Endovascular versus surgical treatment for acute limb ischemia: a systematic review and meta-analysis of clinical trials
Introduction
Acute limb ischemia (ALI) is a life and limb-threatening emergency that results from an abrupt decrease of blood flow to a limb, which threatens tissue viability in patients presenting within two weeks of the acute event. Severe, untreated cases can result in fatal metabolic derangements, limb amputation and death. The three main etiologies of ALI are distal embolization of athero- or thromboembolic material, vascular thrombosis of a high grade underlying lesion (of native vessel, bypass grafts or in-stent thrombosis) or secondary to a traumatic vascular injury (1-4).
The clinical presentation of ALI has the hallmark referred to as the 6 Ps (pallor, pain, pulseless, paralysis, paresthesia and poikilothermia). Clinically, ALI is classified as (I) viable (II) threatened and (III) non-viable tissue. This classification helps to direct therapy in terms of the urgency of intervention, appropriate pre-intervention evaluation and mode of intervention (3,5). While the treatment for non-viable ALI is amputation, treatment options for viable and threatened ALI include endovascular (ENDO) (i.e., intra-arterial thrombolysis, aspiration or rheolytic thrombectomy and/or angioplasty) or surgical (SURG) (i.e., thromboembolectomy, endarterectomy and/or bypass) revascularization.
Several studies have compared ENDO to SURG treatment strategies in this population. The goal of this study is to compare both approaches in regard with mortality and morbidity (limb amputation and recurrent ischemia) based on available evidence in the literature.
Methods
The aim of this meta-analysis was to compare outcomes associated with ENDO versus SURG in patients presenting with ALI (<2 weeks) between 1990 and 2016. The primary endpoints were all cause mortality and limb amputation at 1 month, 6 and 12 months. The secondary endpoint was recurrent ischemia at one year.
The study was performed following procedures recommended by the Cochrane collaboration (6) and is reported in accordance with the recommendations set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (7).
Information sources and search methods
A comprehensive literature search was conducted through the electronic databases MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) for abstracts using various combinations of the terms: acute limb ischemia, SURG revascularization and ENDO revascularization.
Two reviewers (JO, TE) identified articles eligible for further review by performing a screen of abstracts and titles. If a study met the inclusion criteria, the manuscript was obtained and reviewed. In addition, bibliographic references of identified randomized clinical trials and review articles, in order to find randomized clinical trials not identified by the electronic searches, were evaluated.
Studies identification
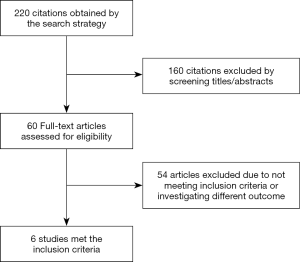
The previously described data sources were searched for possible studies and the search was limited to the English-language literature. Original papers were included and the initial search identified 220 citations. One hundred and sixty citations were excluded by the identifying abstract/title. The final search identified 6 original papers that fulfilled the criteria for inclusion (Figure 1).
Data collection and extraction
Two independent reviewers (JO, TE) extracted data from the included studies by using pre-specified data elements. Data were abstracted on patient demographics and baseline characteristics, study design, sample size, duration of symptoms (<2 weeks), intervention type (ENDO vs. SURG) and type of outcome measures (primary outcomes: mortality and limb amputation as well as secondary outcomes: recurrent ischemia).
One review author extracted the data from included studies and a second author verified the extracted data. The number of events in each trial was extracted when available. Baseline characteristics and study description are reported (Table 1) (4,8-13).

Full table
Risk of bias assessment
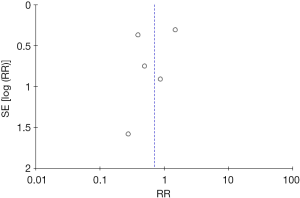
Methodological quality was defined as the control of bias assessed through the reported methods in each individual study using the Cochrane risk of bias tool (14) to assess quality of randomized trials. Newcastle-Ottawa Scale (NOS) (15) was used to assess the quality of observational studies. Two reviewers (JO, TE) independently assessed each study quality by examining risk of bias tool components. No evidence of publication bias was detected based on the symmetry of the funnel plot (Figure 2). There was possible performance bias due to non-blinded studies (Tables 2,3). Disagreements between the reviewers were resolved by discussion or arbitrated with a third coauthor (AA).

Full table
Full table
Statistical analysis and data synthesis
From the abstracted data, the risk ratio (RR) was calculated using the inverse variance method for each study outcome to allow for pooling of similar outcomes. The average effects for the outcomes and 95% confidence intervals (CIs) were obtained using a random effects model, as described by DerSimonian (14). We chose the random effects method as primary analysis because of its conservative summary estimate and incorporation between and within study variance.
To assess heterogeneity of treatment effect among trials, the I2 statistic was used. The I2 statistic represents the proportion of heterogeneity of treatment effect across trials that were not attributable to chance or random error. Hence, a value of 50% or more reflects significant heterogeneity that is due to real differences in study populations, protocols, interventions, and outcomes (14). Sensitivity analyses were performed to assess the effects of selected measures of study designs (i.e., randomized control trial versus observational studies) on pooled effect of ENDO vs. SURG. The influence was estimated by performing a subgroup analysis and test for subgroup differences. The analysis was repeated including randomized studies only and the results remained consistent.
The P value threshold for statistical significance was set at 0.05 for effect sizes. Analyses were conducted using features on RevMan version 5.3.5 (The Nordic Cochrane Center, Copenhagen, Denmark).
Results
A total of 1,773 patients was identified from a total of six studies. Five of those studies were randomized prospective studies, and the sixth study was an observational retrospective study.
Mortality
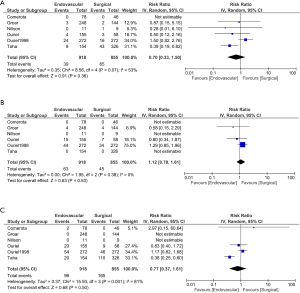
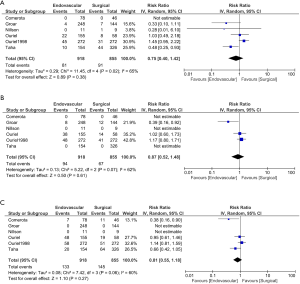
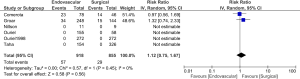
When comparing the two treatment options with respect to effect on mortality, no difference was found. This effect was observed at 1 month (RR for ENDO vs. SURG is 0.70; 95% CI, 0.33 to 1.50), 6 months (RR 1.12; CI, 0.78 to 1.61) or 12 months (RR 0.74; CI, 0.29 to 1.85) (Figure 3). A numerically higher mortality event rate in the SURG option was observed, but it did not reach statistical significance. This was seen at 12 months 96/918 ENDO vs. 165/855 SURG (Figure 3).
Amputation rate
When comparing the two treatment option with respect to amputation rate no difference was found. This effect was observed at 1 month (RR 0.75; CI, 0.40 to 1.42), 6 months (RR 0.87; CI, 0.52 to 1.48) or 12 months (RR 0.81; CI, 0.55 to 1.18) (Figure 4).
Secondary outcomes
Secondary outcome data was limited. Not difference between the two treatment options was observed (RR 1.12; CI, 0.75 to 1.67) (Figure 5)
Discussion
Presentation with ALI is considered a vascular emergency associated with major morbidity and mortality. It is estimated to occur in 1.5 cases/10,000 population per year, complicates 15–20% of chronic limb ischemia and has (3,4) a 30-day mortality rate close to 26% (16). Embolic occlusions can result from either atherosclerotic or thromboembolic debris and for this reason embolic ALI was excluded from some studies comparing local thrombolysis to SURG treatment (9-11).
Tissue plasminogen activator (tPA) is the most common thrombolytic agent used in treating ALI within 1–2 days of presentation (3,4). Intra-arterial thrombolytic therapy is a less invasive approach when compared to SURG revascularization and can be followed by an ENDO or simpler open procedure if required (9-11). However uncertainty exists regarding the optimal revascularization approach (ENDO vs. SURG) as the first line treatment. The 2012 American College of Chest Physicians suggests reperfusion therapy (SURG or ENDO) over no reperfusion therapy and recommends SURG over ENDO for both thrombotic and embolic ALI (17).
Previous studies in the early nineties on treatment for ALI showed ENDO to have comparable results to SURG in terms of mortality, limb amputation and recurrent ischemia at up to one year of follow-up. These results were confirmed by subsequent and more recent studies. These studies also showed that ENDO was associated with worse outcomes when used for treatment of ALI secondary to prosthetic bypass graft occlusion or for treatment of chronic limb ischemia (symptoms of >2 weeks) (9-12,17-20).
This analysis shows no significant difference between the two treatment options in terms of mortality at 1 month, 6 and 12 months, amputation rates or recurrent ischemia at 12 months of follow-up. This study increases the sample size and confirms the equipoise that exists with either an early ENDO or SURG revascularization strategy for ALI. With the lower morbidity that is associated with the ENDO approach, the data presented here support the concept that an ENDO first approach for ALI is a reasonable first line option.
Limitations
This study has several limitations. Although the majority of studies included were randomized, and prospective, one retrospective study was included. All of these studies recruited patients with different co-morbidities and risk factors so heterogeneity as seen in any meta-analysis is a factor to consider.
There was also heterogeneity between the included studies with respect to SURG procedures performed, type and dose of thrombolytic used and type of target vessel (native vs. bypass graft). Finally, there was a lack of uniform reporting of the severity assessment of ALI, which could have an implication on outcomes. The most recent study by Taha and colleagues (12) was the only study that used Rutherford classification of ALI and its results suggested that ENDO might be the preferred first line of treatment for Rutherford 2 native artery/stent failure ALI while SURG might be preferred for bypass graft ALI (12).
The recent advances and improvements in catheter-directed therapy is a factor not well accounted for in this analysis as the majority of the included studies were older and prior to the contemporary ENDO techniques. This is likely to have biased the results in favor of the SURG approach.
Conclusions
In conclusion, this study shows that an ENDO first approach would result in no significant difference versus a SURG approach in terms of mortality and recurrent ischemia and may reduce short-term limb amputation rates for patients with ALI Further randomized trials are required to provide further confirmation of these findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kasirajan K, Ouriel K. Current options in the diagnosis and management of acute limb ischemia. Prog Cardiovasc Nurs 2002;17:26-34. [Crossref] [PubMed]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45 Suppl S:S5-67.
- Lyden SP. Endovascular treatment of acute limb ischemia: review of current plasminogen activators and mechanical thrombectomy devices. Perspect Vasc Surg Endovasc Ther 2010;22:219-22. [Crossref] [PubMed]
- Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med 2012;366:2198-206. [Crossref] [PubMed]
- Katzen BT. Clinical diagnosis and prognosis of acute limb ischemia. Rev Cardiovasc Med 2002;3 Suppl 2:S2-6. [PubMed]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. updated March 2011, The Cochrane Collaboration.
- Higgins JP, G.S.e.C.H.f.S.R.o.I.V.u.M.T.C.C., 2011. Available online: http://www.cochrane-handbook.org
- Comerota AJ, Weaver FA, Hosking JD, et al. Results of a prospective, randomized trial of surgery versus thrombolysis for occluded lower extremity bypass grafts. Am J Surg 1996;172:105-12. [Crossref] [PubMed]
- Nilsson L, Albrechtsson U, Jonung T, et al. Surgical treatment versus thrombolysis in acute arterial occlusion: a randomised controlled study. Eur J Vasc Surg 1992;6:189-93. [Crossref] [PubMed]
- Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med 1998;338:1105-11. [Crossref] [PubMed]
- Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg 1994;220:251-66; discussion 266-8. [Crossref] [PubMed]
- Taha AG, Byrne RM, Avgerinos ED, et al. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg 2015;61:147-54. [Crossref] [PubMed]
- Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg 1996;23:64-73; discussion 74-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcasete-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. November 4, 2012. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Clason AE, Stonebridge PA, Duncan AJ, et al. Morbidity and mortality in acute lower limb ischaemia: a 5-year review. Eur J Vasc Surg 1989;3:339-43. [Crossref] [PubMed]
- Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e669S-90S.
- Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg 1996;23:64-73; discussion 74-5. [Crossref] [PubMed]
- Weaver FA, Comerota AJ, Youngblood M, et al. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus Thrombolysis for Ischemia of the Lower Extremity. J Vasc Surg 1996;24:513-21; discussion 521-3. [Crossref] [PubMed]
- Comerota AJ, Gravett MH. Do randomized trials of thrombolysis versus open revascularization still apply to current management: what has changed? Semin Vasc Surg 2009;22:41-6. [Crossref] [PubMed]