Usefulness of amino terminal pro-B-type natriuretic peptide in evaluating children with cardiac failure
Introduction
Cardiac failure is defined as a clinical syndrome in which the heart is unable to pump enough blood to meet the body’s demands, to dispose of venous return adequately or a combination of both (1). It contributes significantly to childhood morbidity and mortality globally (2,3). The prevalence of heart failure from various studies done within Nigeria has ranged from 5.8–15.5% (4-6), while the mortality rates reported for children from this disease condition has ranged from 5.34–33.2% (5,6), thus identifying heart failure as a significant contributor to childhood mortality.
The cardinal features of childhood heart failure are dyspnea, tachypnea, tachycardia, cardiomegaly and a soft tender hepatomegaly. They form the basis for the clinical diagnosis of heart failure. However, some of these symptoms or signs are also seen in other conditions such as upper respiratory tract infections (URTIs), urinary tract infections (UTIs) and other acute febrile illnesses (7,8).
The natriuretic peptides have been identified as cardiac biomarkers that can more objectively diagnose heart failure and are also useful in the management of the condition (8-10). The plasma levels of these peptides, especially the B-type natriuretic peptide (BNP) and its inactive metabolite, amino-terminal pro B type natriuretic peptide (NT-proBNP) are elevated in subjects with heart failure and increase with worsening degree of heart failure (11,12). The use of these peptides in guiding patient care has been associated with reduced mortality, shorter duration of hospital stay and more event-free post-discharge period (13,14). However, these studies (13,14) were mainly done in adults in whom cardiac failure is of a different etiopathogenesis, compared to cardiac failure in children. The few studies in children (11,12) have not included the etiologic factors of heart failure as seen in our locale such as bronchopneumonia, septicemia and severe anemia (4-6,15). Furthermore, the cut-off values that have been reported have varied widely depending on the study population and the outcome parameter being evaluated. Fonseca et al. (16) in a study on the identification of heart failure amongst adults reported a value of 126 pg/mL, Rusconi et al. (17) in a study on children with chronic heart failure secondary to dilated cardiomyopathy reported a value of >1,000 pg/mL for the identification of moderate to severe degree of heart failure while Ekure et al. (18) gave a cut off of 951 pg/mL for the identification of heart failure in a study on children with structural heart diseases in Nigeria.
This study was therefore carried out to determine the plasma levels of NT-proBNP in children who present with clinical features of cardiac failure in order to relate the levels of this peptide to the severity of cardiac failure, the subject outcome and to also compare the values in these children with those of apparently healthy controls in this locale.
Methods
Study area
The study was carried out in the Children’s Emergency Room (CHER) of the University of Benin Teaching Hospital (UBTH), Benin-City, Edo State. The UBTH provides primary, secondary and tertiary health care services to the entire Edo State and neighbouring states of Delta, Ondo and Kogi States.
The CHER consists of a 13-bed ward and a casualty room. It is open 24 hours every day. All children with emergency conditions present in the casualty where they undergo initial assessment and intervention. They are then admitted into the children’s emergency ward for further management and stabilization before being moved to the main pediatric wards. On the average, 20–30 patients are seen daily.
Ethical approval with protocol number ADM/E22/A/VOLVII/958 was obtained from the Ethics Committee of the UBTH while written informed consent was obtained from parents/guardians of the subjects and controls. Verbal assent was obtained from children aged 8 years and above. This longitudinal study was conducted between May and November 2014.
The subjects were children aged 2 months to 17 years with clinically suspected heart failure determined by the presence of a soft tender hepatomegaly with any two of the following: tachycardia, tachypnea, dyspnea or cardiomegaly, were eligible for the study. The diagnosis of heart failure was based on modified Ross score of ≥3 (19). Those whose parents/caregivers gave consent were recruited for the study. The following children were excluded: those who had kidney disease, defined by glomerular filtration rate of <60 mL/min/1.73 m2 calculated from the serum creatinine levels using the Schwartz formula (20) and obese children with a body mass index (BMI) >95th percentile for the age and sex. The levels of NT-ProBNP are elevated in the presence of renal disease and in obese individuals (21,22). The controls were age matched children being followed up in the clinic for acute illnesses who had a modified Ross score of ≤2, and whose parents gave consent.
Sample size determination
Sample size was determined using the Cochran formula (23) when study population >10,000.
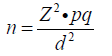
Where: n = minimum sample size when study population >10,000. Z = standard normal deviation for the defined confidence level of 95% =1.96, p = estimated prevalence or known prevalence from a previous study. The prevalence of 15.5% determined by Anah et al. (5) was used. q = proportion of subjects not in heart failure, which is 1 – p. d = margin of error to be tolerated (fixed at 5% or 0.05). A sample size of 201 was thus obtained. When the sample size was determined for study population <10,000, the sample size became 101. An attrition rate of 5% for potential laboratory errors or mishap was allowed for, bringing the final minimum sample size to 125 patients.
Data collection and evaluation
The subjects and controls that met the study criteria were consecutively recruited. A semi-structured researcher-administered proforma was used to obtain relevant demographic and clinical information from the caregiver. A detailed physical examination of the subjects and the controls was done and the presence and degree of severity of heart failure was determined using the modified Ross score (19). The degree of heart failure in the subjects was graded thus: score of 3–6 as mild heart failure, 7–9 as moderate heart and 10–12 as severe heart failure. The socioeconomic status was determined using the scoring system developed by Olusanya et al. (24). The age was categorized into <1 year, ≥1 to <5 years and those ≥5 years.
The anthropometric measurements
The children less than one year of age were weighed without any clothing or diaper, sitting in a bassinet weighing scale with a sensitivity of 0.01 kg. Children aged 1 year or above were weighed wearing their normal clothing without their foot wear or cardigans standing on a Seca® scale (Secagmbh & Co, Germany) with a sensitivity of 0.1 kg. The lengths of all the children were measured using a non-distensible measuring tape from the vertex to the heel placed in the neutral position, having a sensitivity of 0.01 cm. The subjects’ lengths were measured as they presented in respiratory difficulty and were unable to stand up. The controls also had their lengths taken in order to allow for uniformity in both study arms. The BMI of the participants was determined as the ratio of weight (kg)/height2 (m2). The values were then plotted against the percentile chart for the age and sex and the BMI percentile was obtained (25).
Laboratory investigation
Four milliliters (4 mL) of blood was obtained from any accessible superficial vein using a 21G sterile needle into a lithium heparin bottle in a sterile fashion. The samples were immediately centrifuged for 5 minutes at 10,000 r/s. The supernatant was then pipetted into a plain bottle and stored at –20 °C in the refrigerator. They were subsequently used for NT-proBNP and serum creatinine analysis. The levels of NT-proBNP was determined using Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) technique using kits manufactured by Abanova®, Taipei City Taiwan, batch Number E20141127031-3 and Lot Numbers 20141127-9. The analysis of NT-pro BNP was done by a chemical pathologist. The serum creatinine was assayed using a modification of the Kinetic method developed by Jaffe (26). The serum creatinine concentration obtained was used to calculate the glomerular filtration rate (GFR) using the Schwartz formula (23).
While on admission, the patients were followed up and the duration of patients’ stay and the outcome; being discharge, death or discharged against medical advice (DAMA) was recorded.
Data analysis
The data was analyzed using IBM SPSS version 21.0 (SPSS for Window Inc; Chicago, LL, USA) Statistical software. Continuous data such as the age and plasma NT-proBNP were summarized as mean ± standard deviation (SD), while categorized data such as the age-groups and socioeconomic class (SEC) of the subjects and controls were presented as proportions. The chi-square test was used for comparison of the categorized data. The obtained plasma NT-proBNP levels were log transformed before analysis as this converted data are normally distributed unlike the non-transformed data (27) and following analysis, results were reported using the real values. The level of significance of each test was set at P<0.05. The mean values of the log transformed NT-proBNP for various groups were compared with the Student’s t-test. The mean value of the log transformed NT-proBNP for the degrees of severity of heart failure and the SEC were compared using the one-way analysis of variance (ANOVA). A receiver operating curve (ROC) curve was constructed using plasma NT-proBNP obtained from the study participants and the varied cut-off values obtained were used in deriving the positive predictive value (PPV) and negative predictive value (NPV).
Results
A total of 132 subjects and 132 controls were initially recruited for the study. None of the recruited study participants were obese. However, 8 samples, 4 each from the subjects and the controls were lost during storage. Furthermore, 2 samples each from both study arms had eGFR <60 mL/min/1.73 m2 and were excluded from further analysis. Hence, a total of 126 subjects and 126 controls were analyzed for the study.
Socio-demographic characteristics of the subjects and controls
The mean age of the subjects was 2.27±2.91 (range, 0.17–12.00) years while that for the control group was 2.92±3.26 (range, 0.17–13.00) years (t=1.67, P=0.096). There was no statistically significant difference in the gender, age group and socio-economic class distribution between subjects and controls, as shown in Table 1.
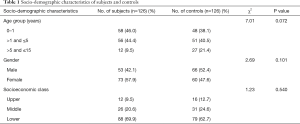
Full table
Causes and severity of heart failure
The distribution of causes of heart failure in the subjects is shown in Table 2. The commonest cause of heart failure was severe anaemia in 53 (42.1%). The group called others included 3 cases of myocarditis and a case of adeno-tonsillitis with obstructive sleep apnea syndrome.
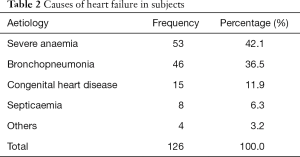
Full table
Of the 126 subjects, 51 (40.5%) had mild heart failure, 60 (47.6%) had moderate heart failure while 15 (11.9%) had severe heart failure. The gender, age group and socio-economic class distribution according to the severity of heart failure is shown in Table 3. More females than males had severe heart failure (P=0.037). Significantly more subjects aged ≤5 years had severe heart failure compared to those >5 years (P=0.037). The socioeconomic class distribution of the subjects according to severity of heart failure was not statistically significant (P=0.783).
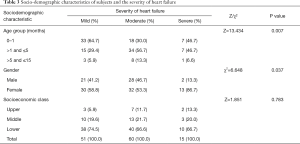
Full table
Mean levels of NT-proBNP in subjects and controls
The mean plasma NT-proBNP in the subjects was 1,137.10±1,234.78 ng/L, while that in controls was 578.00±665.08 ng/L. The difference was statistically significant (P<0.001). The subjects had significantly higher plasma NT-proBNP than controls in all age groups as shown in Table 4.
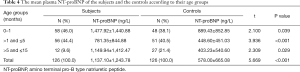
Full table
The subjects with severe heart failure had the highest mean plasma NT-proBNP level 2,514.82±1,612.86 ng/L compared to those with mild 1,203.70±1,249.64 ng/L or moderate 736.05±828.78 ng/L. The difference was statistically significant (P<0.001). Post-hoc analysis showed that mild heart failure vs. severe heart failure (P=0.027), Severe heart failure vs. moderate heart failure (P=0.002).
The mean plasma NT-proBNP was highest in the subjects with CHD (2,297.40 ng/L). There was a statistically significant difference in the distribution of the mean NT-proBNP of the subjects and the cause of heart failure (P<0.001, Table 5).
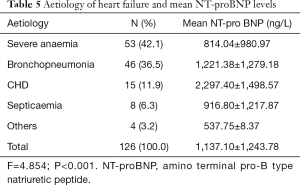
Full table
NT-proBNP in the identification of heart failure
The plasma NT-proBNP levels of the subjects and the controls were used in constructing a ROC to determine a cut-off value for the identification of heart failure as shown in Figure 1. The graph shows that the area under the curve (AUC) was 0.693 with a P<0.001 which is statistically significant.
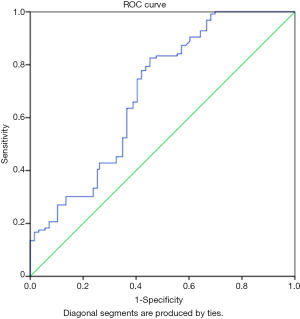
In addition, using the co-ordinate points obtained from the curve, a plasma NT-proBNP level of 315.60 ng/L has a sensitivity of 77.8% and specificity of 57.9% of identifying heart failure. The other cut-off values are as shown in Table 6.

Full table
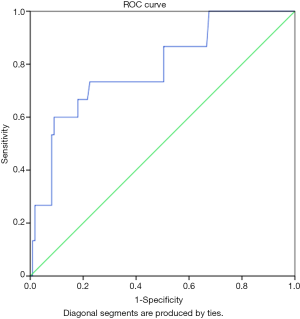
A cut-off value 903.15 ng/L had a sensitivity and specificity of 73.3% and 72.1% respectively for identifying severe heart failure amongst subjects with clinically diagnosed heart failure as shown in Table 7. The AUC was 0.785 (P=0.001, Figure 2).

Full table
Duration of admission and the outcome of subjects according to NT-proBNP level
Of the 126 subjects, 113 (89.7%) were discharged and 4 (3.2%) died while 9 subjects were DAMA; of those that DAMA, 8 (88.9%) did so on account of financial constraints and 1 (11.1%) was to seek faith-based healing. Of the four subjects that died, 2 (50.0%) had moderate heart failure, 1 (25.0%) each had mild and severe class of heart failure respectively.
The subjects who died had the highest mean plasma NT-proBNP of 2,344.20±1,869.52 ng/L compared to those discharged 1,097.83±1,192.30 ng/L and those that DAMA 1,093.59±1,485.46 ng/L (P=0.143).
The mean duration of admission of the subjects was 7.21±6.19 days. The subjects with mild heart failure spent the shortest mean time on admission 6.96±7.82 days compared with those with moderate 7.22±4.80 days and severe heart failure 8.00±5.00 days (F=0.16, P=0.851).
There was a positive correlation between the plasma NT-proBNP and the duration of admission of the subjects but this was not statistically significant [Pearson’s correlation coefficient (r) =0.145, P=0.105].
Discussion
The mean plasma NT-proBNP of the subjects with heart failure was significantly higher than that of the controls in this study. This difference remained irrespective of the age, gender and socioeconomic class. This finding is similar to that reported by Ekure et al. (18), Wong et al. (28), Fonseca et al. (16) and Nevo et al. (29). The similarity could be due to the uniform application of modified Ross criteria in the diagnosis of heart failure in all the studies. This study showed a significant negative correlation between age and the plasma NT-proBNP levels in both study arms which is similar to findings by other researchers (27). This finding has been linked to the association of increasing age with increased adiposity which increases expression of the inactive NPR-C clearance receptor for the peptide, enhancing its removal from circulation and thus lowering plasma levels.
This study revealed that if used in determining the presence of heart failure, a cut-off plasma NT-proBNP level of 315.60 ng/L would correctly identify the presence of the disease in 8 or its absence in 6 out of every 10 persons. This cut off value is lower than the cut-off value of 951 pg/mL for the identification of heart failure in children with structural heart disease reported by Ekure et al. (18) and the 415 pg/mL for the identification of structural heart disease reported by Nevo et al. (29); this could be because these studies evaluated subjects with acquired and congenital forms of structural heart disease whereas the cut-off in this present study was for the identification of heart failure irrespective of the cause. (ng/L being the same as pg/mL)
Subjects with severe heart failure had significantly higher mean NT-proBNP value compared to mild and moderate categories. This finding is akin to reports by Ekure et al. (18), and Sugimoto et al. (11) both noted an increasing plasma NT-proBNP with worsening degree of heart failure. The uniform diagnostic tool for heart failure may have accounted for the similarity in findings. However, unlike other studies (11,17,18) the relationship between plasma NT-proBNP and the severity of heart failure in this study was not uniform as the subjects with moderate heart failure had a lower mean plasma NT-proBNP compared to those with mild heart failure. This may be due to the fact that infants who tend to have higher NT-proBNP values (27) contributed more to subjects with mild and severe forms of heart failure in contrast to those with moderate heart failure.
The mortality rate in this study was 3.2% which is lower than the 5.34% reported previously by Sadoh et al. (30) in the same study area and 11.1% by Anah and co-workers (5). The lower mortality rate in this study may have been accounted for by the extension of credit facilities in the study locale (UBTH) to indigent patients who present in acute emergencies as most of the study participants were from the lower SEC, facilitating prompt medical intervention. Although not statistically significant, the plasma NT-proBNP was highest in the subjects that died compared to those who were discharged. The difference did not reach statistical significance probably because of the small number of mortality. There was no statistically significant relationship between the plasma NT-proBNP and the duration of admission in this study in contrast to previous studies on adult subjects with heart failure (8,9). This could be due to the faster recovery from illnesses in children compared to adults who tend to have co-morbidities.
The children less than five years of age accounted for almost 90% of the subjects in this study which is similar to what has been reported by Anah et al. (5), Adekanmbi et al. (4), as well as Lagunju and co-worker (6). This findings suggests that children less than 5-year continue to bear the brunt of childhood morbidity.
The subjects with CHD had the highest level of mean NT-proBNP compared to the other causes of heart failure, which is similar to what other researchers have reported (29,31,32). This may be due to the fact that CHD being a structural abnormality of the heart may cause more profound release of the cardiac bio-marker when there is heart failure. The relationship between anaemia and plasma NT-proBNP levels has also been evaluated. Hogenhuis et al. (33) and Willis et al. (34) in separate studies reported that anaemia had a direct independent relationship with levels of the peptide. Similarly, Desai et al. (35) stated that after controlling for other known cardiovascular risk factors, there was still a significant inverse relationship between haemoglobin concentration and plasma NT-proBNP. Since severe anemia was the identified cause of the clinical features used in diagnosing and categorizing heart failure in this study, severe anaemia can be said to have caused heart failure as well as elevated the level of NT-proBNP in addition to heart failure in this study. In conclusion, the mean plasma NT-proBNP in children with heart failure was significantly higher than in the controls. The mean plasma NT-proBNP was highest in children with severe heart failure compared to those with moderate or mild heart failure suggesting an ability of NT-proBNP to discriminate between severe heart failure and the less severe categories. It, however, was unable to significantly discriminate between those that died and the ones that were discharged.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval with protocol number ADM/E22/A/VOLVII/958 was obtained from the Ethics Committee of the UBTH while written informed consent was obtained from parents/guardians of the subjects and controls.
References
- Park MK. editor. The Paediatric CardiologyHandbook. London: Mosby Inc., 1997; 58-72.
- McMurray JJ, Petrie MC, Murdoch DR, et al. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J 1998;19 Suppl:P9-16. [PubMed]
- Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22:6A-13A. [Crossref] [PubMed]
- Adekanmbi AF, Ogunlesi TA, Olowu AO, et al. Current trends in the prevalence and aetiology of childhood congestive cardiac failure in Sagamu. J Trop Pediatr 2007;53:103-6. [Crossref] [PubMed]
- Anah MU, Antia-obong AU, Odigwe CO, et al. Heart failure among paediatric emergencies in Calabar. South-eastern Nigeria. Mary Slessor J Med 2004;4:58-62.
- Lagunju IA, Omokhodion SI. Childhood Heart Failure in Ibadan. West Afr J Med 2003;22:42-45. [PubMed]
- Kingue S, Dzudie A, Menanga A, et al. A new look at adult chronic heart failure in Africa in the age of the Doppler echocardiography: experience of the medicine department at Yaounde General Hospital. Ann Cardiol Angeiol (Paris) 2005;54:276-83. [Crossref] [PubMed]
- Palazzuoli A, Antonelli G, Quatrini I, et al. Natriuretic peptides in heart failure: where we are, where we are going. Intern Emerg Med 2011;6:63-8. [Crossref] [PubMed]
- Lipshultz SE, Wilkinson JD, Messiah SE, et al. Clinical research directions in pediatric cardiology. Curr Opin Pediatr 2009;21:585-93. [Crossref] [PubMed]
- Ng LL, Geeranavar S, Jennings SC, et al. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond) 2004;106:129-33. [Crossref] [PubMed]
- Sugimoto M, Manabe H, Nakau K, et al. The role of N-terminal pro-B-type natriuretic peptide in the diagnosis of congestive heart failure in children. - Correlation with the heart failure score and comparison with B-type natriuretic peptide -. Circ J 2010;74:998-1005. [Crossref] [PubMed]
- Wu YR, Chen S, Huang M, et al. N-terminal pro-brain natriuretic peptide in the diagnosis of congestive heart failure in paediatric patients with ventricular septal defects. World J Pediatr 2006;1:40-4.
- Palazzuoli A, Caputo M, Calabrò A, et al. Clinical impact of BNP and other emerging biomarkers in heart failure evaluation and management. Minerva Cardioangiol 2012;60:183-94. [PubMed]
- Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355:1126-30. [Crossref] [PubMed]
- Bondi F, Jaiyesimi F. Heart Failure in an Emergency room setting. Nig J Paediatr 1990;17:1-6.
- Fonseca C, Sarmento PM, Minez A, et al. Comparative value of BNP and NT-proBNP in diagnosis of heart failure. Rev Port Cardiol 2004;23:979-91. [PubMed]
- Rusconi PG, Ludwig DA, Ratnasamy C, et al. Serial measurements of serum NT-proBNP as markers of left ventricular systolic function and remodeling in children with heart failure. Am Heart J 2010;160:776-83. [Crossref] [PubMed]
- Ekure EN, Okoromah CA, Ajuluchukwu JN, et al. Diagnostic usefulness of N-terminal pro-brain natriuretic peptide among children with heart failure in a tertiary hospital in Lagos, Nigeria. West Afr J Med 2011;30:29-34. [Crossref] [PubMed]
- Ross RD. Grading the graders of congestive heart failure in children. J Pediatr 2001;138:618-20. [Crossref] [PubMed]
- Schwartz GJ, Haycock GB, Edelmann CM Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976;58:259-63. [PubMed]
- Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation 1992;86:1081-8. [Crossref] [PubMed]
- Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594-600. [Crossref] [PubMed]
- Cochran WG. Sampling Techniques.2nd ed. New York: John Wiley and Sons, Inc., 1963; 78.
- Olusanya O, Okpere E, Ezimokhai M. The importance of Social class in voluntary fertility control in a developing country. West African J Med 1985;4:205-12.
- Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45-60. [Crossref] [PubMed]
- Kirchka LJ, Park JY. Optical techniques. In: Burtis CA, Ashwood ER, Burns DE, et al. editors. Tietz Fundamentals of Clinical Chemistry. 6th ed. Missouri: Saunders Elseiver; 2008; 71.
- Albers S, Mir TS, Haddad M, et al. N-Terminal pro-brain natriuretic peptide: normal ranges in the pediatric population including method comparison and interlaboratory variability. Clin Chem Lab Med 2006;44:80-5. [Crossref] [PubMed]
- Wong DT, George K, Wilson J, et al. Effectiveness of serial increases in amino-terminal pro-B-type natriuretic peptide levels to indicate the need for mechanical circulatory support in children with acute decompensated heart failure. Am J Cardiol 2011;107:573-8. [Crossref] [PubMed]
- Nevo I, Erlichman M, Algur N, et al. N-terminal pro B–type natriuretic peptide levels in infants and children with acute non-cardiac diseases. Isr Med Assoc J 2011;13:420-4. [PubMed]
- Sadoh WE, Akinsete AM. Epidemiology of childhood heart failure in Benin City. Nig J Cardiol 2006;3:12-5.
- Geiger R, Hammerer-Lercher A, Url C, et al. NT-proBNP concentrations indicate cardiac disease in pediatric patients. Int J Cardiol 2007;123:63-5. [Crossref] [PubMed]
- Moses EJ, Mokhtar AI, Hamzah A, et al. Usefulness of N- terminal pro- B- type natriuretic peptide as a screening tool in identifying patients with congenital heart disease. Lab Med 2011;42:75-80. [Crossref]
- Hogenhuis J, Voors AA, Jaarsma T, et al. Anaemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur J Heart Fail 2007;9:787-94. [Crossref] [PubMed]
- Willis MS, Lee ES, Grenache DG. Effect of anemia on plasma concentrations of NT-proBNP. Clin Chim Acta 2005;358:175-81. [Crossref] [PubMed]
- Desai AS, Bibbins-Domingo K, Shlipak MG, et al. Association between anaemia and N-terminal pro-B-type natriuretic peptide (NT-proBNP): findings from the Heart and Soul Study. Eur J Heart Fail 2007;9:886-91. [Crossref] [PubMed]


