Infarct characterization using CT
Introduction
Cardiac computed tomography (CCT) is considered an accurate imaging technique for the evaluation of coronary artery disease (CAD).
CCT may play the role of a non-invasive gatekeeper to invasive conventional coronary angiography especially in patients with suspected CAD (1-5). In particular, CCT is useful in patients at low-intermediate pre-test probability of CAD (6). The tool had an impressive development in the last decade with improvement of the scanner spatial and temporal resolution. Moreover, a significant reduction in radiation dose has been recently achieved (7-10). Such significant improvements determined an expansion of clinical indications even to non-coronary applications (11). Large clinical trials highlighted the utility and the cost-effectiveness of CCT (12-14). However, the assessment of the morphological pattern of a coronary stenosis should be combined with an evaluation of hemodynamic significance, and eventually related myocardial ischemia, and functional consequences (15). In this clinical context, CCT may integrate additional phases and information in the mainstream examination. CCT and cardiac MR (CMR) may detect and characterize myocardial infarction (MI) with overlapping imaging findings (16).
In this review, we describe the state of the art of CCT in the assessment and characterization of MI focusing on technical aspects, imaging features, and pros and cons in comparison to nuclear medicine and CMR. The review is directed to clinical cardiologists and it can be a focus for radiologists interested in cardiac imaging.
Background
MI is a major cause of death and disability worldwide. According to American Heart Association, CAD caused 1 of every 6 deaths in the United States in 2008 with a remarkable incidence of silent MI (17). According to World Health Organization, the trend of incidence is not expected to abate despite better prevention, diagnosis and treatment (18); nonetheless acute MI prevention and treatment efforts have resulted in favorable declines in the frequency of STEMI and death rates from the major types of acute MI (19).
The heart is a muscle with high energetic demand, which is continuously propelling blood throughout the body. In case of mismatch between oxygen supply and energetic demand, ischemia may follow with a definite sequence of cellular, inflammatory and biochemical effects. MI includes a wide spectrum of pathological stages depending on duration time of ischemia and extension of territories involved. The primary cause of acute MI is the sudden disruption of a coronary unstable atherosclerotic plaque and acute intracoronary thrombosis (20,21). First changes in cells contraction are observed in the first minute of ischemia, however temporary effects are documented after 20 minutes, depending on the robustness and extension of collateral coronary circle (22). After 4 hours from acute event the first gross pathological effects of MI are: edema with increased vascular permeability secondary to inflammation, necrosis (unprogrammed death of cells), and hemorrhage. After 12 hours, the inflammation processes with infiltration of neutrophils start and the myocytes begin to lose nuclei and striations. Within 3 days, the muscle fibers start to disintegrate, the neutrophils die, and the macrophages start to remove the debris of dead cells. Scarring process is defined by the apposition of collagen and ends after two months from the initial event and it is characterized by areas of fibrous tissue (fibrosis) that replace normal cells.
The left ventricle, which is the main target of MI for the primary contribution to heart contraction, can present a reduced functionality with decrease of the ejection fraction. Nonetheless, for a short time period after the beginning of the ischemic process the myocardium is “stunned” in a condition of transient left ventricular dysfunction. A prolonged ischemic state determines the condition of hibernated myocardium, which is partially reversible with revascularization. The restoration of blood flow to the damaged myocardium may also not have positive effects because it may cause an accelerate reperfusion injury due to the activation of oxygen free radicals, microvascular dysfunction and microvascular obstruction (MO). MO or no-reflow phenomenon is caused by edema and osmotic overload with alterations of endothelial cells and cardiomyocytes.
Macroscopically, the left ventricle may present a more round and dilated shape defined as ventricular remodeling. The ischemic process advances like a wavefront from the endocardium to the epicardium and may become transmural (23). A transmural MI involves the entire thickness of the myocardial wall from endocardium to epicardium. The left ventricle may even rupture with dramatic consequences such as hemopericardium and tamponade. Another later consequence may be the thinning of the ventricular wall and the development of an aneurysm. In some cases MI territories may be infiltrated by adipose cells in a process called lipomatous metaplasia. Rest CT perfusion cannot discriminate between lipomatous metaplasia and a rest perfusion defect with viable myocardium because both appear hypodense in this phase. In this case, delayed enhancement (DE) imaging is useful.
CT technical notes
Myocardial perfusion imaging can be assessed with CCT either in a single-step approach for qualitative evaluation of ischaemic myocardium (24) or in a multi-step approach for quantitative analysis of the myocardial blood supply (25).
Early perfusion defects may be displayed during the first passage of the contrast medium in patients with acute, chronic, and subacute MI. Myocardial territories with impaired perfusion have a reduced distribution of contrast medium. Then, a specific region of hypoattenuation can be depicted even during CCT studies aiming solely at the evaluation of coronary arteries with no additional radiation dose to patients or changes in the scanning parameters. Every CT scanner that is able to perform CCT can be employed to assess first-pass defects (Figure 1). Nonetheless, dedicated image filters may improve the delineation of MI areas, which are affected by the hyperattenuating contrast media amount in the left ventricle chamber (26). The contrast in the left ventricle chamber determines beam-hardening artifacts, which may obscure the subtle first-pass subendocardial defects (27,28). The reconstruction of different temporal windows of the cardiac cycle may help to determine if the perfusion defect is true (29).
Dual-energy CT technology may provide image acquisition using more than a single energy X-ray spectrum (30,31). Dual-energy CT represents a promising technique for the integrative analysis of coronary artery morphology and myocardial blood supply; furthermore Dual-energy CT is in good agreement with invasive coronary angiography and SPECT (30). Dual-energy technology enables mapping the myocardial iodine distribution, according to the absorption of X-ray spectra at different energy levels. The colour-maps of iodine distribution are based on both energy spectrum datasets and are superimposed into gray-scale multiplanar reformats of the left ventricle. The approach is not requiring additional scanning time or exceeding radiation dose, if compared to conventional single-energy CCT. Such techniques are usually described as perfusion imaging despite they merely provide a static picture of contrast medium distribution in the myocardium. Moreover, image acquisition covers several segments of the myocardium irrespective of perfusion phase (32,33). In the last years, a dynamic time-resolved scanner technology may exceed the limitations of a static acquisition of data. Such technique provides an effective perfusion imaging with colour-coded maps based on dynamic perfusion CCT performed during adenosine stress at multiple time points of contrast medium distribution through the myocardium (34). Time attenuation curves, perfusion parameters and defects may be analyzed. The recent availability of faster CT systems allowed the dynamic time-resolved perfusion imaging, which enables quantitative measurements of tissue blood flow, similarly to cardiac PET with the use of rubidium-82 (35).
CT techniques for imaging of myocardial viability are based on the pathological background of acute MI, whereas the cell damage leads to the loss of cellular membrane integrity and a subsequent increase in the distribution of contrast medium. In this regard, myocardial viability imaging with CCT is based on the same background and technique of CMR with DE (16,36-41). Iodinated contrast media are thought to accumulate in a way similar to gadolinium during CMR. Nevertheless, a plenty of practical factors such as ECG gating technique, tube parameters, and contrast media protocol may affect DE with CT.
First, ECG gating may be retrospective or prospective. The latter may significantly reduce the radiation dose even for DE purposes (9,42). It is reasonable to expect that a DE-CCT may be performed with a low radiation dose of about 1 mSv (10). Second, the noise inherent to CCT may hamper the assessment of areas of MI especially when a single-energy technique is exploited. In this setting, the use of low kilovoltage has been described to improve the detection of areas of DE within the myocardium in animal experiments (43,44), while tube current does not significantly affect the detection of MI territory, image quality or contrast resolution (45). Martini et al. also demonstrated that the increase of contrast material volume provides a significant improvement in MI image quality (46). Nonetheless, the noise may interfere with the accurate delineation of segments showing DE, especially when obese patients are studied. At least 120 mL of contrast medium should be administered with an optimal delayed scan from 5 to 15 minutes (Figure 2) (16,40,47) or directly after conventional angiography for reperfusion in the attempt to reduce the contrast media use (41). DE-CCT exploits multiplanar reconstruction or maximum intensity projections, with usual thickness from 5 to 10 mm, strict window width and level (29). CCT may evaluate CAD with an initial angiographic scan and subsequently assess the viability of myocardium with a DE scan.
CCT is not accurate in the prediction of myocardial ischemia if compared with CMR or single-photon emission computed tomography (SPECT) (48). Rest CCT can assess only static perfusion defects of infarcted areas of myocardium, as previously described. In this regard, it is widely accepted that the morphological information on a stenosis must be combined with the functional assessment of the perfusion or wall kinetics during provocative tests (49). A stress test may help to determine if a coronary artery stenosis is responsible of a reduction of myocardial perfusion. The stress may be induced pharmacologically or by exercise. It is well known that stress perfusion abnormalities occur before wall motion dysfunction (50). Therefore, additional scans acquired during pharmacologic stress with adenosine or dipyridamole may detect reversible perfusion defects of myocardium (31,51-53). Therefore, the stress-CCT may be indicated: in symptomatic patients at intermediate risk of CAD with non-diagnostic or equivocal ECG result or unable to exercise; in patients with a known coronary artery stenosis, to determine the hemodynamic significance; in patients with coronary artery bypass grafts and recurrent thoracic pain (11). However, as for other methods of provocative testing, stress CCT should be used under safety standards (54,55). CCT and SPECT have an important limitation due to high radiation exposure. Furthermore the use of contrast media should be limited and could be a limitation in patients with impaired renal function. In this case clinical examination together with functional evaluation with echocardiography may represent an alternative solution.
In conclusion, a comprehensive protocol of CCT should include: an initial angiographic scan to assess coronary arteries and myocardium at rest; a second scan with a second bolus of iodine contrast medium, before or after the rest scan, at peak of pharmacologic stress (adenosine 140 µg/min/kg of body weight for 2–5 minutes); a DE acquisition, about 5–10 minutes after the contrast injection, for viability imaging (Table 1).

Full table
Imaging findings
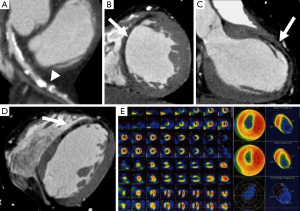
Improved CT scanner technology with high spatial and temporal resolution may detect MI even with standard CT techniques. Characteristics of chronic MI such as perfusion abnormalities, fatty metaplasia with lower attenuation values (<0 HU), calcifications, remodeling of the left ventricle, focal wall thinning, left ventricular thrombus or aneurysm may be easily depicted (Figure 3) (39,56).
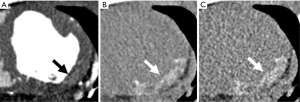
Non-contrast CCT for calcium scoring purpose can already detect chronic MI showing hypoattenuating myocardial regions if compared with nuclear myocardial perfusion imaging with a sensitivity of 92% and a specificity of 72% on a per-patient basis (56,57).
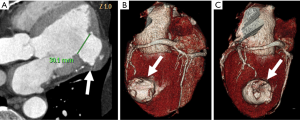
Similarly, contrast-enhanced CCT may detect hypoattenuated areas of MI (58) with a typical ischemic pattern (Figure 4). The distribution is subendocardial or transmural and it is concordant with the ischemic territory as opposed to epicardial or mid-wall distribution of myocarditis pattern (59). Rest dual energy CT provided comprehensive CAD imaging and identified perfusion defects in 90% of cases in comparison to SPECT (60). The major drawback is the visualization of small MI (61).
According to iodinated contrast media distribution, CCT may assess ischemic areas and viable myocardium in a way similar to CMR. The loss of membrane integrity of cells determines hyperenhancing areas. However, the DE may reduce from acute to chronic phases of MI because of the reduction of surrounding edema (62). Acute MI is defined by the ischemic area, which may become necrotic if the microvasculature is not recovered. In this setting, percutaneous coronary angioplasty (PTCA) may not lead to a functional recover because of the severe obstruction of the microvasculature. This is called no reflow phenomenon and it is defined by a central core of hypoattenuation within the hyperenhancing region (63). The decrease in infarct size over time is a process that can also be observed with CT (64). In acute MI the area may be hypoattenuated on first-pass and DE-CCT (65), while in chronic MI, reperfused or not, the scar determines a hyper-enhanced region.
Comprehensive protocol of CCT should be tailored to the patient´s history and to the findings of the first scan (i.e., if the first scan is normal it is not necessary to perform the stress and the DE; if the patient has an intermediate to high pretest it might be preferred to start with the stress and the rest might be avoided according to the findings).
Diagnostic performance
Perfusion and viability may be assessed with CCT (66-69). In the past decades, areas of suspected MI were mainly investigated by nuclear medicine techniques. SPECT and PET provided relevant diagnostic information with significant therapeutic and prognostic implications (70), despite some disadvantages such as attenuation artifacts, radiation dose, and limited off-hours availability. CMR may provide similar results in terms of diagnostic accuracy and long-term prognostic value, with the advantage of the lack of ionizing radiation (71,72).
Echocardiography represents a non-invasive diagnostic technique, which provides information regarding cardiac function and hemodynamics. In the acute settings it plays a role in regional wall motion abnormalities evaluation and for ruling out other etiologies of acute chest pain or dyspnea, including aortic dissection and pericardial effusion. Echocardiography can differentiate normal from infarcted myocardium, with the analysis of wall thickening and wall motion (the pattern of dysfunction may be a reflection of the extent of an infarction). Echocardiography plays a role also in the evaluation of complications of an acute MI like ventricular free wall rupture and pseudoaneurysm formation, ventricular septal rupture and mitral regurgitation (73).
In the next years, image fusion and hybrid scanners will combine more effectively structural and functional information regarding the pathological sequence that goes from stenosis to ischemia (74-78).
New concepts and technical solutions of CT scanner enable comprehensive imaging of MI from coronary stenosis to myocardial tissue damage. A plenty of studies demonstrated the high diagnostic accuracy of CCT (Table 2) in the diagnostic workup of patients with suspected CAD (3-5,8,11,79-83) and low to intermediate risk of CAD (6,84). CCT was also validated by several outcome studies that investigated risk stratification and prognostic value in registry data (85-87). Nonetheless, CCT may also provide functional information including regional heart function and the assessment of MI, ischemia, and viability (88).

Full table
Tanabe et al. demonstrated that Dynamic CT perfusion has the potential to detect abnormal perfused myocardium and severe infarction assessed by SPECT/CMR using comparable cut-off myocardial blood flow (MBF). Authors retrospectively evaluated fifty-three patients who underwent stress dynamic CTP and either SPECT (n = 25) or CMR (n = 28) and found that for detecting the abnormal perfused myocardium, sensitivity and specificity were 80% (95% CI, 71–90) and 86% (95% CI, 76–91) in SPECT (cut-off MBF, 1.23), and 82% (95% CI, 76–88) and 87% (95% CI, 80–92) in CMR (cut-off MBF, 1.25) (69).
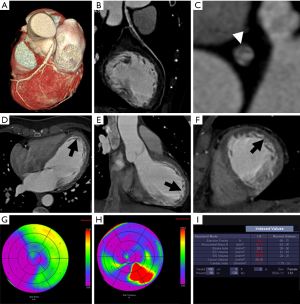
Even non-contrast CCT for calcium scoring purpose can accurately detect chronic MI showing hypoattenuating myocardial regions if compared with nuclear myocardial perfusion imaging with a sensitivity of 92% and a specificity of 72% on a per-patient basis (57). The presence of a myocardial hypo-enhancement region at rest on CCT has a sensitivity and specificity of around 90% to identify patients with a MI (Figures 5-7) (89,90). Rest dual energy CT provided comprehensive CAD imaging and identified perfusion defects in 90% of cases compared to SPECT (60).
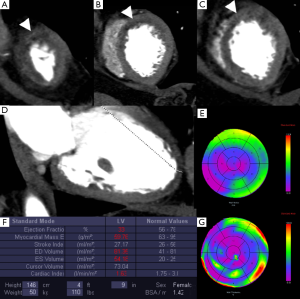
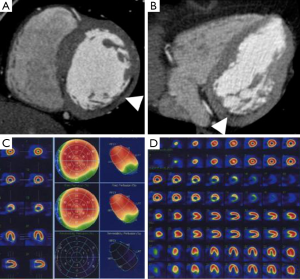
The accuracy in the detection of ischemia was investigated by several studies which compared stress-rest CCT with SPECT and CMR demonstrating a good agreement and similar sensitivity and specificity (30-32,51-53,91-94). In particular, stress perfusion CCT may refine the diagnostic accuracy of CCT alone (52), with increased sensitivity from 83% to 91% and specificity from 71% to 91%. Nevertheless, the current studies are based on small populations and are often biased by the high prevalence of disease in the recruited patients. On the other side, CCT may detect small perfusion defects because it is a technique with better image resolution than SPECT. Iodinated contrast media are reported to determine a vasodilatory effect and to keep specific kinetics, which may cause local hyperemia and improve the ability to detect small areas of MI (95,96).
PTCA has impressively improved the outcome of patients after MI (97). However, the sequelae of MI can determine left ventricular remodeling with reduced ejection fraction, even after restored coronary flow. Left ventricular remodeling is a major determinant of prognosis (98). In this regard, the infarct size and the distribution over myocardial wall may predict left ventricular remodeling (99,100).
SPECT imaging was considered a standard modality to assess the size of myocardial damage after acute MI with an evaluation of residual cardiac segments with perfusion defects (101,102). SPECT was extensively used for this purpose, however the modality may not recognize small perfusion defect in subendocardial infarcts (103). Gadolinium DE-CMR is the current clinical standard for the assessment of left ventricular infarct size (104). CMR may also provide better results in the detection of small infarcts in every clinical condition from acute to chronic settings in comparison to SPECT (105,106). DE- CMR delivers also an excellent prognostic value since it was found to be strongly correlated to the probability of recovered function after revascularization, short and long-term outcome (107,108).
Kim et al. demonstrated that segments with a DE of 75% in the myocardial wall don’t benefit from revascularization (109). CMR may display several aspects of infarction and reperfusion damage: size, interstitial edema, hemorrhage, periinfarct penumbra, MO (110).
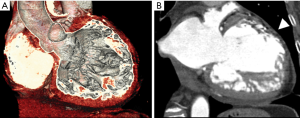
In recent years, CCT has enabled the detection of MI with accurate visualization of infarct size by DE techniques in animal model (38,111) and humans (Figures 8,9) (16). DE may be applied in CCT imaging for the detection of acute and chronic MI with a good accuracy (16,36,112). The dimension of DE territories and perfusion defects are predictive of long-term dysfunction after acute MI (113). Transmural contrast enhancement on CCT without additional administration of contrast media after conventional angiography is a marker of non-viable myocardium (41,114). The pattern of DE may indicate the possibility of functional recovery in patients after MI (115). Moreover, hypoattenuated areas of no-reflow within hyper-enhanced region of DE may be representative of residual perfusion defects after PTCA (116). DE-CCT may evaluate the size of MI immediately after primary PCI without additional contrast media and predict clinical outcome in patients with MI (117). Nevertheless, CCT studies were carried out in small cohorts of patients, although they already have shown promising results.
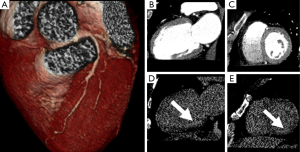
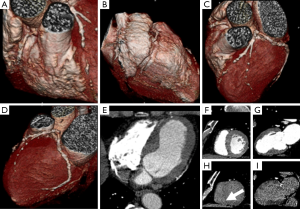
CCT and CMR employ different contrast media, however they may detect and characterize MI with overlapping imaging findings (16). Gadolinium derived contrast media and iodinated contrast media have similar kinetics and distribution in the normal myocardium and in the infarcted territories. Nevertheless, MI characterization by means of CCT may present some issues related to poor contrast resolution when compared to CMR.
CMR remains the non-invasive reference method for evaluating the extent of post-ischemic and non-viable myocardium. Patients with contraindication to CMR (non-compatible pacemakers, defibrillators, or other metal devices) may more easily undergo dedicated CCT studies. CCT is a fast and 24/7 service usually available in emergency context (118), or in clinical daily routine, including the evaluation of coronary arteries and left ventricular function (119).
Outlook
In the last decade, CCT gained rapid advance and unquestionable success in the non-invasive assessment of coronary arteries. CCT was recently applied to the detection of MI, perfusion, and viability. First, several studies were performed in the animal settings with excellent results. Then, human studies were performed on small patients population to assess the reproducibility of the approach. The first promising results are expected to be confirmed on more numerous patients cohort or on a multicenter basis for routine clinical application. The following step could be to place these results in a prognostic context in comparison with CMR which, in current-day practice, is considered the most accurate tool for infarct characterization in clinical setting. The health technology assessment should be also completed with cost-effectiveness and economic sustainability study (120,121). A one stop-shop examination with a profile of first-line imaging could be a solution to cut economic and biological costs in the work-up of patients with suspected or known CAD. Beyond the need to obtain more robust data, another requirement for future studies is the correct selection of patient population. A comprehensive protocol of morphological and functional CCT could be used in patients at intermediate-high probability of CAD in order to reduce procedural time and biological costs. Such protocol needs some additional time, which is exceeding the simple evaluation of coronary arteries of CCT angiographic studies (Table 1). The additional time should take into account both the patient scan (at least 15 minutes to perform a comprehensive protocol) and the reading time of an experienced radiologist (at least 20 minutes to elaborate a complete report) (122). The reporting time could be in part decreased in the future by the use of automated or semi-automated computer applications. If compared to SPECT and CMR, CCT has several advantages: short examination time, wide availability and patient’s acceptance (Table 3). In particular, Dual-energy computed tomography might be promising for the integrative analysis of the coronary artery morphology and the myocardial blood supply; DECT resulted in good agreement with invasive coronary angiography and SPECT. Ruzsics et al. demonstrated that DECT had 92% sensitivity and 93% specificity, with 93% accuracy for detecting any type of myocardial perfusion defect seen on SPECT (30).

Full table
Another relevant issue is that a comprehensive protocol of morphological and functional CCT should be performed with an additional radiation dose administration. The dose administered with CCT should be at least comparable with that of SPECT in perfusion imaging (34) and viability assessment (123), lower than 10 mSv (9). On the other side, CMR with stress and DE imaging may achieve excellent results in absence of potentially dangerous ionizing radiation, despite coronary arteries still cannot be properly assessed (124).
Another potential matter of interest is the timing of examination according to the phase of MI, because the pattern may differ between acute, subacute, chronic or healing phase. In addition, some patients with MI may also present with non-obstructive CAD (125-128) and therefore a comprehensive morphological and functional assessment of the heart should be pursued. Given that, CCT may be employed also in emergency setting, the tool could be an attractive diagnostic option also in this context (129).
Conclusions
CCT achieved promising results in the detection and characterization of MI, keeping into account that morphological, functional, and perfusional information can be not-invasively obtained. CCT may study the entire atherosclerotic process, including coronary plaque and stenosis, myocardial perfusion and viability. On the other side, some efforts should be spent in order to confirm the results in larger populations and to reduce the use of ionizing radiation as low as possible.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nieman K, Cademartiri F, Lemos PA, et al. Reliable non invasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation 2002;106:2051-4. [Crossref] [PubMed]
- Weustink AC, Mollet NR, Neefjes LA, et al. Preserved diagnostic performance of dual-source CT coronary angiography with reduced radiation exposure and cancer risk. Radiology 2009;252:53-60. [Crossref] [PubMed]
- Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of Coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36. [Crossref] [PubMed]
- Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724-32. [Crossref] [PubMed]
- Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135-44. [Crossref] [PubMed]
- Cademartiri F, Maffei E, Palumbo A, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography in patients with low-to-intermediate risk. Radiol Med 2007;112:969-81. [Crossref] [PubMed]
- Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301:500-7. [Crossref] [PubMed]
- Arcadi T, Maffei E, Mantini C, et al. Coronary CT angiography using iterative reconstruction vs. filtered back projection: evaluation of image quality. Acta Biomed 2015;86:77-85. [PubMed]
- Maffei E, Martini C, De Crescenzo S, et al. Low dose CT of the heart: a quantum leap into a new era of cardiovascular imaging. Radiol Med 2010;115:1179-207. [Crossref] [PubMed]
- Achenbach S, Marwan M, Ropers D, et al. Coronary computed tomography angiography with a consistent dose below 1mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340-6. [Crossref] [PubMed]
- di Cesare E, Carbone I, Carriero A, et al. Clinical indications for cardiac computed tomography. From the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM). Radiol Med 2012;117:901-38. [Crossref] [PubMed]
- SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383-91. [Crossref] [PubMed]
- Shah R, Foldyna B, Hoffmann U. Outcomes of anatomical vs. functional testing for coronary artery disease: Lessons from the PROMISE trial. Herz 2016;41:384-90. [Crossref] [PubMed]
- McKavanagh P, Lusk L, Ball PA, et al. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. Eur Heart J Cardiovasc Imaging 2015;16:441-8. [Crossref] [PubMed]
- Budoff MJ, Nakazato R, Mancini GB, et al. CT Angiography for the Prediction of Hemodynamic Significance in Intermediate and Severe Lesions: Head-to-Head Comparison With Quantitative Coronary Angiography Using Fractional Flow Reserve as the Reference Standard. JACC Cardiovasc Imaging 2016;9:559-64. [Crossref] [PubMed]
- Gerber BL, Belge B, Legros GJ, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation 2006;113:823-33. [Crossref] [PubMed]
- Roger VL, Go AS, Lloyd-Jones DM, et al. Executive Summary: Heart Disease and Stroke Statistics 2012 Update: A report From the American Heart Association. Circulation 2012;125:188-97. [Crossref] [PubMed]
- Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html
- McManus DD, Gore J, Yarzebski J, et al. Recent Trends in the Incidence, Treatment, and Outcomes of Patients with ST and Non-ST-Segment Acute Myocardial Infarction. Am J Med 2011;124:40-47. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664-72. [Crossref] [PubMed]
- Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation 2003;108:1772-8. [Crossref] [PubMed]
- Fujita M, Nakae I, Kihara Y, et al. Determinants of collateral development in patients with acute myocardial infarction. Clin Cardiol 1999;22:595-9. [Crossref] [PubMed]
- Rajiah P, Desai MY, Kwon D, et al. MR imaging of myocardial infarction. Radiographics 2013;33:1383-412. [Crossref] [PubMed]
- Rochitte CE, George RT, Chen MY, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE 320 study. Eur Heart J 2014;35:1120-30. [Crossref] [PubMed]
- Bamberg F, Hinkel R, Schwarz F, et al. Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol 2012;47:71-7. [Crossref] [PubMed]
- Mahnken AH, Lautenschläger S, Fritz D, et al. Perfusion weighted color maps for enhanced visualization of myocardial infarction by MSCT: preliminary experience. Int J Cardiovasc Imaging 2008;24:883-90. [Crossref] [PubMed]
- Flohr TG, Klotz E, Allmendinger T, et al. Pushing the envelope: new computed tomography techniques for cardiothoracic imaging. J Thorac Imaging 2010;25:100-11. [Crossref] [PubMed]
- Kitagawa K, George RT, Arbab-Zadeh A, et al. Characterization and correction of beam-hardening artifacts during dynamic volume CT assessment of myocardial perfusion. Radiology 2010;256:111-8. [Crossref] [PubMed]
- Blankstein R, Rogers IS, Cury RC. Practical tips and tricks in cardiovascular computed tomography: diagnosis of myocardial infarction. J Cardiovasc Comput Tomogr 2009;3:104-11. [Crossref] [PubMed]
- Ruzsics B, Schwarz F, Schoepf UJ, et al. Comparison of dual-energy computed tomography of the heart with single photon emission computed tomography for assessment of coronary artery stenosis and of the myocardial blood supply. Am J Cardiol 2009;104:318-26. [Crossref] [PubMed]
- Ko SM, Choi JW, Song MG, et al. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol 2011;21:26-35. [Crossref] [PubMed]
- Cheng W, Zeng M, Arellano C, et al. Detection of myocardial perfusion abnormalities: standard dual-source coronary computed tomography angiography versus rest/stress technetium-99m single photoemission CT. Br J Radiol 2010;83:652-60. [Crossref] [PubMed]
- Weininger M, Schoepf UJ, Ramachandra A, et al. Adenosine-stress dynamic real-time myocardial perfusion and adenosine-stress first-pass dual energy myocardial perfusion CT for the assessment of acute chest pain: initial results. Eur J Radiol 2012;81:3703-10. [Crossref] [PubMed]
- Bastarrika G, Ramos-Duran L, Rosenblum MA, et al. Adenosine stress dynamic myocardial CT perfusion imaging: initial clinical experience. Invest Radiol 2010;45:306-13. [PubMed]
- Lautamäki R, George RT, Kitagawa K, et al. Rubidium-82 PET-CT for quantitative assessment of myocardial blood flow: validation in a canine model of coronary artery stenosis. Eur J Nucl Med Mol Imaging 2009;36:576-86. [Crossref] [PubMed]
- Mahnken AH, Koos R, Katoh M, et al. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. J Am Coll Cardiol 2005;45:2042-7. [Crossref] [PubMed]
- Baks T, Cademartiri F, Moelker AD, et al. Multislice computed tomography and magnetic resonance imaging for the assessment of reperfused acute myocardial infarction. J Am Coll Cardiol 2006;48:144-52. [Crossref] [PubMed]
- Lardo AC, Cordeiro MA, Silva C, et al. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 2006;113:394-404. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J 2008;29:2544-51. [Crossref] [PubMed]
- Nieman K, Shapiro MD, Ferencik M, et al. Reperfused myocardial infarction: contrast-enhanced 64-section CT in comparison to MR imaging. Radiology 2008;247:49-56. [Crossref] [PubMed]
- Habis M, Capderou A, Sigal–Cinqualbre A, et al. Comparison of delayed enhancement patterns on multislice computed tomography immediately after coronary angiography and cardiac magnetic resonance imaging in acute myocardial infarction. Heart 2009;95:624-9. [Crossref] [PubMed]
- Chang HJ, George RT, Schuleri KH, et al. Prospective electrocardiogram-gated delayed enhancement multidetector computed tomography accurately quantifies infarct size and reduces radiation exposure. JACC Cardiovasc Imaging 2009;2:412-20. [Crossref] [PubMed]
- Brodoefel H, Klumpp B, Reimann A, et al. Late myocardial enhancement assessed by 64-MSCT in reperfused porcine myocardial infarction: diagnostic accuracy of low-dose CT protocols in comparison with magnetic resonance imaging. Eur Radiol 2007;17:475-83. [Crossref] [PubMed]
- Mahnken AH, Bruners P, Mühlenbruch G, et al. Low tube voltage improves computed tomography imaging of delayed myocardial contrast enhancement in an experimental acute myocardial infarction model. Invest Radiol 2007;42:123-9. [Crossref] [PubMed]
- Martini C, Maffei E, Palumbo A, et al. Impact of tube current in the quantitative assessment of acute reperfused myocardial infarction with 64-slice delayed-enhancement CT: a porcine model. Radiol Med 2010;115:1003-14. [Crossref] [PubMed]
- Martini C, Maffei E, Palumbo A, et al. Impact of contrast material volume on quantitative assessment of reperfused acute myocardial infarction using delayed-enhancement 64-slice CT: experience in a porcine model. Radiol Med 2010;115:22-35. [Crossref] [PubMed]
- Jacquier A, Boussel L, Amabile N, et al. Multidetector computed tomography in reperfused acute myocardial infarction: assessment of infarct size and no-reflow in comparison with cardiac magnetic resonance imaging. Invest Radiol 2008;43:773-81. [Crossref] [PubMed]
- Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 2008;248:414-23. [Crossref] [PubMed]
- Cademartiri F, La Grutta L, Palumbo A, et al. Computed tomography coronary angiography vs. stress ECG in patients with stable angina. Radiol Med 2009;114:513-23. [Crossref] [PubMed]
- Vliegenthart R, Henzler T, Moscariello A, et al. CT of coronary heart disease: Part 1, CT of myocardial infarction, ischemia, and viability. AJR Am J Roentgenol 2012;198:531-47. [Crossref] [PubMed]
- Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol 2009;54:1072-84. [Crossref] [PubMed]
- Rocha-Filho JA, Blankstein R, Shturman LD, et al. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology 2010;254:410-9. [Crossref] [PubMed]
- Cury RC, Magalhães TA, Borges AC, et al. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol 2010;106:310-5. [Crossref] [PubMed]
- Shaikh K, Wang DD, Saad H, et al. Feasibility, safety and accuracy of regadenoson-atropine (REGAT) stress echocardiography for the diagnosis of coronary artery disease: an angiographic correlative study. Int J Cardiovasc Imaging 2014;30:515-22. [Crossref] [PubMed]
- Greenwood JP, Younger JF, Ridgway JP, et al. Safety and diagnostic accuracy of stress cardiac magnetic resonance imaging vs exercise tolerance testing early after acute ST elevation myocardial infarction. Heart 2007;93:1363-8. [Crossref] [PubMed]
- Rodríguez-Granillo GA, Rosales MA, Renes P, et al. Chronic myocardial infarction detection and characterization during coronary artery calcium scoring acquisitions. J Cardiovasc Comput Tomogr 2010;4:99-107. [Crossref] [PubMed]
- Gupta M, Kadakia J, Hacioglu Y, et al. Non-contrast cardiac computed tomography can accurately detect chronic myocardial infarction: validation study. J Nucl Cardiol 2011;18:96-103. [Crossref] [PubMed]
- Nikolaou K, Knez A, Sagmeister S, et al. Assessment of myocardial infarctions using multidetector-row computed tomography. J Comput Assist Tomogr 2004;28:286-92. [Crossref] [PubMed]
- Rubinshtein R, Miller TD, Williamson EE, et al. Detection of myocardial infarction by dual-source coronary computed tomography angiography using quantitated myocardial scintigraphy as the reference standard. Heart 2009;95:1419-22. [Crossref] [PubMed]
- Ruzsics B, Lee H, Zwerner PL, et al. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia: initial experience. Eur Radiol 2008;18:2414-24. [Crossref] [PubMed]
- Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 2003;361:374-9. [Crossref] [PubMed]
- Mahnken AH, Bruners P, Bornikoel CM, et al. Assessment of myocardial edema by computed tomography in myocardial infarction. JACC Cardiovasc Imaging 2009;2:1167-74. [Crossref] [PubMed]
- Nakazawa G, Tanabe K, Onuma Y, et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am Heart J 2008;155:1150-7. [Crossref] [PubMed]
- Mahnken AH, Bruners P, Kinzel S, et al. Late phase MSCT in the different stages of myocardial infarction: animal experiments. Eur Radiol 2007;17:2310-7. [Crossref] [PubMed]
- Zhang LJ, Peng J, Wu SY, et al. Dual source dual-energy computed tomography of acute myocardial infarction: correlation with histopathologic findings in a canine model. Invest Radiol 2010;45:290-7. [PubMed]
- Nikolaou K, Sanz J, Poon M, et al. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: preliminary results. Eur Radiol 2005;15:864-71. [Crossref] [PubMed]
- Bauer RW, Kerl JM, Fischer N, et al. Dual-energy CT for the assessment of chronic myocardial infarction in patients with chronic coronary artery disease: comparison with 3-T MRI. AJR Am J Roentgenol 2010;195:639-46. [Crossref] [PubMed]
- Ko SM, Song MG, Chee HK, et al. Diagnostic performance of dual-energy CT stress myocardial perfusion imaging: direct comparison with cardiovascular MRI. AJR Am J Roentgenol 2014;203:W605-13. [Crossref] [PubMed]
- Tanabe Y, Kido T, Uetani T, et al. Differentiation of myocardial ischemia and infarction assessed by dynamic computed tomography perfusion imaging and comparison with cardiac magnetic resonance and single-photon emission computed tomography. Eur Radiol 2016;26:3790-801. [Crossref] [PubMed]
- Klocke FJ, Baird MG, Lorell BH, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Society for Nuclear Cardiology. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging: executive summary—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 2003;108:1404-18. [Crossref] [PubMed]
- Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicenter, multivendor, randomized trial. Eur Heart J 2008;29:480-9. [Crossref] [PubMed]
- Mordini FE, Haddad T, Hsu LY, et al. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc Imaging 2014;7:14-22. [Crossref] [PubMed]
- Lancellotti P, Price S, Edvardsen T, et al. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Cardiovasc Imaging 2015;16:119-46. [Crossref] [PubMed]
- Liga R, Vontobel J, Rovai D, et al. Multicentre multi-device hybrid imaging study of coronary artery disease: results from the EValuation of INtegrated Cardiac Imaging for the Detection and Characterization of Ischaemic Heart Disease (EVINCI) hybrid imaging population. Eur Heart J Cardiovasc Imaging 2016;17:951-60. [Crossref] [PubMed]
- Gaemperli O, Schepis T, Kalff V, et al. Validation of a new cardiac image fusion software for three-dimensional integration of myocardial perfusion SPECT and stand-alone 64-slice CT angiography. Eur J Nucl Med Mol Imaging 2007;34:1097-106. [Crossref] [PubMed]
- Pazhenkottil AP, Nkoulou RN, Ghadri JR, et al. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J 2011;32:1465-71. [Crossref] [PubMed]
- Flotats A, Knuuti J, Gutberlet M, et al. Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging 2011;38:201-12. [Crossref] [PubMed]
- Di Carli MF, Dorbala S, Curillova Z, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PETCT imaging. J Nucl Cardiol 2007;14:799-809. [Crossref] [PubMed]
- Miller JM, Dewey M, Vavere AL, et al. Coronary CT angiography using 64 detector rows: methods and design of the multi-centre trial CORE-64. Eur Radiol 2009;19:816-28. [Crossref] [PubMed]
- Weustink AC, Mollet NR, Neefjes LA, et al. Diagnostic accuracy and clinical utility of noninvasive testing for coronary artery disease. Ann Intern Med 2010;152:630-9. [Crossref] [PubMed]
- La Grutta L, Toia P, Galia M, et al. Role of Cardiac Computed Tomography in the Evaluation of Coronary Artery Stenosis in Patients With Ascending Aorta Aneurysm Detected at Transthoracic Echocardiography. J Comput Assist Tomogr 2016;40:393-7. [Crossref] [PubMed]
- Task Force Members1, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003.
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation 2010;122:e525-55. [Crossref] [PubMed]
- Marwan M, Hausleiter J, Abbara S, et al. Multicenter Evaluation Of Coronary Dual-Source CT angiography in patients with intermediate Risk of Coronary Artery Stenoses (MEDIC): study design and rationale. J Cardiovasc Comput Tomogr 2014;8:183-8. [Crossref] [PubMed]
- Pundziute G, Schuijf JD, Jukema JW, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;49:62-70. [Crossref] [PubMed]
- Min JK, Dunning A, Lin FY, et al. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr 2011;5:84-92. [Crossref] [PubMed]
- Aldrovandi A, Maffei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol 2009;19:1653-60. [Crossref] [PubMed]
- Lin FY, Min JK. Assessment of cardiac volumes by multidetector computed tomography. J Cardiovasc Comput Tomogr 2008;2:256-62. [Crossref] [PubMed]
- Schepis T, Achenbach S, Marwan M, et al. Prevalence of first-pass myocardial perfusion defects detected by contrast enhanced dual-source CT in patients with non-ST segment elevation acute coronary syndromes. Eur Radiol 2010;20:1607-14. [Crossref] [PubMed]
- Feuchtner GM, Plank F, Pena C, et al. Evaluation of myocardial CT perfusion in patients presenting with acute chest pain to the emergency department: comparison with SPECT-myocardial perfusion imaging. Heart 2012;98:1510-7. [Crossref] [PubMed]
- Nagao M, Matsuoka H, Kawakami H, et al. Quantification of myocardial perfusion by contrast-enhanced 64-MDCT: characterization of ischemic myocardium. AJR Am J Roentgenol 2008;191:19-25. [Crossref] [PubMed]
- George RT, Arbab-Zadeh A, Miller JM, et al. Adenosine stress 64- and 256 row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging 2009;2:174-82. [Crossref] [PubMed]
- Okada DR, Ghoshhajra BB, Blankstein R, et al. Direct comparison of rest andadenosine stress myocardial perfusion CT with rest and stress SPECT. J Nucl Cardiol 2010;17:27-37. [Crossref] [PubMed]
- Ho KT, Chua KC, Klotz E, Panknin C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc Imaging 2010;3:811-20. [Crossref] [PubMed]
- Baile EM, Pare PD, D’Yachkova Y, et al. Effect of contrast media on coronary vascular resistance: contrast-induced coronary vasodilation. Chest 1999;116:1039-45. [Crossref] [PubMed]
- Bell MR, Lerman LO, Rumberger JA. Validation of minimally invasive measurement of myocardial perfusion using electron beam computed tomography and application in human volunteers. Heart 1999;81:628-35. [Crossref] [PubMed]
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13-20. [Crossref] [PubMed]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161-72. [Crossref] [PubMed]
- Mansencal N, Tissier R, Deux JF, et al. Relation of the ischaemic substrate to left ventricular remodelling by cardiac magnetic resonance at 1.5 T in rabbits. Eur Radiol 2010;20:1214-20. [Crossref] [PubMed]
- Palazzuoli A, Beltrami M, Gennari L, et al. The impact of infarct size on regional and global left ventricular systolic function: a cardiac magnetic resonance imaging study. Int J Cardiovasc Imaging 2015;31:1037-44. [Crossref] [PubMed]
- Medrano R, Lowry RW, Young JB, et al. Assessment of myocardial viability with 99mTc sestamibi in patients undergoing cardiac transplantation: a scintigraphic/pathological study. Circulation 1996;94:1010-7. [Crossref] [PubMed]
- Gibbons RJ, Miller TD, Christian TF. Infarct size measured by single photon emission computed tomographic imaging with (99m) Tc-sestamibi: a measure of the efficacy of therapy in acute myocardial infarction. Circulation 2000;101:101-8. [Crossref] [PubMed]
- Bartosik J, El-Ali HH, Nilsson U, et al. Subendocardial versus transmural ischaemia in myocardial perfusion SPECT--a Monte Carlo study. Clin Physiol Funct Imaging 2006;26:343-50. [Crossref] [PubMed]
- Wagner A, Mahrholdt H, Thomson L, et al. Effect of time, dose, and inversion time for acute myocardial infarct size measurements based on magnetic resonance imaging-delayed contrast enhancement. J Am Coll Cardiol 2006;47:2027-33. [Crossref] [PubMed]
- Rubinshtein R, Miller TD, Williamson EE, et al. Detection of myocardial infarction by dual-source coronary computed tomography angiography using quantitated myocardial scintigraphy as the reference standard. Heart 2009;95:1419-22. [Crossref] [PubMed]
- Perazzolo Marra M, Lima JAC, Illiceto S. MRI in acute myocardial infarction. Eur Heart J 2011;32:284-93. [Crossref] [PubMed]
- Beek AM, Kuhl HP, Bondarenko O, et al. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol 2003;42:895-901. [Crossref] [PubMed]
- Saeed M, Hetts S, Wilson M. Reperfusion injury components and manifestations determined by cardiovascular MR and MDCT imaging. World J Radiol 2010;2:1-14. [Crossref] [PubMed]
- Kim RJ, Wu E, Rafael A, et al. The use of contrast enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445-53. [Crossref] [PubMed]
- Midiri M, La Grutta L, Grassedonio E, et al. Non invasive imaging of myocardial infarction with computed tomography and magnetic resonance. Curr Vasc Pharmacol 2015;13:64-77. [Crossref] [PubMed]
- Hoffmann U, Millea R, Enzweiler C, et al. Acute myocardial infarction: contrast-enhanced multi-detector row CT in a porcine model. Radiology 2004;231:697-701. [Crossref] [PubMed]
- Choe YH, Choo KS, Jeon ES, et al. Comparison of MDCT and MRI in the detection and sizing of acute and chronic myocardial infarcts. Eur J Radiol 2008;66:292-9. [Crossref] [PubMed]
- Lessick J, Dragu R, Mullak D, et al. Is functional improvement after myocardial infarction predicted with myocardial enhancement patterns at multidetector CT? Radiology 2007;244:736-44. [Crossref] [PubMed]
- Habis M, Capderou A, Ghostine S, et al. Acute myocardial infarction early viability assessment by 64-slice computed tomography immediately after coronary angiography: comparison with low-dose dobutamine echocardiography. J Am Coll Cardiol 2007;49:1178-85. [Crossref] [PubMed]
- Sato A, Hiroe M, Nozato T, et al. Early validation study of 64-slice multidetector computed tomography for the assessment of myocardial viability and the prediction of left ventricular remodeling after acute myocardial infarction. Eur Heart J 2008;29:490-8. [Crossref] [PubMed]
- Paul J-F, Wartski M, Caussin C, et al. Late defect on delayed contrast-enhanced multi-detector row CT scans in the prediction of SPECT infarct size after reperfused acute myocardial infarction: initial experience. Radiology 2005;236:485-9. [Crossref] [PubMed]
- Sato A, Nozato T, Hikita H, et al. Prognostic value of myocardial contrast delayed enhancement with 64-slice multidetector computed tomography after acute myocardial infarction. J Am Coll Cardiol 2012;59:730-8. [Crossref] [PubMed]
- Boussel L, Ribagnac M, Bonnefoy E, et al. Assessment of acute myocardial infarction using MDCT after percutaneous coronary intervention: comparison with MRI. AJR Am J Roentgenol 2008;191:441-7. [Crossref] [PubMed]
- Cury RC, Nieman K, Shapiro MD, et al. Comprehensive assessment of myocardial perfusion defects, regional wall motion, and left ventricular function by using 64-section multidetector CT. Radiology 2008;248:466-75. [Crossref] [PubMed]
- La Grutta L, La Grutta S, Galia M, et al. Acceptance of noninvasive computed tomography coronary angiography: for a patient-friendly medicine. Radiol Med 2014;119:128-34. [Crossref] [PubMed]
- Meyer M, Nance JW Jr, Schoepf UJ, et al. Cost-effectiveness of substituting dual-energy CT for SPECT in the assessment of myocardial perfusion for the workup of coronary artery disease. Eur J Radiol 2012;81:3719-25. [Crossref] [PubMed]
- Kachenoura N, Gaspar T, Lodato JA, et al. Combined assessment of coronary anatomy and myocardial perfusion using multidetector computed tomography for the evaluation of coronary artery disease. Am J Cardiol 2009;103:1487-94. [Crossref] [PubMed]
- Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation 2007;116:1290-305. [Crossref] [PubMed]
- Di Cesare E, Cademartiri F, Carbone I, et al. Clinical indications for the use of cardiac MRI. By the SIRM Study Group on Cardiac Imaging. Radiol Med 2013;118:752-98. [Crossref] [PubMed]
- Reynolds HR, Srichai MB, Iqbal SN, et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 2011;124:1414-25. [Crossref] [PubMed]
- Fishbein MC, Siegel RJ. How big are coronary atherosclerotic plaques that rupture? Circulation 1996;94:2662-6. [Crossref] [PubMed]
- Cademartiri F, La Grutta L, Palumbo A, et al. Imaging techniques for the vulnerable coronary plaque. Radiol Med 2007;112:637-59. [Crossref] [PubMed]
- Pasupathy S, Air T, Dreyer RP, et al. Systematic review of patients presenting with suspected myocardial infarction and non obstructive coronary arteries. Circulation 2015;131:861-70. [Crossref] [PubMed]
- Nieman K, Hoffmann U. Cardiac computed tomography in patients with acute chest pain. European Heart Journal 2015;36:906-14. [Crossref] [PubMed]


