Inflammatory and infectious aortic diseases
Introduction
Aortitis, inflammation of the aorta, is most commonly due to large-vessel vasculitides including giant cell and Takayasu’s arteritis (Table 1) (1). Prompt diagnosis and treatment with glucocorticoids is essential to avoid the profound disability that can occur if these entities are left untreated. Infectious aortitis is rare but life-threatening, and in contrast to vasculitides, must be treated promptly with antibiotics and potentially surgical or endovascular repair. The clinical presentation of infectious and inflammatory aortitis is variable and nonspecific, which can make diagnosis of these disorders challenging and delayed. We review the epidemiology, pathophysiology, presentation, diagnosis and management of aortitis.

Full table
Infectious diseases
Mycotic aneurysms
Prevalence and etiology
Mycotic aneurysms reflect fewer than 1% of aortic aneurysms that are surgically repaired, are more common in men, and are more likely to rupture than non-infected aneurysms (2-5). Mycotic aneurysms are more likely to occur in the aorta than other arteries (6-8). Previously, MAs were associated with endocarditis, β-hemolytic group A streptococci, pneumococci, and Haemophilus influenza (8,9). Since the introduction of more targeted antibiotic regimens, MAs are more commonly associated with intravascular intervention and intravenous drug abuse, and Staphylococcus and Salmonella (6,9). Syphilitic aortitis is rare but may cause aortic wall thickening and aneurysmal dilatation, and is typically limited to the ascending and thoracic aorta (10).
MAs are thought to arise due to degeneration of the arterial wall (8). MAs tend to more rapidly progress compared with non-infected aneurysms: the acute inflammation in response to pathogenic infection resulting in neutrophilic infiltration of the arterial wall (4,11,12) leads to the activation of collagenolytic and elastolytic enzymes and concomitant breakdown and saccular dilation (13-15). This process leads to the characteristic saccular appearance of MAs.
Infection is due to inoculation of the arterial wall, which may occur in the setting of iatrogenic, self-inflicted, or traumatic arterial wall injury, or from extension of an infectious extravascular source adjacent the arterial wall (16,17). Bacteria may seed the arterial wall injury either from bacteremia or septic emboli (18). Typically, infection initiates in a nidus such as in an ulcerated atherosclerotic plaque or in the vasa vasorum (12). The vasa vasorum is thought to be key in the pathogenesis of MA formation; due to its small lumen size and slower flow, it is more susceptible to bacterial colonization (19,20). The vasa vasorum is more pronounced in larger arteries, which may explain why the aorta is the most common site of MA formation.
Diagnosis
Early diagnosis and rapid triage for intervention is key to reducing mortality from MA (21,22). However, diagnosis is challenging given the low prevalence and nonspecific symptoms. Clinical signs may include fever and laboratory abnormalities including elevated erythrocyte sedimentation rate and leukocytosis (23). Bacteremia is common, though cultures may be negative, particularly after antibiotics have been given. Symptoms include pain or a pulsatile mass (23). In the setting of pre-existing endocarditis, prior invasive procedures, intravenous drug use or immunocompromise should increase suspicion (8,24).
Non-invasive cross-sectional imaging is essential in the diagnosis of MA. Computed tomography angiography (CTA) has arisen as the imaging modality of choice owing to its excellent resolution allowing for three-dimensional reconstruction and its rapid acquisition; magnetic resonance imaging (MRI) may also be used (25-27). Certain features may distinguish MA from non-infected aneurysms, including serial imaging which may reveal rapid progression typical of infected aneurysms. Other characteristic features include contrast-enhancement and a saccular outpouching configuration, whereas non-infected atherosclerotic aneurysms tend to be fusiform (28). Saccular configuration may also suggest imminent rupture, alerting the need for further urgent diagnostic workup. Other features include irregularity of the arterial wall and peri-aortic gas, edema, mass or stranding (28). MAs also tend to have higher uptake on FDG-PET imaging of 4.5 SUVmax or more compared with non-infected aneurysms (29). FDG-PET boasts high sensitivity and its potentially high false-positive rate can be improved with simultaneous CT.
Treatment and prognosis
Due to its elusive presentation, MA is often difficult to treat because of delayed diagnosis. Rapid diagnosis and treatment is key, as aortic MA is associated with 15–50% mortality (7-9,24). No randomized trials are available to guide management, though treatment involves antibiotics and surgical intervention. Antibiotics are tailored based on culture sensitivity when available. When cultures are not available, consultation with one’s infectious disease specialists is suggested, as there are regional differences in antibiotic resistance. No consensus exists regarding the duration of antibiotic course, with some advocating for life-long suppressive antibiotics while others suggest at minimum a 6–8-week post-operative course (30).
Aortic MAs usually necessitate surgical repair, with two possible available approaches including extraanatomic bypass (EAB) and in situ graft placement. By tradition, EAB has been used for infrarenal aneurysms and in situ graft placement for thoracic or suprarenal aneurysms. While EAB avoids graft placement within an infected field, it may lead to aortic stump disruption, amputation, and reinfection (2,3,21,31). Several graft materials are available for in situ graft placement, such as silver-coated grafts, cryopreserved arterial allografts, rifampicin-impregnated grafts, and autogenous vein grafts (2,32-34).
Endovascular stenting is one treatment option, though somewhat controversial: placing foreign material to an infected site, without prior debridement, may carry a risk of stent infection, malposition with endoleak, and potential rupture (35). After an initial report in 1998 of endovascular stent placement for a thoracic MA (36), a number of small series have presented examples of successful endovascular repair of mycotic thoracic and abdominal aneurysms, though patients with aortobronchial and aortoenteric fistulas have worse outcomes after repair (37,38). A large multicenter retrospective series of endovascular repair of 130 aortic MAs in 123 patients reported 91% 1-month survival and 75% 1-year survival at 1 year; only 6 were converted to open repair (39). Open surgery remains the gold standard intervention (40), though endovascular treatment can be considered as a temporizing measure particularly for critically ill patients (41).
Nonaneurysmal infectious aortitis
Infectious aortitis most commonly presents as a MA, though there are case reports of nonaneurysmal infection (42). Nonaneurysmal infectious aortitis is more difficult to diagnose on imaging than MA, given the lack of aortic dilation, but even so may be complicated by rupture (43,44). A more recent case report described achieving an elusive diagnosis of infectious nonaneurysmal infectious aortitis by using broad-range polymerase chain reaction and DNA sequencing, which allowed for identification of the microbial species despite negative blood cultures (45).
Aortic graft infection
Prevalence and etiology
The incidence of graft infection after aortic aneurysm repair is low, below 0.5%, and is equally likely with open or endovascular repair, despite the finding that open repair was more likely to be complicated perioperative septicemia, pneumonia, and surgical site infection (46). Infections within the first 3 post-operative months are considered early and those after 3 months are considered late. Patients with perioperative infections are at higher risk for graft infection, suggesting seeding by bacteremia (46). For patients who present with infection within 3 months of repair, contamination during endograft placement is considered the likely source (47). Aortoenteric and aortobronchial fistulas are a common cause of infection and portend a poorer prognosis (Figure 1) (48). Culprit organisms include Staphylococcus, Streptococcus and Propionibacterium species, Enterobacter cloacae, Escherichia coli, Pseudomonas aeruginosa, and Listeria monocytogenes (47,49).
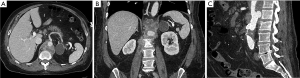
In one study, graft infection was more common after thoracic endovascular aneurysm repairs (EVAR) (5%) compared to aortic aneurysm repairs (0.3%) (49), though another study showed no significant difference in rates (47). Emergent repairs are more likely to be complicated by graft infection than elective procedures (47).
Diagnosis
Clinically, patients present with constitutional symptoms including pain, fever and chills, which may prompt suspicion for postoperative infection (49,50). Aortic graft infections occurring more than 3 months after repair are initially evaluated with CT or MRI, which may reveal ectopic gas, peri-graft inflammation and fluid, thickening of adjacent bowel, and pseudoaneurysm formation at the graft anastomosis (51). Imaging may reveal contained rupture or abscess formation (49). Imaging is problematic in the first 3 months following repair, as peri-graft fluid and inflammation may be normally present. Radionuclide scanning, particularly with white blood cell labeling, may helpful to diagnose early vascular graft infection (51,52).
Treatment and prognosis
Treatment approaches may be conservative with intravenous antibiotics alone or operative in addition to antibiotics, with surgical excision and extra-anatomic bypass considered gold standard (48). One meta-analysis questioned whether surgery should be the gold standard approach (53). Another retrospective study reported that in situ prosthetic reconstruction had better mortality rates compared with extra-anatomic bypass (54). Though all available data is limited to retrospective reviews and thus subject to bias, so no conclusions can be drawn. Reported mortality after graft infection ranges from 20% to 40% (46,48,55).
Inflammatory diseases
Noninfectious vasculitis
Prevalence and etiology
Large-vessel vasculitis typically involves the aorta and its proximal branches and is most commonly caused by Takayasu’s disease or giant cell arteritis; more rarely, it may be a due to Behcet’s disease, sarcoidosis, Kawasaki disease, rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, Cogan syndrome, or Wegener’s granulomatosis (56). It is thought that giant cell and Takayasu’s arteritis share similar underlying pathology mechanisms. Whereas Takayasu’s disease and giant cell arteritis involve T cells, macrophages and antigen-presenting cells, other vasculitides involve autoantibodies (57). As in infectious aortitis, large-vessel vasculitis involves an inflammatory process of the vasa vasorum, though in contrast to MA, T cell-mediated vasculitides do not usually lead to degeneration of the elastic lamina that would lead to aneurysm formation (57).
Giant cell arteritis and Takayasu’s disease affect different populations. Giant cell arteritis, carries a lifetime risk of 1% in women and 0.5% in men in the United States (58), and nearly always presents in people over 50 years of age, with a predilection for people of Scandinavian decent (59). In contrast, Takayasu’s disease presents primarily in women between 10 and 40 years of age (60,61), and predominates in Asia while being rare in the US and Europe . It has a worldwide distribution, with the greatest prevalence in Asia (60,62).
Diagnosis
Giant cell arteritis, associated with polymyalgia rheumatica, is suspected in patients over 50 years of age with new headache, claudication of the jaw or tongue or upon swallowing, unexplained fever or anemia, or abrupt visual disturbances (Figure 2). Patients are often found on physical exam to have tender areas or nodules of the scalp and tenderness to palpation of the temporal artery with decreased pulsation (63). Laboratory evaluation is nonspecific, though the C-reactive protein and erythrocyte sedimentation rate are usually high (64). The gold standard diagnostic test is a temporal artery biopsy, performed in an outpatient office setting. Unilateral biopsy is usually performed first, followed by biopsy of the contralateral side if the first study is negative. Histologic assessment reveals inflammation typically involving T cells and macrophages, and is most often transmural but may also be more localized, for example to the vasa vasorum; infiltration by eosinophils or neutrophils is rare (65). Giant cells, calcification, and laminar necrosis may be seen along the internal elastic lamina. Biopsy may be falsely negative in roughly a third of patients (66). Color Doppler ultrasound is a noninvasive modality to assess the temporal artery, and has been proposed by some to replace invasive biopsy (67), though the gold standard remains histologic evaluation.
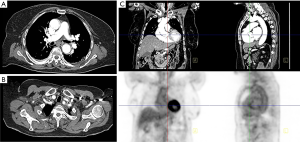
Large-vessel giant cell arteritis, the form of the disease involving the aorta, does not typically involve the temporal arteries, resulting in false negative histology and elusive diagnosis (68). Among patients with giant cell arteritis, two thirds develop aortitis (69), more than 10% develop large vessel stenosis and nearly 20% develop aortic aneurysm and dissection (70). Large-vessel giant cell arteritis impacts patients at a younger age (68 years) compared with cranial giant cell arteritis (76 years). Whereas cranial giant cell arteritis is associated with jaw claudication and headaches, large-vessel giant cell arteritis is associated with upper extremity claudication and asymmetric blood pressures (71). However, these features may not be present, and rather only constitutional symptoms noted. Large vessel involvement may only be detected incidentally on imaging.
Whereas conventional angiography was used previously to characterize the large vessel changes in giant cell arteritis, noninvasive imaging with MR and CT angiography and PET has arisen as primary modalities. Active disease is characterized by wall thickening, mural enhancement on venous phase imaging, and FDG-avidity; the aorta and its proximal branches are typically involved with skip areas of stenosis and dilation (72). MR angiography can demonstrate mural edema, seen as T2-bright signal or T1-weighted enhancement, and “edema-weighted” MR angiography may detect inflammatory changes (73). PET imaging can localize affected vessels, and FDG-avidity is most often detected in the aortic arch (74).
Takayasu’s arteritis (Figure 3) typically begins in the left proximal or middle subclavian artery, but progresses to involve the aorta and pulmonary arteries in half of patients (75). Onset is typically subacute, beginning with low grade fevers, fatigue, and weight loss; as the disease progresses, signs and symptoms may include limb claudication, decreased pulses, and discrepant blood pressures due to involvement of the subclavian arteries, hypertension due to involvement of the renal arteries, abdominal pain and diarrhea due to involvement of the mesenteric arteries, angina due to involvement of the carotid arteries or aorta, chest pain and dyspnea due to involvement of the pulmonary arteries, and neurologic symptoms due to involvement of the carotid and vertebral arteries (76). Other signs and symptoms include carotidynia, bruits, and arthralgias.
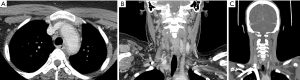
There are no diagnostic laboratory tests for Takayasu’s disease; C-reactive protein and the erythrocyte sedimentation rate may be elevated, but these tests are not sensitive or specific (76). Thus, diagnosis hinges on the clinical presentation and imaging. Conventional angiography may be used when direct four-limb blood pressure measurement is desired, or when catheter-directed therapy is planned. Otherwise, noninvasive imaging with CT and MR angiography is preferred, as these modalities will demonstrate mural changes in addition to arterial stenoses (77). Imaging findings in Takayasu’s disease are similar to those in giant cell arteritis, with similar patterns of vascular involvement (78). Not only are Takayasu’s and giant cell arteritis similar on imaging; histopathological findings are also indistinguishable (79). Therefore, diagnosis is based on the patient age, with patients under 40 years old diagnosed with Takayasu’s arteritis, and those over 40 years likely to have giant cell arteritis.
Treatment and prognosis
Once giant cell arteritis is suspected, patients are treated with glucocorticoids, regardless of whether a biopsy has been performed, with daily prednisone regimens shown to be more effective than alternate-day regimens (80). Placebo-controlled studies are not possible, as the consequences of not treating (e.g., blindness) are so dire. If the disease is well controlled, glucocorticoids can be tapered. The optimal method to monitor patients is not defined, as laboratory tests are nonspecific. Relapse may be re-treated with glucocorticoids. To treat resistant disease, or to decrease the dose of corticosteroids, other agents including methotrexate, tocilizumab, anti-TNF therapy, and cyclophosphamide may be considered. Low-dose daily aspirin is indicated in all patients with giant cell arteritis to reduce cranial ischemic complications (81).
Studies suggest that giant cell arteritis does not carry a higher mortality rate compared to the unaffected population. However, if aortic dissection or aneurysm occurs, there is an increase in mortality with a hazard ratio of 3.4 (82). In contrast to patients with cranial giant cell arteritis, those with large-vessel giant cell arteritis suffer more relapses and require high doses of corticosteroids for longer treatment durations (71). It is unknown whether steroid treatment impacts the course of aortitis and the risk of developing aneurysm.
As in giant cell arteritis, the gold standard treatment of Takayasu’s arteritis is daily oral glucocorticoids (83). Imaging may be used to monitor treatment efficacy (84). For resistant disease, other medications may be considered, including methotrexate, leflunomide, mycophenolate mofetil, tocilizumab, azathioprine, and anti-TNF agents. In late cases with irreversible arterial stenosis, revascularization may be considered if there are ischemic symptoms. Angioplasty is preferred to stent placement and can be effective in aortic stenosis to improve and ankle-brachial index and symptoms (85). Takayasu’s arteritis is chronic and progressive, with periods of remissions and exacerbations. Five-year survival is roughly 90% (86). Among patients who require revascularization, 20-year survival is nearly 75% (87).
Inflammatory aortic aneurysm
Inflammatory aortic aneurysm is a noninfectious entity, representing up to 10% of abdominal aortic aneurysms, distinguished from atherosclerotic aneurysms by the extent of peri-aortic fibrosis and mural thickening (88). The etiology of inflammatory aortic aneurysm is unknown, though roughly half of cases may be due to IgG4-related disease, and thus related to mediastinal or retroperitoneal fibrosis (89). Clinically, inflammatory aneurysm may present with back pain, weight loss, fatigue, and elevated erythrocyte sedimentation rate, more commonly in men (90). Classic imaging findings include a low density, mildly enhancing soft tissue mass anterolateral to and surrounding the calcified aortic wall (91). When rupture occurs, inflammatory aortic aneurysms may confer a higher operative mortality compared with atherosclerotic aneurysms (92). Inflammatory and atherosclerotic aneurysms are managed similarly, with either surgical or endovascular repair. Endovascular repair of inflammatory aneurysms can decrease early post-operative mortality rates, though persistent peri-aortic inflammation can lead to associated morbidity such as renal failure due to hydronephrosis (93).
Conclusions
Aortitis may be due to infectious or inflammatory causes, and primarily involves the vasa vasorum. All entities can result in nonspecific constitutional symptoms, and diagnosis can be elusive. Any localizing symptom should raise suspicion and prompt work up with cross sectional imaging, which can detect inflammation of the aortic wall and other findings suggesting particular diagnoses. Also, a careful history may reveal associated symptoms, such as headache in giant cell arteritis, or potential infectious sources in mycotic aneurysm. Differentiating infectious and inflammatory aortitis is essential, as infectious causes require intervenous antibiotics and surgical intervention whereas inflammatory aortitis is treated with corticosteroids. Early diagnosis is essential, as delays in treatment result in grave outcomes.
Acknowledgements
Funding: R Oklu acknowledges support from National Institutes of Health (R01-HL137193, R01-EB24403, R21-EB021148, R03-CA172738) and Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gornik HL, Creager MA. Aortitis. Circulation 2008;117:3039-51. [Crossref] [PubMed]
- Maeda H, Umezawa H, Goshima M, et al. Primary infected abdominal aortic aneurysm: surgical procedures, early mortality rates, and a survey of the prevalence of infectious organisms over a 30-year period. Surg Today 2011;41:346-51. [Crossref] [PubMed]
- Oderich GS, Panneton JM, Bower TC, et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg 2001;34:900-8. [Crossref] [PubMed]
- Parkhurst GF, Dekcer JP. Bacterial aortitis and mycotic aneurysm of the aorta; a report of twelve cases. Am J Pathol 1955;31:821-35. [PubMed]
- Sommerville RL, Allen EV, Edwards JE. Bland and infected arteriosclerotic abdominal aortic aneurysms: a clinicopathologic study. Medicine (Baltimore) 1959;38:207-21. [Crossref] [PubMed]
- Brown SL, Busuttil RW, Baker JD, et al. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1984;1:541-7. [Crossref] [PubMed]
- McCready RA, Bryant MA, Divelbiss JL, et al. Arterial infections in the new millenium: an old problem revisited. Ann Vasc Surg 2006;20:590-5. [Crossref] [PubMed]
- Stengel A, Wolferth CC. Mycotic (bacterial) aneurysms of intravascular origin. Arch Intern Med (Chic) 1923;31:527-54. [Crossref]
- Mandell G, Bennett J, Dolin R. Mandell, Douglas, And Bennett's Principles and Practice of Infectious Diseases. 7th edition. Churchill Livingstone, 2010.
- Roberts WC, Ko JM, Vowels TJ. Natural history of syphilitic aortitis. Am J Cardiol 2009;104:1578-87. [Crossref] [PubMed]
- Maleszewski JJ. Inflammatory ascending aortic disease: perspectives from pathology. J Thorac Cardiovasc Surg 2015;149:S176-83. [Crossref] [PubMed]
- Stone JR, Bruneval P, Angelini A, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol 2015;24:267-78. [Crossref] [PubMed]
- Buckmaster MJ, Curci JA, Murray PR, et al. Source of elastin-degrading enzymes in mycotic aortic aneurysms: bacteria or host inflammatory response? Cardiovasc Surg 1999;7:16-26. [Crossref] [PubMed]
- Okamoto T, Akaike T, Suga M, et al. Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem 1997;272:6059-66. [Crossref] [PubMed]
- Potempa J, Watorek W, Travis J. The inactivation of human plasma alpha 1-proteinase inhibitor by proteinases from Staphylococcus aureus. J Biol Chem 1986;261:14330-4. [PubMed]
- Garb M. Appendicitis: an unusual cause of infected abdominal aortic aneurysm. Australas Radiol 1994;38:68-9. [Crossref] [PubMed]
- Rubery PT, Smith MD, Cammisa FP, et al. Mycotic aortic aneurysm in patients who have lumbar vertebral osteomyelitis. A report of two cases. J Bone Joint Surg Am 1995;77:1729-32. [Crossref] [PubMed]
- Osler W. The Gulstonian Lectures, on Malignant Endocarditis. Br Med J 1885;1:467-70. [Crossref] [PubMed]
- Clarke JA. An X-Ray Microscopic Study of the Vasa Vasorum of the Normal Human Thoracic Aorta. Z Anat Entwicklungsgesch 1964;124:261-7. [Crossref] [PubMed]
- Solomon GF, Moos RH, Stone GC, et al. Peripheral Vasoconstriction Induced by Emotional Stress in Rats. Angiology 1964;15:362-5. [Crossref] [PubMed]
- Chan FY, Crawford ES, Coselli JS, et al. In situ prosthetic graft replacement for mycotic aneurysm of the aorta. Ann Thorac Surg 1989;47:193-203. [Crossref] [PubMed]
- Muller BT, Wegener OR, Grabitz K, et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg 2001;33:106-13. [Crossref] [PubMed]
- Reddy DJ, Ernst CB. Infected aneurysms. In: Rutherford RB. editor. Vascular Surgery. 4th edition. Philadelphia: WB Saunders, 1995:1139-53.
- Deipolyi AR, Bailin A, Khademhosseini A, et al. Imaging findings, diagnosis, and clinical outcomes in patients with mycotic aneurysms: single center experience. Clin Imaging 2016;40:512-6. [Crossref] [PubMed]
- Czum JM, Corse WR, Ho VB. MR angiography of the thoracic aorta. Magn Reson Imaging Clin N Am 2005;13:41-64. v. [Crossref] [PubMed]
- Deipolyi AR, Rho J, Khademhosseini A, et al. Diagnosis and management of mycotic aneurysms. Clin Imaging 2016;40:256-62. [Crossref] [PubMed]
- McGuigan EA, Sears ST, Corse WR, et al. MR angiography of the abdominal aorta. Magn Reson Imaging Clin N Am 2005;13:65-89. v-vi. [Crossref] [PubMed]
- Lee WK, Mossop PJ, Little AF, et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics 2008;28:1853-68. [Crossref] [PubMed]
- Murakami M, Morikage N, Samura M, et al. Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography–Computed Tomography for Diagnosis of Infected Aortic Aneurysms. Ann Vasc Surg 2014;28:575-8. [Crossref] [PubMed]
- Hollier LH, Money SR, Creely B, et al. Direct replacement of mycotic thoracoabdominal aneurysms. J Vasc Surg 1993;18:477-84; discussion 485. [Crossref] [PubMed]
- Moneta GL, Taylor LM Jr, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg 1998;175:396-9. [Crossref] [PubMed]
- Clagett GP, Valentine RJ, Hagino RT. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg 1997;25:255-66; discussion 267-70. [Crossref] [PubMed]
- Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg 2003;196:435-41. [Crossref] [PubMed]
- Vogt PR, Brunner-La Rocca HP, Carrel T, et al. Cryopreserved arterial allografts in the treatment of major vascular infection: a comparison with conventional surgical techniques. J Thorac Cardiovasc Surg 1998;116:965-72. [Crossref] [PubMed]
- Jones KG, Bell RE, Sabharwal T, et al. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg 2005;29:139-44. [Crossref] [PubMed]
- Semba CP, Sakai T, Slonim SM, et al. Mycotic aneurysms of the thoracic aorta: repair with use of endovascular stent-grafts. J Vasc Interv Radiol 1998;9:33-40. [Crossref] [PubMed]
- Jaffer U, Gibbs R. Mycotic thoracoabdominal aneurysms. Ann Cardiothorac Surg 2012;1:417. [PubMed]
- Kritpracha B, Premprabha D, Sungsiri J, et al. Endovascular therapy for infected aortic aneurysms. J Vasc Surg 2011;54:1259-65. [Crossref] [PubMed]
- Sörelius K, Mani K, Björck M. Endovascular Treatment of Mycotic Aortic Aneurysms: A European Multicenter Study. J Vasc Surg 2015;61:836. [Crossref]
- Lau C, Gaudino M, de Biasi AR, et al. Outcomes of open repair of mycotic descending thoracic and thoracoabdominal aortic aneurysms. Ann Thorac Surg 2015;100:1712-7. [Crossref] [PubMed]
- Oshima H, Yamamoto K, Komori K, et al. Usefulness of bridging thoracic endovascular aortic repair and sac irrigation followed by open repair in patients with mycotic thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2014;148:2422-4. [Crossref] [PubMed]
- Lopes RJ, Almeida J, Dias PJ, et al. Infectious thoracic aortitis: a literature review. Clin Cardiol 2009;32:488-90. [Crossref] [PubMed]
- Bardin JA, Collins GM, Devin JB, et al. Nonaneurysmal suppurative aortitis. Arch Surg 1981;116:954-6. [Crossref] [PubMed]
- Stephens CT, Pounds LL, Killewich LA. Rupture of a nonaneurysmal aorta secondary to Staphylococcus aortitis: a case report and review of the literature. Angiology 2006;57:506-12. [Crossref] [PubMed]
- Kanemitsu S, Shimono T, Nakamura A, et al. Molecular diagnosis of nonaneurysmal infectious aortitis. J Vasc Surg 2011;53:472-4. [Crossref] [PubMed]
- Vogel TR, Symons R, Flum DR. The incidence and factors associated with graft infection after aortic aneurysm repair. J Vasc Surg 2008;47:264-9. [Crossref] [PubMed]
- Cernohorsky P, Reijnen MM, Tielliu IF, et al. The relevance of aortic endograft prosthetic infection. J Vasc Surg 2011;54:327-33. [Crossref] [PubMed]
- Murphy EH, Szeto WY, Herdrich BJ, et al. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg 2013;58:1179-85. [Crossref] [PubMed]
- Heyer KS, Modi P, Morasch MD, et al. Secondary infections of thoracic and abdominal aortic endografts. J Vasc Interv Radiol 2009;20:173-9. [Crossref] [PubMed]
- Smeds MR, Duncan AA, Harlander-Locke MP, et al. Treatment and outcomes of aortic endograft infection. J Vasc Surg 2016;63:332-40. [Crossref] [PubMed]
- Modrall JG, Clagett GP. The role of imaging techniques in evaluating possible graft infections. Semin Vasc Surg 1999;12:339-47. [PubMed]
- Liberatore M, Iurilli AP, Ponzo F, et al. Clinical usefulness of technetium-99m-HMPAO-labeled leukocyte scan in prosthetic vascular graft infection. J Nucl Med 1998;39:875-9. [PubMed]
- O’Connor S, Andrew P, Batt M, et al. A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 2006;44:38-45. e8.
- Ducasse E, Calisti A, Speziale F, et al. Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg 2004;18:521-6. [Crossref] [PubMed]
- Capoccia L, Speziale F, Menna D, et al. Preliminary Results from a National Enquiry of Infection in Abdominal Aortic Endovascular Repair (Registry of Infection in EVAR-RI EVAR). Ann Vasc Surg 2016;30:198-204. [Crossref] [PubMed]
- Miller DV, Maleszewski JJ. The pathology of large-vessel vasculitides. Clin Exp Rheumatol 2011;29:S92-8. [PubMed]
- Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med 2003;349:160-9. [Crossref] [PubMed]
- Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum 2011;63:633-9. [Crossref] [PubMed]
- Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum 2009;61:1454-61. [Crossref] [PubMed]
- Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129-34. [Crossref] [PubMed]
- Lupi-Herrera E, Sanchez-Torres G, Marcushamer J, et al. Takayasu's arteritis. Clinical study of 107 cases. Am Heart J 1977;93:94-103. [Crossref] [PubMed]
- Koide K. Takayasu arteritis in Japan. Heart Vessels Suppl 1992;7:48-54. [Crossref] [PubMed]
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-8. [Crossref] [PubMed]
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001;45:140-5. [Crossref] [PubMed]
- Cavazza A, Muratore F, Boiardi L, et al. Inflamed temporal artery: histologic findings in 354 biopsies, with clinical correlations. Am J Surg Pathol 2014;38:1360-70. [Crossref] [PubMed]
- Ashton-Key MR, Gallagher PJ. False-negative temporal artery biopsy. Am J Surg Pathol 1992;16:634-5. [Crossref] [PubMed]
- Schmidt WA, Kraft HE, Vorpahl K, et al. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997;337:1336-42. [Crossref] [PubMed]
- Brack A, Martinez-Taboada V, Stanson A, et al. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999;42:311-7. [Crossref] [PubMed]
- Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012;71:1170-6. [Crossref] [PubMed]
- Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003;48:3522-31. [Crossref] [PubMed]
- Muratore F, Kermani TA, Crowson CS, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology (Oxford) 2015;54:463-70. [Crossref] [PubMed]
- Khan A, Dasgupta B. Imaging in Giant Cell Arteritis. Curr Rheumatol Rep 2015;17:52. [Crossref] [PubMed]
- Flamm SD, White RD, Hoffman GS. The clinical application of 'edema-weighted' magnetic resonance imaging in the assessment of Takayasu's arteritis. Int J Cardiol 1998;66 Suppl 1:S151-9; discussion S161.
- Blockmans D, Stroobants S, Maes A, et al. Positron emission tomography in giant cell arteritis and polymyalgia rheumatica: evidence for inflammation of the aortic arch. Am J Med 2000;108:246-9. [Crossref] [PubMed]
- Yamada I, Shibuya H, Matsubara O, et al. Pulmonary artery disease in Takayasu’s arteritis: angiographic findings. AJR Am J Roentgenol 1992;159:263-9. [Crossref] [PubMed]
- Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med 1994;120:919-29. [Crossref] [PubMed]
- Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol 2004;16:31-7. [Crossref] [PubMed]
- Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore) 2009;88:221-6. [Crossref] [PubMed]
- Michel BA, Arend WP, Hunder GG. Clinical differentiation between giant cell (temporal) arteritis and Takayasu's arteritis. J Rheumatol 1996;23:106-11. [PubMed]
- Hunder GG, Sheps SG, Allen GL, et al. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med 1975;82:613-8. [Crossref] [PubMed]
- Nesher G, Berkun Y, Mates M, et al. Low-dose aspirin and prevention of cranial ischemic complications in giant cell arteritis. Arthritis Rheum 2004;50:1332-7. [Crossref] [PubMed]
- Kermani TA, Warrington KJ, Crowson CS, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis 2013;72:1989-94. [Crossref] [PubMed]
- Kerr GS. Takayasu’s arteritis. Rheum Dis Clin North Am 1995;21:1041-58. [PubMed]
- Sueyoshi E, Sakamoto I, Hayashi K. Aortic aneurysms in patients with Takayasu's arteritis: CT evaluation. AJR Am J Roentgenol 2000;175:1727-33. [Crossref] [PubMed]
- Rao SA, Mandalam KR, Rao VR, et al. Takayasu arteritis: initial and long-term follow-up in 16 patients after percutaneous transluminal angioplasty of the descending thoracic and abdominal aorta. Radiology 1993;189:173-9. [Crossref] [PubMed]
- Hall S, Barr W, Lie JT, et al. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore) 1985;64:89-99. [Crossref] [PubMed]
- Miyata T, Sato O, Koyama H, et al. Long-term survival after surgical treatment of patients with Takayasu’s arteritis. Circulation 2003;108:1474-80. [Crossref] [PubMed]
- Walker DI, Bloor K, Williams G, et al. Inflammatory aneurysms of the abdominal aorta. Br J Surg 1972;59:609-14. [Crossref] [PubMed]
- Kasashima S, Zen Y, Kawashima A, et al. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol 2008;32:197-204. [Crossref] [PubMed]
- Zaheer A. Inflammatory aortic aneurysm. Pearls and Pitfalls in Cardiovascular Imaging: Pseudolesions, Artifacts, and Other Difficult Diagnoses. Cambridge University Press, 2015:206-9.
- Iino M, Kuribayashi S, Imakita S, et al. Sensitivity and specificity of CT in the diagnosis of inflammatory abdominal aortic aneurysms. J Comput Assist Tomogr 2002;26:1006-12. [Crossref] [PubMed]
- Pennell RC, Hollier LH, Lie JT, et al. Inflammatory abdominal aortic aneurysms: a thirty-year review. J Vasc Surg 1985;2:859-69. [Crossref] [PubMed]
- Maeda H, Umezawa H, Hattori T, et al. Early and late outcomes of inflammatory abdominal aortic aneurysms: comparison with the outcomes after open surgical and endovascular aneurysm repair in literature reviews. Int Angiol 2013;32:67-73. [PubMed]


