Advanced 3-D analysis, client-server systems, and cloud computing—Integration of cardiovascular imaging data into clinical workflows of transcatheter aortic valve replacement
TAVR and the need for imaging
Degenerative aortic stenosis (AS) is highly prevalent in the aging populations of industrialized countries, and advanced symptomatic stages are associated with poor prognosis (1-3). Open surgical valve replacement has been the only established treatment with documented significant improvement of long-term outcome. However, many of the older patients with AS are considered high-risk or ineligible for surgery because of often multiple co-morbidities (2). For these patients, transcatheter aortic valve replacement (TAVR) has emerged as a treatment alternative. Clinical registries and randomized trials have demonstrated good short and medium term results in high-risk and inoperable patient populations (4-8). However, results from recent cost-analysis are incomplete (9-11).
By definition, the TAVR procedure is characterized by a lack of exposure and visualization of the operative field. Percutaneous vascular access is obtained from the iliac or subclavian arteries, with large delivery catheters required for insertion of the crimped valve (≤18-F sheaths, outer diameter of approximately 7 mm). Because of the frequently advanced peripheral artery disease (PAD), access is associated with significant risk of vascular complications (12). If PAD precludes peripheral access, alternative access routes including trans-aortic and transapical access can be considered (13-16). The stent-valve is advanced and deployed within the aortic annulus either by balloon inflation or passive expansion and needs to be firmly anchored within the aortic root, without injuring the surrounding soft tissue. Matching the size of the prosthesis to that of the aortic annulus, optimal positioning of the stent/valve relative to the annulus, and proper alignment with the vessel axis are critical in order to avoid complications, including post-procedural aortic insufficiency, device embolization, and coronary occlusion (17,18). Pre- and intra-procedural imaging is therefore critical for patient selection, pre-procedural planning, and intra-operative decision-making, both regarding access site/route and deployment zone. Key imaging modalities are conventional angiography and echocardiography, which are used prior and during the procedure. In addition, multidetector computed tomography (CT) has assumed an important incremental role before TAVR (19).
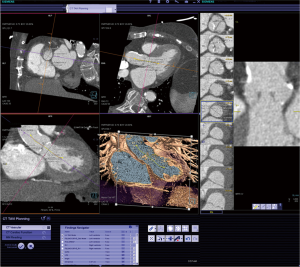
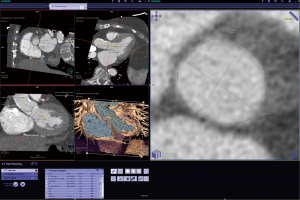
An important characteristic of CT is the rapid acquisition of a 3-D data volume with the ability for subsequent 3-D reconstruction of the acquired data (Figures 1-5). A typical TAVR CT dataset consists of arterial phase acquisition of the complete aorta, and images of the aortic root reconstructed in 8 to 10 cardiac phases with a limited field of view. This results in a large study of 3,500 to 4,500 single DICOM images, or about 2.5 Gigabytes of image data. For tortuous vascular structure (e.g., iliac arteries) and complex non-circular structures (e.g., the oval-shaped annulus) 3-D reconstruction and derived diameter measurements allow to identify precise minimal and maximal diameter, circumference, and area (20,21). While the differences between 2-D and 3-D derived measurements are small, they may have impact on device selection and deployment (22,23). Because of its tomographic nature and superior soft tissue resolution, the CT dataset also allows precise evaluation of the structures surrounding the annulus including qualitative and quantitative assessment of calcification (24-26).
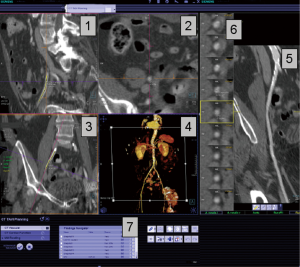
The analysis of CT data involves extensive manual reconstruction by the physician during direct interaction with the dataset rather than simple review of saved images. This requires experience with interactive 3-D interpretation, advanced analysis software and extensive IT support for distribution and archiving of the original and modified data. Automatic case preparation with post-processing of image data, will likely increase efficacy in clinical routine. Steps include segmentation of the aorta and presentation of the resulting centerline or automatic measurements of aortic valve plane and coronary ostia. These computations result in further binary volumetric data, which may extend the original image data by the factor of 2.5.
Clinical workflows and corresponding analysis steps
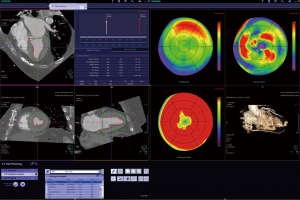
The process of CT image acquisition and analysis in the context of TAVR has evolved and is increasingly standardized, similar to clinical end-point definition, which are well defined (27-29). Details of image acquisition techniques are beyond the focus of this manuscript, but are described elsewhere (29-30). Briefly, acquisition protocols include imaging of the entire aorta including the iliac vessels. As part of this acquisition, imaging of the aortic root requires ECG-synchronization in order to avoid motion artifact. CT acquisition is associated with injection of iodine-based contrast material and radiation exposure (31-33). After initial general review including non-cardiac structures, the cardiovascular analysis is focused on the access site/route and aortic root deployment zone. The peripheral access arteries (iliac and axillary arteries) are evaluated for luminal size, calcified and non-calcified atherosclerotic plaque of the vessel wall, and vessel tortuosity (Figures 4,5). If the review identifies extensive PAD with limited access, alternative access routes are evaluated. This included the assessment of the ascending aorta for potential direct trans-aortic access, and evaluation of the LV apex for potential transapical approach (13-16). Because TAVR stent/valve prostheses are available in limited sizes, precise measurement of the aortic annulus is critical. Specifically, the annulus is measured as the plane at the lowest insertion point of the aortic valve leaflets and minimal/maximal diameter, circumference, and area are described (Figures 1,2). In addition, the spatial relation of the coronary ostia to the annulus is assessed. Lastly it is important to define an angiographic plane orthogonal to the valve plane in order to ensure optimal positioning/alignment of the prosthesis with the vessels axis (34,35). While severity of stenosis (AVA), valvular and left ventricular function (LVEF) are primarily evaluated with echocardiography, multi-phase reconstruction (4-D) of data acquired with retrospectively-gated protocols allow limited functional analysis with CT (Figure 3). Cine-loop reconstruction of image planes at the annulus and aortic valve allow to identify phases with maximum annular diameter and aortic valve opening area, respectively. These images are used for planimetry (36,37).
The analysis process is best organized into a multi-step workflow, which involves manual or semi-automated aortic root segmentation, centerline reconstruction of the aorta and iliac arteries, and additional 4-D functional analysis of ventricular and valvular function. While manual analysis with standard, basic 3-D software is possible, it is time-intensive and operator-dependent. Increasingly, semi-automated software systems pre-analyze the data, providing the evaluating physician with a structured template for final analysis and documentation/reporting (Table 1).

Full Table
Supporting software and network structure
Analysis software
In most clinical centers, selection of patients suitable for the TAVR procedure involves an interdisciplinary team of physicians, including Cardiologists, Cardiothoracic surgeons, and Imaging specialists/Radiologists. After an initial clinical appointment, subsequent testing including imaging is performed. The imaging specialist analyzes the data, prepares a report including saved key images and presentation states. The interventional cardiologist and surgeon reviews the accumulation data, which is used for decision making and planning of the procedure. All these single steps do not necessarily happen at the same place and time, they may be performed independently at different places in the hospital at different time points. Therefore, organization of the huge amount of accumulation data in a centralized archive and customized reading software models optimized for the workflow of individual groups of clinicians supports the clinical pathway.
As described above, the 3-dimensional CT datasets require complex, interactive reconstruction/reformation during analysis (19). Several reconstruction techniques are used. A basic technique for cardiovascular analysis is reconstruction along and perpendicular to the center-axis/center-line of ventricular or vascular structures. These ‘double-oblique’ reconstructions of thin slice cuts through the dataset allow precise diameter measurements. In particular in non-circular structures, e.g., the oval-shaped aortic annulus, such 3-D derived diameter measurements allow to identify minimal and maximal diameter, circumference, and area. These cross-sectional images can be obtained manually by adjusting the plane at selected points along the vessel axis. An advanced approach, now standard on dedicated workstations, is semi-automated identification of the vessels axis or centerline, which subsequently allows to slide a double-oblique perpendicular plane along the vessel and display the cross-section at any point along the vessel. Another frequently used technique is segmentation of the cavity of a vascular structure, e.g., the left ventricle (LV) or aortic root. This allows description of the enclosed volume. Additional analysis involves ‘4-D data’, i.e. data reconstructed at multiple phases of the cardiac cycle. Integration and display of multiple phases into cine-loops allows review of valvular and ventricular function. If segmentation of e.g., the LV is performed in multiple phases, analysis including automated calculation of LVEF becomes possible. With each of these techniques, the images can be displayed along thin cut-planes placed in the 3-D volume (similar to planes defined with echocardiography or angiography). Alternatively, use of several types of volume rendering techniques allows a shaded display of the entire 3-D dataset with focus on individual structures. These images allow easy 3-D orientation and are very useful for display and presentation of results.
Advanced software programs typically initiate semi-automated analysis in the background/at the server level once the data is sent from the CT scanner. Based on mathematical models, this analysis creates preliminary presentation states, e.g., identification of the vessel centerline or segmentation of the aortic root and cardiac chambers. On the basis of these initial data and additional automated post-processing algorithms at each step, the systems support a stepwise, structured review and analysis of the data and generation of secondary images, presentation states, and reports at the time of review by the imaging specialist/clinician. As described above, in the case of TAVR, this approach includes measurements of the root, entire aorta, and iliac arteries. More advanced systems are increasingly automated and provide more pre-processing as well as structured documentation and reporting of the data.
IT architecture
The evolving large imaging data files play a central role in pre-procedural planning and peri-procedural guidance. Organization of data from these multi-step workflows is complex. Optimally, the individual parts are integrated into a comprehensive image data file. Storage should maintain not only the originally acquired images, but also saved images, and presentation states allowing to recreate the presentation and e.g., measurements for different interpreters. While this is possible with advance 3-D workstations, these systems have traditionally allowed only limited sharing of data. Initial systems basically were independent workstations with a full copy of the software and direct connection to the CT scanner. The images would be transferred to this local workstation, where data manipulation, analysis, and initial storage would take place. Saved individual images could be sent to other equivalent workstations and/or the central archival (PACS), where they would be added to the archived data. Because of the high cost of these dedicated 3-D workstations, the number in each hospital was typically relatively low (necessitating the majority of clinicians to review images in the ‘reading room’).
The need to share complex data including presentation states, findings and reports creates new challenges for data storage, post-processing and sharing. It requires a complex infrastructure/network spanning across multiple locations in large hospital systems. This has been achieved by client-server solutions, where a single dataset is localized and modified on a powerful central server, while the local workstation used only the access point and command structure. These systems allow access to the data from several, less expensive, clinical workstations. An important aspect is the feature of saving and sharing result data between different participants in the clinical pathway, including basic or advanced images, presentation states, or post-processed data objects (segmentation masks representing anatomical structures, registration matrices between studies of different time points or different modalities).
This IT network structure allows interactive sharing of results, with each analysis step leaving a trace on the central server and adding data to the original data set. This is important for complex scenarios including TAVR, because the analysis process is typically performed by more than one investigator, separated by time and location. For example, an initial review by an imaging specialist/radiologist is followed by additional analysis by the interventionalist/surgeon. The evolving central dataset, including data added during analysis, is available for review and use at multiple locations. In addition to review, segmentation masks may be fused with those of other modalities and used during the procedure for orientation or navigation (e.g., root angulations and CT images for C-arm orientation). However, storage and sharing with 3-D analysis client-server solutions is still limited to a small number of users with access and knowledge of the advance software.
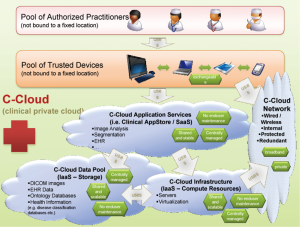
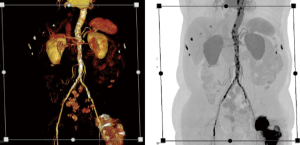
Institution-wide storage and sharing are established features of PACS systems, which are designed to provide easy access to all images, but rely on relatively basic axial review and simple additional 3D- and 4D-analysis tools. Providing an integrated solution combining features of advanced client-server analysis systems and PACS requires a complex IT architecture with bi-directional communication between a central archiving system, PACS systems, integrated client-server networks with different complex software applications, and multiple individual workstations used across the hospital system (38,39) (Figures 6-10). The system needs to be integrated into work-lists/work-flows of different practitioners. Such integration will allow sharing of advanced visualization, but the requirements for data storage and computing power for rendering and post-processing of multi-dimensional image data reach a complete new level. Data is distributed to larger image archiving systems, which allows long-term archiving for DICOM and non-DICOM images, as well as for non-imaging data into a patient specific file. The core storage provides access to all data generated in the healthcare system, which can be visualized in a patient-centric fashion on the electronic medical record (EMR) or more advanced dedicated systems. An emerging solution of this complex task is the use of multi-server systems (‘server-farms’), with connection of large number of servers integrated into existing work-flows. These systems are a form of cloud computing, with the totality of the integrated systems being consistent with a “private medical-grade cloud”
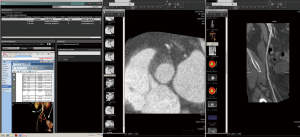
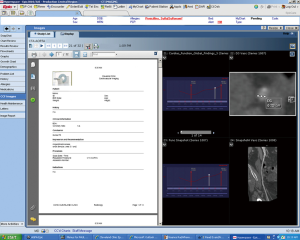
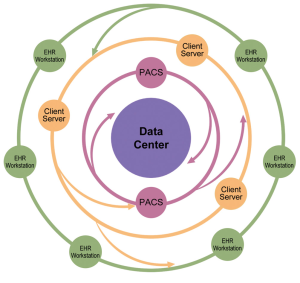
These systems put large requirements in terms of integration and usability on the imaging software architecture. The hospital controls the images and can implement own strategies for data security. Large hospital systems may be able to maintain a ‘private cloud’. However, because of the rapid increase in imaging data volume, it may be beneficial to reduce the amount of storage in existing on-site data centers if technically possible. In the context of faster innovation cycles, use of third party data service could be an alternative, which would enable hospitals to eliminate the need for hardware and software updates, but on the other hand create new dependencies and questions of data security. Vendor lock-in could be a problem, which could cause high follow-up costs when a vendor/system needs to be exchanged and data needs to be migrated. At the current time, several vendors provide such cloud based imaging services, but basic challenges, including data safety and compatibility, are not yet fully solved. Therefore the private clinical image cloud seems to be a valid approach until vendors provide a better standardization of their cloud imaging products.
While technically complex, the clinical advantages of these systems are obvious. These systems allow to maintain a central data file which can be shared between several groups of practitioners (Imaging Specialist/Radiologist, Clinician/Cardiologist/Cardiothoracic Surgeon), accessed and modified at multiple locations (reading room, physician office, operating room). As long as integration is established, different practitioners involved in the diagnosis and treatment could contribute and access the data from department-specific information and image management systems. For example, the Radiologist may work from a PACS study list, with integrated additional advanced software programs. Specialized interventionalists typically make use of the data with advanced software programs, bypassing the PACS system. The majority of clinicians generally rely on more limited access to the imaging data, optimally through the electronic health record (EHR). The network could include a single or multiple hospitals. Additional servers may allow access to the data for clinical research, maintaining a data structure compliant with respective regulations.
Conclusions
TAVR exemplifies the increased amount of patient-specific imaging data generated with modern imaging. Sharing of this image information has become a critical part of novel treatment approaches, but has also created huge demands on advanced data network solutions. The creation of a mobile, comprehensive longitudinal imaging file, as part of the EMR, which follows the patient and can be updated along the course of diagnosis and treatment is a critical development, with cloud technologies providing a possible solution. However, data demonstrating that such data has impact on decision-making or outcome is still lacking.
Acknowledgements
We acknowledge the contributions of Marion Tomasko, Graphic Department, Heart&Vascular Institute, Cleveland Clinic.
Disclosure: MZ is employee of Siemens.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [PubMed]
- Kapadia SR, Goel SS, Svensson L, et al. Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg 2009;137:1430-5. [PubMed]
- Leon MB, Smith CR, Mack M, et al. PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [PubMed]
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69-76. [PubMed]
- Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. [PubMed]
- Gurvitch R, Wood DA, Tay EL, et al. Transcatheter aortic valve implantation. Durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 2010;122:1319-27. [PubMed]
- Neyt M, Van Brabandt H, Devriese S, et al. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2012;2. pii: e001032.
- Reynolds MR, Magnuson EA, Wang K, et al. PARTNER Investigators. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation 2012;125:1102-9. [PubMed]
- Watt M, Mealing S, Eaton J, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart 2012;98:370-6. [PubMed]
- Kurra V, Schoenhagen P, Roselli EE, et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1258-64. [PubMed]
- da Gama Ribeiro V, Vouga L, Markowitz A, et al. Vascular access in transcatheter aortic valve implantation. Int J Cardiovasc Imaging 2011;27:1235-43. [PubMed]
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3:359-66. [PubMed]
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008;86:46-54; discussion 54-5. [PubMed]
- Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5. [PubMed]
- Al Ali AM, Altwegg L, Horlick EM, et al. Prevention and management of transcatheter balloon-expandable aortic valve malposition. Catheter Cardiovasc Interv 2008;72:573-8. [PubMed]
- Jilaihawi H, Kashif M, Fontana G, et al. Cross-sectional computed tomographic assessment improves accuracy of aortic annular sizing for transcatheter aortic valve replacement and reduces the incidence of paravalvular aortic regurgitation. J Am Coll Cardiol 2012;59:1275-86. [PubMed]
- Schoenhagen P, Numburi U, Halliburton SS, et al. Three-dimensional imaging in the context of minimally invasive and transcatheter cardiovascular interventions using multi-detector computed tomography: from pre-operative planning to intra-operative guidance. Eur Heart J 2010;31:2727-40. [PubMed]
- Tops LF, Wood DA, Delgado V, et al. Noninvasive Evaluation of the Aortic Root With Multislice Computed Tomography: Implications for Transcatheter Aortic Valve Replacement. JACC Cardiovasc Imaging 2008;1:321-30. [PubMed]
- Akhtar M, Tuzcu EM, Kapadia SR, et al. Aortic root morphology in patients undergoing percutaneous aortic valve replacement: evidence of aortic root remodeling. J Thorac Cardiovasc Surg 2009;137:950-6. [PubMed]
- Messika-Zeitoun D, Serfaty JM, Brochet E, et al. Multimodal assessment of the aortic annulus diameter. Implication for transcatheter aortic valve implanation. J Am Coll Cardiol 2010;55:186-94. [PubMed]
- Delgado V, Ng AC, van de Veire NR, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 2010;31:1114-23. [PubMed]
- Cueff C, Serfaty JM, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011;97:721-6. [PubMed]
- Latsios G, Gerckens U, Buellesfeld L, et al. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv 2010;76:431-9. [PubMed]
- John D, Buellesfeld L, Yuecel S, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010;3:233-43. [PubMed]
- Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17. [PubMed]
- Vahanian A, Alfieri OR, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2008;34:1-8. [PubMed]
- Achenbach S, Delgado V, Hausleiter J, et al. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366-80. [PubMed]
- Schoenhagen P, Hausleiter J, Achenbach S, et al. Computed tomography in the evaluation for transcatheter aortic valve implantation (TAVI). Cardiovasc Diagn Ther 2011;1:44-56.
- Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74. [PubMed]
- Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23. [PubMed]
- Halliburton SS, Schoenhagen P. Cardiovascular imaging with computed tomography: responsible steps to balancing diagnostic yield and radiation exposure. JACC Cardiovasc Imaging 2010;3:536-40. [PubMed]
- Kurra V, Kapadia SR, Tuzcu EM, et al. Pre-procedural imaging of aortic root orientation and dimensions comparison between X-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc Interv 2010;3:105-13. [PubMed]
- Gurvitch R, Wood DA, Leipsic J, et al. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:1157-65. [PubMed]
- de Heer LM, Budde RP, Mali WP, et al. Aortic root dimension changes during systole and diastole: evaluation with ECG-gated multidetector row computed tomography. Int J Cardiovasc Imaging 2011;27:1195-204. [PubMed]
- O'Brien B, Schoenhagen P, Kapadia SR, et al. Integration of Three-Dimensional Imaging Data in the Assessment of Aortic Stenosis: Impact on Classification of Disease Severity. Circ Cardiovasc Imaging 2011;4:566-73. [PubMed]
- Cowie MR, Chronaki CE, Vardas P. e-Health innovation: time for engagement with the cardiology community. Eur Heart J 2012. [Epub ahead of print]. [PubMed]
- Fernandez-Bayó J. IHE profiles applied to regional PACS. Eur J Radiol 2011;78:250-2. [PubMed]