Cardiac troponin and outcome in decompensated heart failure with preserved ejection fraction
IntroductionOther Section
Heart failure (HF) is a growing healthcare issue in developed countries due to its high prevalence, mortality, morbidity, and cost of care. In the United States, the population prevalence of HF was 2.42% in 2012 and is projected to increase by 23% in 2030 to 2.97% (1).
Nearly half of the patients with the clinical syndrome of HF have a normal or near-normal left ventricular ejection fraction (LVEF), referred to as heart failure with preserved ejection fraction (HFpEF) (2-6). HFpEF appears to carry a similar short- and long-term mortality to HF with reduced ejection fraction following acute HF hospitalization (3,7-9). This has prompted a search for biomarkers to help risk stratify patients, in order to guide more aggressive in-hospital management and post-discharge follow up (10).
Cardiac troponin (cTn) has been established as an effective prognostic marker in several arenas, including acute HF exacerbation with a predominantly reduced EF (11-13). Myocardial injury and detectable cTn levels have been previously identified in patients with HFpEF (4,10,14,15). However, studies assessing the prognostic value of cTn level elevation, in hospitalized patients with HFpEF, are limited. The aim of this study is to describe the short-, intermediate-, and long-term outcomes associated with cTn level elevation in patients with acute HFpEF decompensation.
MethodsOther Section
A retrospective study of consecutive patients admitted for HFpEF decompensation, not associated with acute coronary syndrome (ACS), between January 2010 and December 2013, was performed. HFpEF was diagnosed by the presence of signs and symptoms of HF, normal or near-normal LVEF (≥50%), and evidence of cardiac dysfunction by echocardiography. The diagnosis was confirmed, by an independent cardiologist, after chart review.
Patients with myocardial ischemia (i.e., tachyarrhythmias, illicit drug toxicity, severe hypertension defined as systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥110 mmHg, and shock) or myocardial injury, not secondary to HF, were excluded. Patients who required initiation of, or were previously on, hemodialysis were excluded. In patients with multiple admissions for HFpEF exacerbation, the first admission was used.
Two cTnI levels, within 6 hours of presentation, were required for inclusion in order to detect the rising or falling pattern. Patients with an increase or decrease of more than 20% in cTnI levels were excluded. The peak cTnI level was used. Other lab tests were measured on initial presentation. cTnI level was measured using ADVIA Centaur cTnI assay (Siemens Healthcare Diagnostics, NY). The assay has an analytical measuring range of 0.008 ng/mL to 50 ng/mL, a 99th percentile of 0.04 ng/mL, and a coefficient of variance (CV) of 10% at a level of 0.03 ng/mL.
Patients with troponin level elevation were compared to patients with normal troponin level. Troponin level elevation was defined as a cTnI level of 0.04 ng/mL or greater. Normal troponin level was defined as an undetectable cTnI level or a level <0.04 ng/mL (below the 99th percentile value of the reference population for the test). The primary outcome was short-, intermediate-, and long-term all-cause mortality. The secondary outcomes were differences in B-type natriuretic peptide level (BNP), length of stay, and readmission rates between the two groups.
Clinical and demographic data were retrospectively abstracted from medical records. LVEF was assessed by 2D echocardiography using biplane method of disks (modified Simpson’s rule). Survival status was obtained from medical records and social security death index database.
Continuous variables were presented as means ± SDs or medians (interquartile Q1–Q3), depending on their distribution. Categorical variables were presented as frequencies and percentages. Continuous variables were compared using analysis of variance or Mann-Whitney U-test, depending on the distribution of the variable. Categorical variables were compared using chi-squared analysis.
Cox proportional hazard models were used to determine the predictors of the primary outcome and are expressed as hazard ratios (HR) and 95% confidence intervals (CI). Multivariate cox proportional hazard model was adjusted for age, sex, race, history of coronary artery disease, diabetes mellitus, atrial fibrillation, stroke, hypertension, anemia, smoking, creatinine level, BNP level, and statistically significant variables in the univariate models. Kaplan-Meier curves were used to illustrate the difference in survival function between the two groups. Spearman’s coefficient of rank correlation (rho) was used to assess the correlation between cTnI level and BNP, length of stay, and number of readmissions.
The areas under the curve (AUC) of the receiver operating characteristics (ROC) were calculated for initial BNP and peak cTnI levels. The optimum cutoff point for BNP level, to predict 2-year mortality, was that with the maximum combined sensitivity and specificity. The statistical significance of the difference between the areas under BNP and cTnI ROC curves was compared using Hanley and McNeil method.
All probabilities were 2-sided and P values <0.05 were considered statistically significant. All data were analyzed using MedCalc version 16.8 (MedCalc Software bvba, Belgium) and SPSS 22 (IBM Corp., USA).
ResultsOther Section
Out of the 432 patients evaluated for study inclusion, 363 patients met the inclusion criteria. Fifty-nine patients were excluded for requiring hemodialysis, 5 for incomplete data, 2 for severe hypertension, 1 for cardiogenic shock, 1 for illicit drug use, and 1 for tachyarrhythmia. Overall, the patients were mostly elderly, female and overweight. There was a high prevalence of systemic hypertension, anemia, diabetes, and atrial fibrillation (Table 1). Overall, cardiac cTnI level was detected in 338 patients (93%) and ranged between 0.008–0.76 ng/mL, with a mean level of 0.077±0.11 ng/mL.

Full table
One hundred eighty-eight patients (52%) had troponin level elevation (cTnI level of 0.04 ng/mL or more), and 175 patients (48%) had normal troponin level (cTnI level <0.04 ng/mL). Characteristics of the patients, according to whether they had troponin level elevation or normal troponin level, are summarized in Table 1. There were small yet statistically significant differences between the two groups. Patients with troponin level elevation were more likely to be males, African Americans, and smokers, and to have higher BNP and serum creatinine levels.
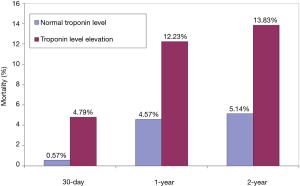
Patients with troponin level elevation had a higher 30-day (4.8% vs. 0.6%, P=0.014), 1-year (12.2% vs. 4.6%, P=0.009), and 2-year mortality (13.8% vs. 5.1%, P=0.005) when compared to patients with normal troponin level (Figure 1). Two-year Kaplan-Meier survival curves demonstrate the survival difference between the two groups (Figure 2).

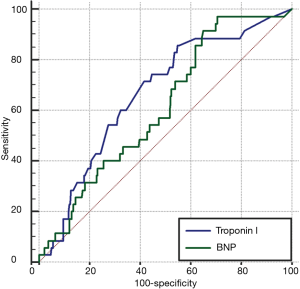
BNP levels were higher in patients with troponin level elevation when compared to patients with normal troponin level (820±950 pg/dL vs. 478±465 pg/dL, P<0.0001). There was a statistically significant positive correlation between cTnI level and BNP level (Spearman’s rho 0.27, 95% CI: 0.17–0.37, P<0.0001,). A BNP level >287 pg/mL was found to be the optimum criterion for predicting 2-year mortality with 91.4% sensitivity and 35.1% specificity. The area under the cTnI ROC curve was higher than that of BNP (Figure 3). However, this difference was statistically insignificant (P=0.345).
The median length of stay was 5 days per admission, and had a weak positive correlation with cTnI level (Spearman’s rho 0.11, 95% CI: 0.01–0.21, P=0.036). Longer length of stay was seen in patients with troponin level elevation; however, this difference was statistically insignificant. During the study period, more than half of the patients had at least one readmission for HFpEF decompensation. There was no significant difference in the number of readmissions between the two groups. Neither the number of readmissions nor the length of stay were independent predictors of mortality.
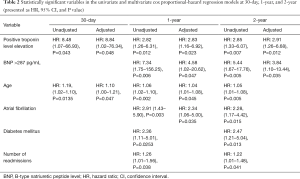
Univariate cox proportional-hazard regression models showed that troponin level elevation was associated with an increased risk of 30-day (HR 8.48, 95% CI: 1.07–66.93, P=0.043), 1-year, (HR 2.82, 95% CI: 1.26–6.31, P=0.012) and 2-year mortality (HR 2.85, 95% CI: 1.33–6.07, P=0.007). It also identified BNP level >287 pg/mL, age, history of atrial fibrillation, diabetes mellitus, and number of readmissions as statistically significant predictors of mortality (Table 2).
Full table
Multivariate cox proportional-hazard regression model identified troponin level elevation as an independent predictor of 30-day, 1-year, and 2-year mortality. Additionally, BNP level >287 pg/mL, age, and history of atrial fibrillation were identified as independent predictors of 1-year and 2-year mortality (Table 2).
DiscussionOther Section
In the present study, more than half of the hospitalized patients with HFpEF decompensation, not associated with ACS, had troponin level elevation. The presence of troponin level elevation was associated with an 8-fold higher short-term mortality and a 3-fold higher intermediate- and long-term mortality. This association was independent of other clinical and laboratory risk factors seen in HFpEF decompensation. These findings suggest that cTnI measurement, in hospitalized patients with HFpEF decompensation, carries an important prognostic value during patients’ early evaluation and is useful for early risk assessment.
The pathophysiological mechanism of myocardial injury and cTn elevation in HFpEF is not fully understood and is probably multifactorial. Potential contributing mechanisms include subendocardial ischemia, neurohormonal activation, inflammatory cytokine release, myocardial stretching, increased wall stress, oxidative stress, and altered myocyte calcium handling. The end result of these mechanisms is myocyte necrosis, apoptosis, or troponin degradation and release from viable cells (16).
The clinical characteristics of the patients in the current study were in agreement with previous studies of patients with HFpEF (2-4,7,9). They were more likely to be elderly, female, and overweight. Also, they were more likely to have hypertension, diabetes, anemia, atrial fibrillation and elevated creatinine levels. On examining the relative prognostic impacts of comorbidities on mortality, we found that age, history of atrial fibrillation, history of stroke, and diabetes were independent predictors of mortality.
Previous studies have reported variable all-cause mortality rates in patients with HFpEF. This variability appears to be dependent on the study design and setting. In population-based observational studies, the reported 1-year mortality rate was 22–29% (3,7,17). On the other hand, more recent randomized controlled trials (RCTs) reported a 3-time lower 1-year mortality rate of less than 10% (18-24). This difference was confirmed by a meta-analysis of 31 observational studies and RCTs. The mortality rate was 146 deaths per 1,000 patient-years in the non-RCTs alone, and 101 deaths per 1,000 patient-years in the RCTs alone (25). Regardless of this difference, the mortality burden of HFpEF is significant. In the present study, after excluding certain patient subsets, the 30-day mortality rate was 2.75% and was comparable to the previously reported in-hospital and 30-day mortality (4,7,9,26). The 1-year mortality rate was 8.54%, and was comparable to the mortality rate in the RCTs. Additionally, the 2-year mortality rate was found to be 9.64%.
In the present study, there was a statistically significant positive correlation between BNP and cTnI levels. Additionally, a BNP level >287 pg/mL was found to be an independent predictor of intermediate- and long-term mortality. A similar relationship was previously seen in the acute decompensated HF national registry (ADHERE), where an elevated admission BNP level was found to be an independent predictor of mortality in 18,164 patients with HFpEF decompensation (27). There was also a statistically positive correlation between cTnI level and the length of stay, with a trend towards longer lengths of stay in patients with troponin level elevation. More than half of the patients had at least one readmission for HFpEF decompensation in the study period. This highlights the substantial morbidity and cost of care for such patients.
Currently available pharmacological therapies have failed to demonstrate a meaningful survival benefit in HFpEF despite their success in patients with HF with reduced ejection fraction (28). In our study, there was no significant difference between the medications on discharge between the two groups. Neither beta blockers nor angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) had a statistically significant effect on mortality.
The findings in our study provide important clinical significance. The presence of troponin level elevation was associated with statistically significant worse short- and long-term mortality, independent of common clinical and laboratory risk factors seen in HFpEF decompensation, including glomerular filtration rate and the presence of chronic kidney disease. Additionally, an elevated BNP level >287 pg/dL was independently associated with higher intermediate- and long-term mortality. Both cTnI and BNP levels provide a valuable tool for early risk stratification in patients with HFpEF decompensation.
Several limitations in our study must be acknowledged. The most important limitation is the retrospective design. The patients evaluated for study inclusion were only those with a discharge diagnosis of HFpEF, at the discretion of the discharging physicians. Hence, the sample size in our study might not represent the actual number of patients hospitalized with this diagnosis due to issues with documentation and under-diagnosis. Another limitation is that the coronary anatomy was not routinely evaluated, and it is uncertain if some patients with ACS were included. Two cTnI levels were required for inclusion to detect the rising and falling pattern in an attempt to rule out patients with ACS, and the highest troponin I level in our data was only 0.76 ng/mL.
In an effort to decrease the significant effect of end-stage renal disease and extreme fluid overload in patients with acute renal failure on cTnI level elevation, we excluded patients who required or were on hemodialysis; however, such patients represent a substantial proportion of patients with HFpEF. Similarly we excluded patients with severe hypertension and cardiogenic shock. The number of readmissions in our study only represents readmissions to our institution, and might underestimate the actual number of readmissions.
ConclusionsOther Section
In hospitalized patients with HFpEF decompensation, troponin level elevation was associated with higher short-, intermediate-, and long-term mortality. Additionally, a BNP level >287 pg/dL was associated with higher intermediate- and long-term mortality. This highlights the importance of cTnI and BNP level measurements for early risk stratification. Further prospective studies are needed to assess the relationship between cTnI and BNP levels and outcomes in patients with HFpEF.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Patient’s data and outcomes were retrospectively retrieved from hospital’s medical records. Informed consent was not required due to the retrospective design. All data were de-identified and secured appropriately.
ReferencesOther Section
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606-19. [Crossref] [PubMed]
- Gurwitz JH, Magid DJ, Smith DH, et al. Contemporary prevalence and correlates of incident heart failure with preserved ejection fraction. Am J Med 2013;126:393-400. [Crossref] [PubMed]
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9. [Crossref] [PubMed]
- Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65-75. [Crossref] [PubMed]
- Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail 2013;6:279-86. [Crossref] [PubMed]
- Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424-31. [Crossref] [PubMed]
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260-9. [Crossref] [PubMed]
- Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006;47:76-84. [Crossref] [PubMed]
- Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768-77. [Crossref] [PubMed]
- Perna ER, Aspromonte N, Cimbaro Canella JP, et al. Minor myocardial damage is a prevalent condition in patients with acute heart failure syndromes and preserved systolic function with long-term prognostic implications: a report from the CIAST-HF (Collaborative Italo-Argentinean Study on cardiac Troponin T in Heart Failure) study. J Card Fail 2012;18:822-30. [Crossref] [PubMed]
- Peacock WF 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117-26. [Crossref] [PubMed]
- Xue Y, Clopton P, Peacock WF, et al. Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail 2011;13:37-42. [Crossref] [PubMed]
- Pascual-Figal DA, Manzano-Fernández S, Boronat M, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail 2011;13:718-25. [Crossref] [PubMed]
- Jhund PS, Claggett BL, Voors AA, et al. Elevation in high-sensitivity troponin T in heart failure and preserved ejection fraction and influence of treatment with the angiotensin receptor neprilysin inhibitor LCZ696. Circ Heart Fail 2014;7:953-9. [Crossref] [PubMed]
- Santhanakrishnan R, Chong JP, Ng TP, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2012;14:1338-47. [Crossref] [PubMed]
- Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071-8. [Crossref] [PubMed]
- Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998-1005. [Crossref] [PubMed]
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383-92. [Crossref] [PubMed]
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268-77. [Crossref] [PubMed]
- Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013;309:781-91. [Crossref] [PubMed]
- Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338-45. [Crossref] [PubMed]
- Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 2006;114:397-403. [Crossref] [PubMed]
- Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456-67. [Crossref] [PubMed]
- Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-81. [Crossref] [PubMed]
- Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750-7. [Crossref] [PubMed]
- Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009;73:1893-900. [Crossref] [PubMed]
- Fonarow GC, Peacock WF, Phillips CO, et al. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol 2007;49:1943-50. [Crossref] [PubMed]
- Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 2014;115:79-96. [Crossref] [PubMed]


