A peripheral blood gene expression score is associated with plaque volume and phenotype by intravascular ultrasound with radiofrequency backscatter analysis: results from the ATLANTA study
Introduction
Atherosclerosis is the result of complex interactions between genetic susceptibility and the environment. Genetic susceptibility is transcribed into a dynamic pattern of RNA gene expression under the influence of environmental factors, resulting in a large number of intermediate phenotypes that ultimately lead to the development of atherosclerosis. Such intermediate phenotypes include vascular permeability, vascular adhesion, vascular reactivity, blood pressure, lipoprotein levels and innate and adaptive immunity. Traditional clinical evaluation is based on the assessment of risk factors based on such intermediate phenotypes including blood pressure, cholesterol, high-density lipoprotein (HDL) cholesterol and diabetes combined into global risk assessment scores, such as the Framingham Risk Score (FRS) (1,2). More recently, several extensive, genome-wide association studies attempted to identify variants in the human genome that carry susceptibility for the development of coronary artery disease (CAD) and cardiovascular events (3-6).
Interestingly, less is known about the relationship between gene expression patterns in circulating blood cells and coronary atherosclerosis. We recently published the first validation of a composite gene expression score for the diagnosis of obstructive CAD, defined as 50% or greater percent diameter stenosis based on invasive coronary X-ray angiography from a different, larger population (N=526) (7). In this study we found that a composite gene expression score based on age, sex and 23 gene expression values derived from an independent development cohort, was associated with the severity of angiographic CAD and provided incremental diagnostic value beyond the Diamond-Forrester classification system. However, while that study assessed the relationship between obstructive CAD and peripheral gene expression, it did not directly evaluate the relationship to atherosclerotic plaque volume and composition.
Intravascular ultrasound with radiofrequency backscatter analysis (IVUS/VH) has been validated for quantitative measurements of coronary plaque volume and composition and has been shown to predict outcomes and treatment effects (8-10). Therefore, the objective of the present study was to assess the relationship between this previously validated GES and coronary plaque volume and composition by IVUS/VH. We hypothesized that a higher GES would be associated with greater plaque volume and more advanced, more vulnerable plaque phenotype as assessed by IVUS/VH.
Methods
General study design
The ATLANTA study program (Assessment of Tissue characteristics, Lesion morphology and hemodynamics by Angiography with fractional flow reserve, intravascular ultrasound and virtual histology and Non-invasive computed Tomography in Atherosclerotic plaques) was a prospective, single-center, investigator-initiated study approved by the Institutional Review Board of Piedmont Healthcare. All participants provided informed consent according to institutional protocols. The overall study design of phase I has been published previously (11); here we report combined results from a gene-expression sub-study of Phase I and Phase II (ClinicalTrials.gov NCT00817102). Briefly, the study enrolled symptomatic males and females between ages 18 and 90 presenting with stable angina symptoms with at least one coronary arterial plaque with percent diameter stenosis of 40-99%. Each patient underwent invasive X-ray angiography, IVUS/VH, fractional flow reserve (FFR) measurements, and coronary computed tomography angiography. The study lesion was identified for each patient based on identifiable standard anatomical landmarks (i.e. septal, diagonal, obtuse marginal branches) amongst all imaging modalities in order to facilitate co-registration. Blood was collected and batched for core laboratory measurements of biomarkers. Since the gene expression algorithm was developed and validated only in non-diabetic subjects, we excluded diabetic patients from the current analysis.
Lipoprotein and biomarker measurements
All patients underwent fasting lipid profile measurements including total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides using standard enzymatic methods. Additionally, all patients had high sensitivity C-reactive protein (hsCRP) measurements based on the highly sensitive Near Infrared Particle Immunoassay rate methodology (Beckman-Coulter, Brea, CA). Finally, all patients underwent lipoprotein-associated phospholipase A2 (Lp-PLA2) testing using enzyme-linked immunosorbent assay (ELISA) through a commercial laboratory (Boston Heart Lab; Boston, MA). All measurements were done at one time-point in one laboratory to maintain consistency.
Evaluation of peripheral blood gene expression
We utilized a previously validated, commercially available, composite peripheral blood gene expression score for the assessment of CAD (Corus® CAD; CardioDx, Inc; Palo Alto, CA) (7,12). This score is based on patient age, sex, and expression levels of 23 genes that have been associated with obstructive CAD, and yields a score of 1-40, with higher scores associated with higher likelihood of obstructive CAD.
Blood collection, RNA purification and RT-PCR
Whole blood samples were collected in PAXgene® tubes prior to coronary angiography and IVUS/VH, and treated according to the manufacturer’s instructions, then frozen at –20 °C. RNA purification was performed with an automated method based on the Agencourt RNAdvance system (7,12). All PCR reactions were run in triplicate and median values were used for score calculation as described (7).
IVUS-VH image acquisition
After intracoronary injection of nitroglycerin (median total dose per case: 200 mcg; Interquartile range, 200-400 mcg) and after placing a guiding catheter in the target coronary artery, a 3.2 F, 20 MHz ultrasound catheter (Eagle Eye; Volcano Inc; Rancho Cordova, CA) was inserted and was advanced at least 2 cm beyond the most distal portion of the target lesion. Automated pullback was performed at a rate of 0.5 mm/sec (R-100; Volcano Inc; Rancho Cordova, CA). The electrocardiographic signal was simultaneously recorded for the reconstruction of the radiofrequency backscatter information using In-Vision Gold (Volcano Inc; Rancho Cordova, CA).
IVUS/VH image analysis
De-identified IVUS/VH datasets were analyzed by an experienced cardiologist (GV) using dedicated software (pcVH 3.0.394, Volcano Inc, Rancho Cordova, CA) on a dedicated workstation using Simpson’s rule. Semi-automatic contouring of the luminal boundary and the external elastic lamina was performed in each frame. Based on a previously validated algorithm (8), the software classified each pixel as dense calcium (DC; white), fibrous tissue (FI; green), fibro-fatty tissue (FF; light green) or necrotic core (NC; red) (Figure 1A-D). Total volume and percentage of each of the four components was measured in the entire pullback.
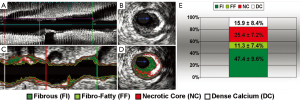
Plaque composition by intravascular ultrasound with radiofrequency backscatter analysis (IVUS/VH). Grayscale IVUS dataset in longitudinal (A) and cross-sectional views (B) and corresponding IVUS/VH datasets (C,D) are shown; Percent contribution of each IVUS/VH plaque component in current study population (E), presented as mean ± standard deviation (n=18 subjects)
Quantitative analysis
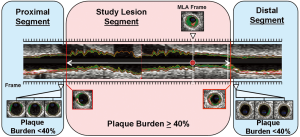
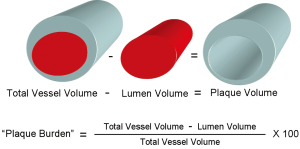
We measured geometrical and compositional variables in each distinct plaque within the region of the entire IVUS/VH pullback. As seen in Figure 2, the starting point of a plaque was defined as the site where plaque burden exceeded 40% in three consecutive frames and termination point was defined as the site where plaque burden fell below 40% in three consecutive frames. This definition of IVUS/VH plaque was based on current, international recommendations for IVUS/VH measurements and it was similar to the large, prospective PROSPECT study (10,13). Geometrical variables included minimal lumen diameter (MLD), percent diameter stenosis (%DS), minimal lumen area (MLA) and percent area stenosis (%AS). %DS and %AS were calculated based on a proximal and distal reference segment to correct for vessel tapering. Absolute total plaque volume was measured in each plaque, as well as the volume and percent of each of the four plaque components. Plaque burden was also calculated in 3 dimensions at each frame across the entire length of the plaque rather than only at the point of maximal stenosis. Plaque burden was calculated as the difference between the vessel volume and the luminal volume expressed as a percentage of the vessel volume (Figure 3).
Ethical standards
The experiments described in the study comply with the current laws of the United States and were approved by the Institutional Review Board of Piedmont Healthcare as described. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Statistical analysis
Associations between lipoprotein measurements, biomarkers, gene expression score and plaque variables was determined using Spearman rank correlation and an associated exact P-value. A P-value ≤0.05 was considered statistically significant. Due to the limited sample size, only correlations with gene expression score were analyzed to help reduce multiple testing limitations and false discovery. To account for multiple comparisons, a permutation test was used to estimate the likelihood of the observed results under the null hypothesis. Statistical analyses were conducted independently at the Piedmont Heart Institute in Atlanta, GA by the investigators and also by a biostatistician at CardioDx, Inc. The investigators at the Piedmont Heart Institute were responsible for data analysis, interpretation and the writing of the manuscript.
Results
General patient characteristics
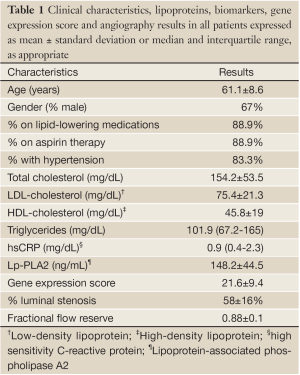
From the ATLANTA study cohort, 18 patients had simultaneous gene expression score and interpretable IVUS/VH data available constituting the study population for the present analysis. Mean age was 61.1±8.6 years; 67% were male. Clinical characteristics, lipids, biomarkers, and angiography results of the patients are shown in Table 1. Angiographically, average luminal diameter stenosis was 58±16%; range 33% to 80%. Ten patients had non-obstructive CAD (<70% diameter stenosis) and 8 had obstructive CAD (≥70% diameter stenosis). Mean FFR of the study lesions was 0.88±0.1; 3 patients had FFR<0.75. Medication usage included high rates of statin therapy (89%) and antiplatelet therapy (94%).
Full Table
Gene expression data
Average GES (Table 1) on a previously validated scale of 1-40 was 21.6±9.4; with a range of 5-34. Of the 18 patients, 5 patients had a low score (<15).
IVUS/VH characteristics of patients
We assessed a total of 1,158 mm of coronary anatomy by IVUS/VH (mean 64.4±10.9 mm per patient). Plaque geometrical and compositional variables based on IVUS/VH are shown in Table 2 and Figure 1E. Of the 18 vessels interrogated by IVUS/VH, 15 had MLA <4 mm2. Although angiographically there were 8 study lesions of ≥70% stenosis, by IVUS/VH none had %DS >70%.
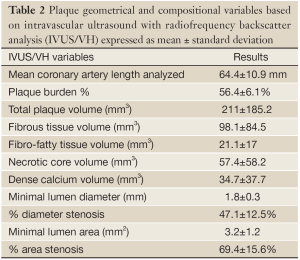
Full Table
Gene expression score and plaque volume and composition
Peripheral gene expression as measured by the composite score was significantly correlated by non-parametric Spearman rank correlation with volumes of necrotic core (R2=0.56; P=0.015), dense calcium (R2=0.60; P=0.007) non-calcified plaque (R2=0.50; P=0.036) and total plaque (R2=0.55; P=0.018) (Table 3; Figure 4). None of these IVUS-VH measurements showed significant association with lipid measurements (LDL/HDL, total cholesterol or triglycerides). Permutation of the composite gene expression scores over 1,000 trials resulted in only 39 (3.9%) showing 4 or more IVUS-VH parameters as significantly associated with the GES.
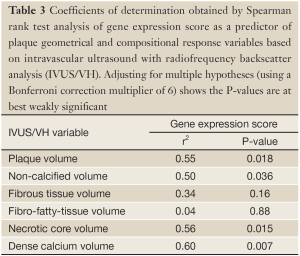
Full Table
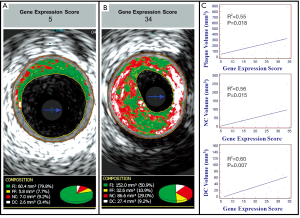
Discussion
To the best of our knowledge, this is the first study to evaluate the relationship between peripheral blood gene expression and coronary arterial plaque measurements by IVUS/VH. The main finding of the study is that altered expression of 23 genes, mostly inflammatory genes and genes involved with innate and acquired immunity, as measured by a previously validated composite score, is associated with more necrotic core, dense calcium and larger plaque volume based on IVUS/VH measurements.
Development and progression of atherosclerosis
There are several key elements in the development of atherosclerosis (14). The initiating factor is the deposition of atherogenic lipoprotein particles in the subintima, which triggers a reactive, mal-adaptive inflammatory response. It has been shown that lipoprotein deposition is the primary, and inflammation is the secondary process in atherosclerosis (15). Circulating monocytes are recruited to the subintima and they are differentiated to resident macrophages with phagocytic capabilities to remove atherogenic lipids through scavenger receptors, such as CD36 and SR-B1 (16-18). Interestingly, since macrophages in the vessel wall cannot shut down lipoprotein uptake via scavenger receptors and cellular reverse cholesterol transport mechanisms cannot keep up with the uptake of oxidized lipoproteins, resident macrophages accumulate atherogenic lipoproteins, turn into foam-cells and ultimately, they undergo apoptosis and necrosis, leading to the development of a necrotic core (19,20). Apoptosis and necrosis is followed by an active healing process through calcification, which is mediated by osteoblastic transformation of vascular smooth muscle cells under the influence of oxidized LDL, angiotensin II, TNF-alpha and others (21-24). While these early stages of the atherosclerotic process are characterized by positive remodeling and minimal luminal compromise (increased plaque volume; Glagov phenomenon), late stages are characterized by progressive fibrosis with negative remodeling and severe luminal compromise (25,26).
Coronary atherosclerosis imaging by IVUS/VH
Recently, IVUS/VH has been introduced as an invasive method for coronary atherosclerotic plaque characterization (8-11,27). IVUS remains the reference standard for the measurement of geometrical characteristics of coronary plaques, such as MLD, %DS, MLA, %AS, plaque burden and remodeling index. Furthermore, capitalizing on radiofrequency backscatter data, IVUS/VH can also provide some tissue characterization and based on such characteristics, voxels can be characterized as fibrous tissue, fibro-fatty tissue, necrotic core and dense calcium (Figure 1A-D). This approach has been validated both ex-vivo and in-vivo and can quantify the volume and percentage of each of the four components (8).
Association of gene expression with the biology of atherosclerosis
Our study found that a composite gene expression score was correlated with the volume of necrotic core, dense calcium volume and total plaque volume, as measured by IVUS/VH. Likely, in patients with higher gene expression scores, a higher volume of necrotic core and dense calcium, which are typically closely correlated by IVUS/VH, resulted in a higher total plaque volume. Interestingly, the majority of the 23 genes that are part of the composite score are related to inflammation and this supports the notion that this gene expression pattern reflects an overall inflammatory response to lipoprotein deposition in coronary atherosclerotic plaques. The 23 genes of the composite score can be divided into 6 different terms, or groups (Table 4). Genes in Term 1 (Table 4) are related to innate immunity, more specifically to neutrophil activation and apoptosis. Importantly, CASP5 gene expression is up-regulated in CAD, which may represent apoptosis leading to the development of the necrotic core (28). Thus, this may provide an important link between our finding of the gene expression score and its association with necrotic core by IVUS/VH. Furthermore, the TLR4 gene is expressed by macrophages which are key components in the pathogenesis of atherosclerosis. Genes in Term 2 (Table 4) are also related to innate immunity and cell necrosis, again related to neutrophils in chronic inflammatory conditions (S100A8, S100A12) and cell necrosis (CLEC4E), again potentially providing a link between the gene expression score and necrotic core by IVUS/VH. Term 3 genes (Table 4) are also related to innate immunity, more specifically to natural killer (NK) cell activation, normalized for T-cell specific genes. KLRC4 activates NK cells and inhibits T- and B-cells; NK-cell activation and reduced lymphocyte count have been implicated in atherosclerosis (29). Term 4 (Table 4) is related to adaptive immunity and reflects T- and B-cell activation; however, the role of lymphocytes in atherosclerosis is less clear at this time. Finally, Term 5 and Term 6 consist of genes with unknown functions (Table 4).
An apparent paradox is that atherosclerotic plaques, especially advanced plaques like the ones characterized by a necrotic core, mostly contain resident macrophages in the lipid-rich necrotic core as well as in the fibrous cap. In fact, the presence of a large amount of macrophages, mostly in the fibrous cap, is one of the hallmarks of “vulnerable plaques” from a histopathologic point of view (30). In addition, activated T-cells expressing HLA-DR4 have also been demonstrated in plaques, mostly in the shoulder regions (31). This apparent paradox may suggest that an overall broader inflammatory response, involving neutrophils, NK-cells, T-cells and B-cells and monocytes/macrophages is mounted as a maladaptive response to lipoprotein retention.

Full Table
Previous studies
While there have not been a large number of studies evaluating the relationship between peripheral gene expression and CAD, our study is consistent with previous studies and extend on those observations. We have previously validated this composite gene expression score in a separate patient population for the diagnosis of obstructive CAD (>50% luminal diameter stenosis) using invasive coronary X-ray angiography as reference standard (7). In that study of 526 non-diabetic patients referred for invasive coronary angiography, there was a linear relationship between the composite GES and the severity of angiographic CAD, and the addition of the GES to the Diamond-Forrester classification scheme improved the area under the ROC curve from 0.66 to 0.72 (P=0.003). While invasive coronary angiography cannot directly evaluate plaque volume, overall luminal stenosis is related to the total amount of plaque. The present study with IVUS/VH-based plaque volume and component measurements extends on these findings.
In summary, in this preliminary, hypothesis-generating study of the relationship between peripheral gene expression measured by a previously validated composite score and plaque volume and composition by IVUS/VH, we found that the GES was associated with necrotic core, dense calcium and total plaque volume. These findings are consistent with the inflammatory hypothesis of atherosclerosis and suggest that this gene expression score, which is mostly related to inflammatory genes, reflects overall inflammatory activity and a potentially more vulnerable plaque phenotype.
Limitations
Our study has several limitations. First, while this study was prospective and gene expression analysis was done prospectively, it was a single-center study with a relatively small sample size and we were only able to analyze a limited number of biomarkers and IVUS/VH parameters due to the limitations imposed by the multiple hypotheses (Bonferroni) correction on the P-values. We tried to mitigate these issues by using a non-parametric analysis method, as well as by using permutation testing. The permutation analysis suggests that the correlations between plaque volume and characteristics are unlikely to be due to chance, although relationships between individual IVUS-VH parameters and the GES need further work. Thus, this should be regarded as a preliminary, hypothesis-generating study and additional studies in larger cohorts will be needed for validation; Second, we only performed IVUS/VH in one coronary artery and the influence of atherosclerotic plaques in other coronary arteries and other vascular beds could not be evaluated; Third, we excluded patients with diabetes, as the composite gene expression score was developed in non-diabetic subjects; Furthermore, most of the patients were on lipid-lowering medications and non-steroidal anti-inflammatory agents, which may alter gene expression patterns and may explain the lack of correlation between traditional lipoproteins and plaque components; Finally, the full clinical predictive value of IVUS/VH-derived plaque features is still actively being investigated.
Conclusions
To our knowledge, this preliminary report is the first prospective study to evaluate the relationship between peripheral gene expression measured by a previously validated composite score and the volume and composition of coronary atherosclerotic plaques by IVUS/VH. Given the multiple factors that influence atherosclerosis, we found that a predominantly inflammatory gene expression pattern was moderately associated with overall larger plaque burden and with more advanced atherosclerosis, characterized by more necrotic core and dense calcium by IVUS/VH. In this hypothesis-generating study, our results are consistent with the inflammatory hypothesis of atherosclerosis and suggest that this composite gene expression score is not only predictive of obstructive CAD as has been shown in the past, but also predictive of larger atherosclerotic plaque burden with a more vulnerable phenotype.
Acknowledgements
We would like to thank the CardioDx, Inc. reference laboratory staff for performing the CorusCAD gene expression test.
Disclosure: The ATLANTA studies were supported in part by grants to Dr. Voros from Abbott Vascular, Volcano Inc., Vital Images, Siemens Medical Solutions, and CardioDx. Bela Asztalos is employed by Boston Heart Diagnostics. Michael Elashoff, John Blanchard and Steven Rosenberg are employees of CardioDx, Inc.
References
- D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743-53.
- Wilson PW, D’Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47.
- Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med 2009;361:2518-28.
- Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 2007;316:1491-3.
- Kathiresan S, Melander O, Anevski D, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med 2008;358:1240-9.
- Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med 2007;357:443-53.
- Rosenberg S, Elashoff MR, Beineke P, et al. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med 2010;153:425-34.
- Nair A, Kuban BD, Tuzcu EM, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002;106:2200-6.
- Serruys PW, García-García HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008;118:1172-82.
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35.
- Voros S, Rinehart S, Qian Z, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv 2011;4:198-208.
- Wingrove JA, Daniels SE, Sehnert AJ, et al. Correlation of peripheral-blood gene expression with the extent of coronary artery stenosis. Circ Cardiovasc Genet 2008;1:31-8.
- Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478-92.
- Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832-44.
- Nakashima Y, Fujii H, Sumiyoshi S, et al. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol 2007;27:1159-65.
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 2001;108:785-91.
- Hansson GK, Libby P, Schönbeck U, et al. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 2002;91:281-91.
- Matsumoto A, Naito M, Itakura H, et al. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A 1990;87:9133-7.
- Ball RY, Stowers EC, Burton JH, et al. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis 1995;114:45-54.
- Björkerud S, Björkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol 1996;149:367-80.
- Armstrong ZB, Boughner DR, Drangova M, et al. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc Res 2011;90:165-70.
- Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 2006;99:1044-59.
- Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial 2007;20:103-9.
- Speer MY, Yang HY, Brabb T, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 2009;104:733-41.
- Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371-5.
- Schoenhagen P, Ziada KM, Vince DG, et al. Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol 2001;38:297-306.
- Garcia-Garcia HM, Gonzalo N, Regar E, et al. Virtual histology and optical coherence tomography: from research to a broad clinical application. Heart 2009;95:1362-74.
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ 2007;14:10-22.
- Braun NA, Covarrubias R, Major AS. Natural killer T cells and atherosclerosis: form and function meet pathogenesis. J Innate Immun 2010;2:316-24.
- Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 2010;30:1282-92.
- Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2009;29:1714-7.


