et al. Prediction of long-term segmental and global functional recovery of hibernating myocardium after revascularisation based on low dose dobutamine and late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014;16:83. [Crossref] [PubMed]
- Pegg TJ, Selvanayagam JB, Jennifer J, et al. Prediction of global left ventricular functional recovery in patients with heart failure undergoing surgical revascularisation, based on late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:56. [Crossref] [PubMed]
- Kancharla K, Weissman G, Elagha AA, et al. Scar quantification by cardiovascular magnetic resonance as an independent predictor of long-term survival in patients with ischemic heart failure treated by coronary artery bypass graft surgery. J Cardiovasc Magn Reson 2016;18:45. [Crossref] [PubMed]
- Bettencourt N, Ferreira ND, Leite D, et al. CAD detection in patients with intermediate-high pre-test probability: low-dose CT delayed enhancement detects ischemic myocardial scar with moderate accuracy but does not improve performance of a stress-rest CT perfusion protocol. JACC Cardiovasc Imaging 2013;6:1062-71. [Crossref] [PubMed]
- Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol 2009;54:1072-84. [Crossref] [PubMed]
- Goetti R, Feuchtner G, Stolzmann P, et al. Delayed enhancement imaging of myocardial viability: low-dose high-pitch CT versus MRI. Eur Radiol 2011;21:2091-9. [Crossref] [PubMed]
- Esposito A, Palmisano A, Antunes S, et al. Cardiac CT With Delayed Enhancement in the Characterization of Ventricular Tachycardia Structural Substrate: Relationship Between CT-Segmented Scar and Electro-Anatomic Mapping. JACC Cardiovasc Imaging 2016;9:822-32. [Crossref] [PubMed]
- George RT, Arbab-Zadeh A, Miller JM, et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging 2012;5:333-40. [Crossref] [PubMed]
- Bastarrika G, Ramos-Duran L, Rosenblum MA, et al. Adenosine-stress dynamic myocardial CT perfusion imaging: initial clinical experience. Invest Radiol 2010;45:306-13. [PubMed]
- Gweon HM, Kim SJ, Kim TH, et al. Evaluation of reperfused myocardial infarction by low-dose multidetector computed tomography using prospective electrocardiography (ECG)-triggering: comparison with magnetic resonance imaging. Yonsei Med J 2010;51:683-91. [Crossref] [PubMed]
- Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol 2005;45:2042-7. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Rosales MA, Baum S, et al. Early assessment of myocardial viability by the use of delayed enhancement computed tomography after primary percutaneous coronary intervention. JACC Cardiovasc Imaging 2009;2:1072-81. [Crossref] [PubMed]
- Sato A, Nozato T, Hikita H, et al. Prognostic value of myocardial contrast delayed enhancement with 64-slice multidetector computed tomography after acute myocardial infarction. J Am Coll Cardiol 2012;59:730-8. [Crossref] [PubMed]
- Watabe H, Sato A, Nishina H, et al. Enhancement patterns detected by multidetector computed tomography are associated with microvascular obstruction and left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J 2016;37:684-92. [Crossref] [PubMed]
- Wang R, Zhang Z, Xu L, et al. Low dose prospective ECG-gated delayed enhanced dual-source computed tomography in reperfused acute myocardial infarction comparison with cardiac magnetic resonance. Eur J Radiol 2011;80:326-30. [Crossref] [PubMed]
- Jacquier A, Boussel L, Amabile N, et al. Multidetector computed tomography in reperfused acute myocardial infarction. Assessment of infarct size and no-reflow in comparison with cardiac magnetic resonance imaging. Invest Radiol 2008;43:773-81. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Carrascosa P, Cipriano S, et al. Beam hardening artifact reduction using dual energy computed tomography: implications for myocardial perfusion studies. Cardiovasc Diagn Ther 2015;5:79-85. [PubMed]
- Carrascosa P, Capunay C, Rodriguez-Granillo GA, et al. Substantial iodine volume load reduction in CT angiography with dual-energy imaging: insights from a pilot randomized study. Int J Cardiovasc Imaging 2014;30:1613-20. [Crossref] [PubMed]
- Carrascosa P, Leipsic JA, Capunay C, et al. Monochromatic image reconstruction by dual energy imaging allows half iodine load computed tomography coronary angiography. Eur J Radiol 2015;84:1915-20. [Crossref] [PubMed]
- Meinel FG, De Cecco CN, Schoepf UJ, et al. First-arterial-pass dual-energy CT for assessment of myocardial blood supply: do we need rest, stress, and delayed acquisition? Comparison with SPECT. Radiology 2014;270:708-16. [Crossref] [PubMed]
- Carrascosa PM, Deviggiano A, Capunay C, et al. Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. Eur J Radiol 2015;84:637-42. [Crossref] [PubMed]
- De Cecco CN, Harris BS, Schoepf UJ, et al. Incremental value of pharmacological stress cardiac dual-energy CT over coronary CT angiography alone for the assessment of coronary artery disease in a high-risk population. AJR Am J Roentgenol 2014;203:W70-7. [Crossref] [PubMed]
- Wang R, Yu W, Wang Y, et al. Incremental value of dual-energy CT to coronary CT angiography for the detection of significant coronary stenosis: comparison with quantitative coronary angiography and single photon emission computed tomography. Int J Cardiovasc Imaging 2011;27:647-56. [Crossref] [PubMed]
- Rodriguez-Granillo GA, Carrascosa P, Cipriano S, et al. Myocardial signal density levels and beam-hardening artifact attenuation using dual-energy computed tomography. Clin Imaging 2015;39:809-14. [Crossref] [PubMed]
- Truong QA, Thai WE, Wai B, et al. Myocardial scar imaging by standard single-energy and dual-energy late enhancement CT: Comparison with pathology and electroanatomic map in an experimental chronic infarct porcine model. J Cardiovasc Comput Tomogr 2015;9:313-20. [Crossref] [PubMed]
- Kartje JK, Schmidt B, Bruners P, et al. Dual energy CT with nonlinear image blending improves visualization of delayed myocardial contrast enhancement in acute myocardial infarction. Invest Radiol 2013;48:41-5. [Crossref] [PubMed]
- Wichmann JL, Hu X, Kerl JM, et al. Non-linear blending of dual-energy CT data improves depiction of late iodine enhancement in chronic myocardial infarction. Int J Cardiovasc Imaging 2014;30:1145-50. [Crossref] [PubMed]
- Kurobe Y, Kitagawa K, Ito T, et al. Myocardial delayed enhancement with dual-source CT: advantages of targeted spatial frequency filtration and image averaging over half-scan reconstruction. J Cardiovasc Comput Tomogr 2014;8:289-98. [Crossref] [PubMed]
- Wichmann JL, Bauer RW, Doss M, et al. Diagnostic accuracy of late iodine-enhancement dual-energy computed tomography for the detection of chronic myocardial infarction compared with late gadolinium-enhancement 3-T magnetic resonance imaging. Invest Radiol 2013;48:851-6. [Crossref] [PubMed]
- Zhao L, Ma X, Delano MC, et al. Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography. Eur Radiol 2013;23:1034-43. [Crossref] [PubMed]
- Shiozaki AA, Senra T, Arteaga E, et al. Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr 2013;7:173-81. [Crossref] [PubMed]
- Axsom K, Lin F, Weinsaft JW, et al. Evaluation of myocarditis with delayed-enhancement computed tomography. J Cardiovasc Comput Tomogr 2009;3:409-11. [Crossref] [PubMed]
- Deux JF, Mihalache CI, Legou F, et al. Noninvasive detection of cardiac amyloidosis using delayed enhanced MDCT: a pilot study. Eur Radiol 2015;25:2291-7. [Crossref] [PubMed]
- Langer C, Lutz M, Eden M, et al. Hypertrophic cardiomyopathy in cardiac CT: a validation study on the detection of intramyocardial fibrosis in consecutive patients. Int J Cardiovasc Imaging 2014;30:659-67. [Crossref] [PubMed]
- Fiechter M, Stehli J, Fuchs TA, et al. Impact of cardiac magnetic resonance imaging on human lymphocyte DNA integrity. Eur Heart J 2013;34:2340-5. [Crossref] [PubMed]
- Lancellotti P, Nchimi A, Delierneux C, et al. Biological Effects of Cardiac Magnetic Resonance on Human Blood Cells. Circ Cardiovasc Imaging 2015;8:e003697. [Crossref] [PubMed]
- Chrysochou C, Power A, Shurrab AE, et al. Low risk for nephrogenic systemic fibrosis in nondialysis patients who have chronic kidney disease and are investigated with gadolinium-enhanced magnetic resonance imaging. Clin J Am Soc Nephrol 2010;5:484-9. [Crossref] [PubMed]
- Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents-current status. Neuroradiology 2016;58:433-41. [Crossref] [PubMed]
- Roberts DR, Lindhorst SM, Welsh CT, et al. High Levels of Gadolinium Deposition in the Skin of a Patient With Normal Renal Function. Invest Radiol 2016;51:280-9. [PubMed]
- Katznelson R, Djaiani GN, Minkovich L, et al. Prevalence of claustrophobia and magnetic resonance imaging after coronary artery bypass graft surgery. Neuropsychiatr Dis Treat 2008;4:487-93. [Crossref] [PubMed]
- Eshed I, Althoff CE, Hamm B, et al. Claustrophobia and premature termination of magnetic resonance imaging examinations. J Magn Reson Imaging 2007;26:401-4. [Crossref] [PubMed]
- Lee HJ, Im DJ, Youn JC, et al. Assessment of myocardial delayed enhancement with cardiac computed tomography in cardiomyopathies: a prospective comparison with delayed enhancement cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2017;33:577-84. [PubMed]
- Lee HJ, Im DJ, Youn JC, et al. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016;280:49-57. [Crossref] [PubMed]
- Genders TS, Petersen SE, Pugliese F, et al. The optimal imaging strategy for patients with stable chest pain: a cost-effectiveness analysis. Ann Intern Med 2015;162:474-84. [Crossref] [PubMed]
- Turchetti G, Kroes MA, Lorenzoni V, et al. The cost-effectiveness of diagnostic cardiac imaging for stable coronary artery disease. Expert Rev Pharmacoecon Outcomes Res 2015;15:625-33. [Crossref] [PubMed]
- Pontone G, Andreini D, Guaricci AI, et al. The STRATEGY Study (Stress Cardiac Magnetic Resonance Versus Computed Tomography Coronary Angiography for the Management of Symptomatic Revascularized Patients): Resources and Outcomes Impact. Circ Cardiovasc Imaging 2016.9. [PubMed]
- Moschetti K, Petersen SE, Pilz G, et al. Cost-minimization analysis of three decision strategies for cardiac revascularization: results of the "suspected CAD" cohort of the european cardiovascular magnetic resonance registry. J Cardiovasc Magn Reson 2016;18:3. [Crossref] [PubMed]


