Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies
Introduction
In 2007, a joint task force of the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology and the World Heart Federation proposed the Universal Definition of myocardial infarction (MI), which was an expert consensus document categorizing MI into five subtypes (1). The Third Universal Definition released in 2012, was an update to the 2007 document and backed the MI subtype classification as per the 2007 Universal Definition (2). Type 1 MI is caused by an acute atherothrombotic coronary event following plaque rupture. Type-2 MI is an entity where a condition other than coronary artery disease (CAD) contributes to a critical imbalance between oxygen supply (such as hypoxemia, anemia, or hypotension) and demand (such as tachycardia, tachyarrhythmias, or hypertension). In clinical practice it may be difficult to distinguish type 2 MIs from other non-ischemic conditions, such as Takotsubo cardiomyopathy and myocarditis (3). This difficulty resulted in significant variation in the prevalence of type 2 MI across studies, ranging from 1.6% to 29.6% (4-7). Although evidence-based treatment recommendations are established for type-1 MI, there is a lack of similar recommendations for type 2 MI. Recent studies have shown that compared to type 1 MI, noninvasive strategies are more often followed in type 2 MI and these patients also receive fewer cardioprotective drugs (8,9). While some studies have shown that type 2 MI is associated with higher mortality rates (10), others have shown mortality comparable to type 1 MI after multivariate adjustment (11). Although isolated studies comparing outcomes between type 1 and type 2 MI exist, a meta-analysis of these studies will provide useful information.
Methods
Eligibility criteria and data extraction
All observational studies through 30 June 2016 that compared presenting symptoms, baseline characteristics, interventions, and mortality outcomes between type 1 and type 2 MI were identified by conducting a search in the databases of PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE using the search terms “Type-1 myocardial infarction”, “versus”, “Type-2 myocardial infarction”, and “Demand Ischemia” (5,8,9,11-16). We also searched the major cardiovascular conference proceedings, bibliographies of original trials, meta-analyses and review articles. Studies were included that met the following criteria:
- Comparison of patients with type-1 versus type-2 MI.
- Data for outcome variables of interest.
Studies not comparing type 1 MI and type 2 MI, reviews, duplicate studies, non-English language articles, case reports, and articles that did not assess outcomes were excluded. The meta-analysis was performed as per the recommendations of the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17-19).
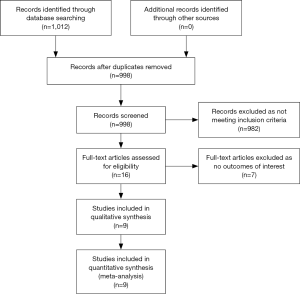
Two authors (S.G. and S.V) independently extracted the data from observational studies using standardized protocol and disagreements were resolved by discussions with the other authors. The primary outcomes of interest were in hospital, 30-day, and one-year mortality, as well as the 30-day major adverse cardiac event (MACE) rate. We also compared the presenting symptoms, the presenting EKG findings, interventions, and comorbidities in pooled cohorts of type -1 and type -2 MI patients. We compared outcome variables when at least two studies reported them. The search strategy and algorithm are shown in Figure 1.
The above authors (S.G. and S.V) independently conducted the quality assessment of the included studies. Disagreements were resolved by discussion or consensus. The methodological quality was assessed using the Newcastle-Ottawa form (20), which was a valid instrument designed to assess the quality of cohort studies. The Newcastle-Ottawa form assigns a maximum of four points for selection, two points for comparability and three points for exposure or outcome. Newcastle-Ottawa form scores of 7 were considered as high-quality studies and of 5–6 as moderate quality (20). The scores of the included studies are summarized in Table 1.

Full table
Statistical analysis
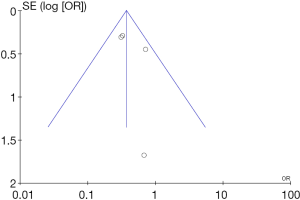
Study dates, design, sample size, inclusion/exclusion criteria, outcomes and comorbidities in both groups were extracted from all of the studies (Tables 2,3). The meta-analysis for outcomes was performed using Revman version 5.3 (Cochrane, Oxford, UK). The random-effects pooled risk ratios (RRs) were calculated using the DerSimonian and Laird method. Heterogeneity was defined as the proportion of total variation observed between trials due to differences among them rather than to sampling error, and was assessed using Cochrane’s Q statistic and I^2 values (21). An I^2 value of <25% was considered low, while I^2 >75% was considered high. Publication bias is visually assessed by funnel plot for inpatient mortality (Figure 2).

Full table
Full table
Characteristics of included studies
ACS, acute coronary syndrome; MI, myocardial infarction; LBBB, left bundle branch block; RWMA, regional wall motion abnormalities; cTnI, cardiac troponin I; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
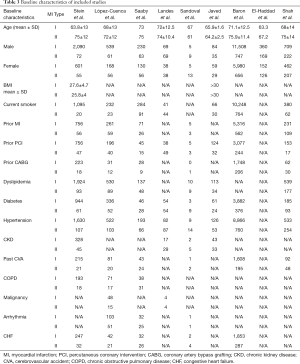
Baseline characteristics of included studies
MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure.
Funnel plot for the outcome “in hospital mortality”.
Results
Study population
A total of 1,012 articles were identified in the literature search, of which 16 were retrieved and reviewed. A total of 9 publications were identified for inclusion (Figure 1). Outcomes were abstracted and meta-analyzed if reported by a minimum of two studies.
Patient characteristics and presentation
The selected studies yielded a total of 25,872 patients for the meta-analysis. The final cohort for type 2 MI consisted of 2683 patients (10%). Compared to type 1 MI, type 2 MI patients were older (mean age 74 years for type 2 vs. 69.82 years for type 1) and more likely to be female (46% vs. 32.75%, respectively). More patients with type 2 MI presented with atypical symptoms of dyspnea (25% for type 2 vs. 2.4% for type 1) and arrhythmia, and were more often diagnosed with non-ST-elevation MI (NSTEMI) (70.0% for type 2 vs. 44.1% for type 1). The type 2 MI cohort contained more diabetic patients than the type-1 MI cohort (29.18% vs. 25.61% respectively). The prevalence of chronic kidney disease was significantly higher in patients with type 2 MI (35% vs. 13.2%). The type 2 MI group also had a higher incidence of hypertension compared to type 1 MI (60.46% vs. 52%). However, smoking was more prevalent in patients with type 1 (54.46%) compared to type 2 MI (43.7%). Histories of congestive heart failure (CHF) and prior MI were more common in type 2 MI compared to type 1 MI (21% vs. 10% for CHF) and (36.7% vs. 30% for prior MI). Patients with type 2 MI had an overall higher incidence of cardiovascular disease and other comorbidities, particularly chronic obstructive pulmonary disease (COPD) and CVA.
Causes of type 2 MI
The most common trigger associated with type 2 MI was operative stress (20%), followed by sepsis (19%), arrhythmia (18.63%), heart failure (15%), and anemia (12%). The most common associated arrhythmia was tachyarrhythmia, especially atrial fibrillation. In the majority of patients, more than one trigger was identified.
In-hospital management
Patients with type 2 MI were less often referred for primary or non-primary angiography. In those patients who underwent coronary angiography, 13.7% had a percutaneous intervention in type 2 MI cohort compared to 64% in type 1. These patients were also at an increased risk of complications during PCI and were sent for urgent coronary artery bypass grafting (CABG).
In- and out-of-hospital outcome
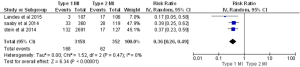
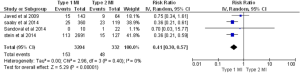
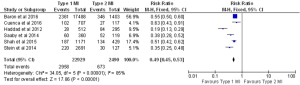
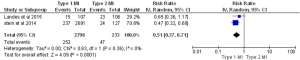
In almost all the studies, mortality was reported as a significant outcome along with the MACE rate, (major adverse cardiac event) which is a composite of death, reinfarction, CVA or urgent revascularization. Three studies reported 30-day mortality, five studies reported in-hospital mortality, and four studies reported one-year mortality. Two studies reported 30-day MACEs. In-hospital and 30-day death rates were almost three times higher in patients with type 2 MI compared to type 1 MI [in-hospital: 15% for type 2 vs. 4.7% for type 1, P<0.00001 (Figure 3): 30-day: 17.6% for type 2 vs. 5.3% for type 1, P<0.00001, (Figure 4)]. The one-year mortality rate was significantly higher for type 2 MI: 27% of these patients died at the end of one year compared to 13% of type 1 patients (P<0.00001) (Figure 5). The 30-day MACEs, including death, re-MI, CVA or urgent revascularization were significantly higher in patients with type 2 compared to type 1 MI (20% vs. 9%, P<0.0001) (Figure 6). Given the large sample size of the study “Baron et al.” and possibly contributing to heterogeneity, it was excluded and analysis of one year mortality repeated. Results were still consistent with higher mortality in type 2 MI (RR =0.41, 95% CI, 0.36–0.47, P<0.00001).
Discussion
The medical literature contains many arguments about the diagnosis and implications of type 2 MI and the terminology is still a matter of debate amongst many clinicians worldwide. The cardiac troponin measurement has been an attractive test for detecting whether a patient has had an MI; however, widespread use of coronary angiography has shown that many patients with elevated troponin do not have evidence of plaque rupture or erosion of the intima with overlying thrombus formation in the coronary vessels. The Third Universal Definition of MI consensus document defines type-2 MI as a condition in which a supply-demand imbalance leads to myocardial injury with necrosis that is not caused by acute coronary syndrome, including arrhythmias, aortic dissection, severe aortic valve disease, hypertrophic cardiomyopathy, shock, respiratory failure, severe anemia, hypertension with or without left ventricular hypertrophy, coronary spasm, coronary embolism or vasculitis, or coronary endothelial dysfunction without CAD (22). Although multiple studies have shown increased mortality with type 2 MI, clarity in the management guidelines is still not established. The true incidence of type 2 MI is unknown partly due to the vague diagnostic criteria leading to the physician reluctance in applying them in clinical practice, thus resulting in difficulty conducting prospective trials, and current ICD coding system not recognizing type 2 MI.
The results of the current meta-analysis with data derived from observational studies demonstrate the following for type-2 MI: (I) the short and intermediate-term mortality rates are three times higher than for type-1 MI; (II) patients tend to be older, are more often female, and have a higher prevalence of cardiac and non-cardiac comorbidities; (III) it more frequently presents with atypical symptoms and NSTEMI; and (IV) percutaneous coronary interventions are performed less often in type-2 MI patients compared to those with type-1 MI.
In our meta-analysis baseline characteristics were notably different in the type-2 MI cohort compared to type-1. The type-2 MI patients were considerably older and were more frequently female. They also had a higher prevalence of the traditional coronary risk factors of diabetes mellitus, hypertension, hyperlipidemia, and other comorbidities, including COPD, peripheral vascular disease, chronic kidney disease. Smoking, however was more prevalent in the type-1 MI patients. The association of comorbidities observed in our study is comparable to that seen in a study by Gupta et al., in which similar clinical variables of advanced age, poor functional status, and renal failure were associated with type-2 MI during the postoperative period (23).
Our study found that operative stress was the most common trigger of type-2 MI. It was followed by sepsis, tachyarrhythmia, especially atrial fibrillation, heart failure, and anemia. This is a novel finding differs from the one found by Javed et al. and Haddad et al. where sepsis was the most common trigger. Elevation in troponin I can occur after noncardiac surgery in patients without CAD (24). In a study by Gualandro et al. (25), nearly 50% of patients with postoperative acute coronary syndrome had no evidence of plaque rupture. In another study by Sametz et al. (26), perioperative catecholamine changes and hypercoagulable status were present. In a recent case series, 10 out of 17 patients with sepsis and type-2 MI did not have coronary artery disease (27). This result supports the hypothesis that other mechanisms play a role in the observed myocardial injury. The inflammatory markers tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) which are released in acute illness, are known to cause myocardial depression and might explain the release of troponin (28). Inflammatory mediators likely increase the permeability of endothelial monolayer to macromolecules resulting in troponin leakage and contributing to microvascular dysfunction.
PCIs are less often utilized in patients with type-2 MI. Possible reasons for this could be the time utilized for treatment of underlying triggering mechanisms such as sepsis, wide variation in clinical practices due to a lack of guidelines, a conservative approach taken by treating physicians due to multiple coexisting comorbidities, or the presence of potential contraindications to anticoagulation. As type-2 MI patients have high cardiovascular risk score, it is necessary to recognize the subset of patients like those with MI in postoperative period in whom invasive therapeutic strategies could be implemented (29-32).
In-hospital and 30-day mortality were three times higher in type-2 MI patients compared to type-1 in the present meta-analysis. The 30-day mortality rate of 17.6% was slightly greater than that reported by Devereaux et al.; this variation may be because only perioperative MI patients were included in the latter study. One-year mortality was also significantly higher in type-2 MI patients conceivably because these patients are sicker and present with greater comorbidity.
A recent study evaluated the concordance between type-1 and type-2 MIs per the Universal Definition of MI classification system and ICD-9 coding for the diagnosis of MI and found that ICD-9 coded MIs represented only a small percentage of arbitrated MIs, principally due to lack of coding for type-2 (33). Similarly, Lofthus et al. retrospectively compared each inpatient encounter with a final primary diagnosis of acute MI at two hospitals for one year, and adjudicated each encounter according to the Universal Definition of MI. They found that nearly 25% of patient encounters with a primary coded diagnosis of acute MI did not have type-1 MI. These observations lend support to the need for clear diagnostic criteria, and management guidelines for type 2 MI. As the WHO endorses the ICD coding system as the standard diagnostic tool for epidemiology, health management, and clinical purposes, the inclusion of type-2 MI in the ICD-10 coding system is necessary (34). Both ACC and the AHA have requested ICD-10-CM (International Statistical Classification of Diseases and Related Health Problems, 10th revision, Clinical Modification) codes for specific MI subtypes to globalize the clinical MI profile, specifically for type-2 (35). The addition of type-2 MI in future ICD codes would enable research focused on epidemiology, management, and outcomes using the available databases.
Our study had several limitations. The included studies were observational studies, and propensity matching was available only in a few of them. There were also varying numbers of patients in each group among the different trials. Patients outside the coronary care units were not included, and this could have affected the mortality rates. The limited number of type-2 MI patients also limits the power of this study, and the treatment strategies for type-2 MI patients were at the discretion of treating physicians, which could be a source of selection bias.
Conclusions
Type-2 MI is a frequent entity and compared to type-1 MI, it is more common in females, older individuals, and in patients with multiple comorbidities. It also tends to result in a higher mortality rate. Invasive treatment strategies are less often utilized for type-2 MI, and these patients are often denied guideline-directed medical therapy. Given the complexity of MI patients and the insufficient data on type-2 MI, the inclusion of type-2 MI in the ICD-10 codes is warranted in order to enable research focused on its’ epidemiology, management, and outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-38. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart 2012;7:275-95. [Crossref] [PubMed]
- Alpert JS, Thygesen KA, White HD, et al. Diagnostic and therapeutic implications of type 2 myocardial infarction: review and commentary. Am J Med 2014;127:105-8. [Crossref] [PubMed]
- Melberg T, Burman R, Dickstein K. The impact of the 2007 ESC-ACC-AHA-WHF Universal definition on the incidence and classification of acute myocardial infarction: a retrospective cohort study. Int J Cardiol 2010;139:228-33. [Crossref] [PubMed]
- Javed U, Aftab W, Ambrose JA, et al. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol 2009;104:9-13. [Crossref] [PubMed]
- Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med 2013;126:789-97. [Crossref] [PubMed]
- Gonzalez MA, Eilen DJ, Marzouq RA, et al. The universal classification is an independent predictor of long-term outcomes in acute myocardial infarction. Cardiovasc Revasc Med 2011;12:35-40. [Crossref] [PubMed]
- Saaby L, Poulsen TS, Diederichsen AC, et al. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med 2014;127:295-302. [Crossref] [PubMed]
- Sandoval Y, Smith SW, Apple FS. Type 2 myocardial infarction: the next frontier. Am J Med 2014;127:e19. [Crossref] [PubMed]
- Bonaca MP, Wiviott SD, Braunwald E, et al. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation 2012;125:577-83. [Crossref] [PubMed]
- López-Cuenca A, Gomez-Molina M, Flores-Blanco PJ, et al. Comparison between type-2 and type-1 myocardial infarction: clinical features, treatment strategies and outcomes. J Geriatr Cardiol 2016;13:15-22. [PubMed]
- Baron T, Hambraeus K, Sundstrom J, et al. Type 2 myocardial infarction in clinical practice. Heart 2015;101:101-6. [Crossref] [PubMed]
- Stein GY, Herscovici G, Korenfeld R, et al. Type-II myocardial infarction--patient characteristics, management and outcomes. PLoS One 2014;9:e84285. [Crossref] [PubMed]
- Landes U, Bental T, Orvin K, et al. Type 2 myocardial infarction: A descriptive analysis and comparison with type 1 myocardial infarction. J Cardiol 2016;67:51-6. [Crossref] [PubMed]
- El-haddad H RE, Swett K. prognostic implications of type 2 myocardial infarctions. world J cardiovasc Dis 2012.237-41.
- Shah AS, McAllister DA, Mills R, et al. Sensitive troponin assay and the classification of myocardial infarction. Am J Med 2015;128:493-501.e3. [Crossref] [PubMed]
- Higgins J GS. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. 2008.
- Moher D CD, Eastwood S. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [Crossref] [PubMed]
- Moher D LA, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581-98. [Crossref] [PubMed]
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011;124:381-7. [Crossref] [PubMed]
- Kim M, Son M, Lee DH, et al. Troponin-I Level After Major Noncardiac Surgery and Its Association With Long-Term Mortality. Int Heart J 2016;57:278-84. [Crossref] [PubMed]
- Gualandro DM, Campos CA, Calderaro D, et al. Coronary plaque rupture in patients with myocardial infarction after noncardiac surgery: frequent and dangerous. Atherosclerosis 2012;222:191-5. [Crossref] [PubMed]
- Sametz W, Metzler H, Gries M, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Invest 1999;29:582-7. [Crossref] [PubMed]
- Ammann P, Fehr T, Minder EI, et al. Elevation of troponin I in sepsis and septic shock. Intensive Care Med 2001;27:965-9. [Crossref] [PubMed]
- Kumar A, Thota V, Dee L, et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996;183:949-58. [Crossref] [PubMed]
- Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med 2011;154:523-8. [Crossref] [PubMed]
- de Araújo Gonçalves P, Ferreira J, Aguiar C, et al. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J 2005;26:865-72. [Crossref] [PubMed]
- Banihashemi B, Goodman SG, Yan RT, et al. Underutilization of clopidogrel and glycoprotein IIb/IIIa inhibitors in non-ST-elevation acute coronary syndrome patients: the Canadian global registry of acute coronary events (GRACE) experience. Am Heart J 2009;158:917-24. [Crossref] [PubMed]
- Fox KA, Anderson FA Jr, Dabbous OH, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 2007;93:177-82. [Crossref] [PubMed]
- Díaz-Garzón J, Sandoval Y, Smith SW, et al. Discordance between ICD-Coded Myocardial Infarction and Diagnosis according to the Universal Definition of Myocardial Infarction. Clin Chem 2017;63:415-9. [Crossref] [PubMed]
- Mendis S, Thygesen K, Kuulasmaa K, et al. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 2011;40:139-46. [Crossref] [PubMed]
- Coordination and Maintenance Committee Meeting. CDC. ICD-10; 2016 March 9-10.