Advanced endografting techniques: snorkels, chimneys, periscopes, fenestrations, and branched endografts
Introduction
Endovascular aneurysm repair (EVAR) has revolutionized the treatment of abdominal aortic aneurysms (AAA). Multiple randomized trials have shown EVAR to have lower rates of morbidity and mortality when compared with open surgical AAA repair in the early perioperative period. The EVAR 1 trial and DREAM studies have shown both modalities to be equivalent after 2 years (1,2). However, the 15-year EVAR 1 data reveals that after 8 years, both total mortality and aneurysm-related mortality were higher in the EVAR group than in the open repair group. After 8 years, total mortality was 53% in the EVAR group and 46% in the open repair group (P=0.048). The aneurysm-related mortality was 5% in the EVAR group and 1% in the open repair group (P=0.0064) (3). This increased mortality was attributed to long-term aneurysm sac enlargement in the EVAR group compared to the open repair group, a fact that highlights the importance of long-term patient monitoring. Nevertheless, EVAR remains a viable option for most patients with AAA.
Technical challenges
The anatomy of the aneurysm from the proximal neck to the access vessels may create technical challenges for endovascular repair. Upwards of 30% of patients with AAAs have unsuitable proximal neck morphology for conventional endovascular repair (4-7). Anatomies considered unsuitable for conventional infrarenal stent grafting include short or absent necks, angulated necks, conical necks, or large necks exceeding size availability for current stent grafts.
A number of advanced endovascular techniques and devices have been developed to circumvent these challenges. These include snorkeling procedures such as chimneys, periscopes, and sandwich techniques; “homemade” or “back-table” fenestrated endografts as well as manufactured, customized fenestrated endografts; and more recently, physician modified branched devices. Furthermore, new devices in the pipeline are under investigation, such as “off-the-shelf” fenestrated stent grafts, branched stent grafts, lower profile devices, and novel sealing designs, have the potential of solving many of the aforementioned problems.
Any number of technical challenges may arise during standard endovascular repair of aortic aneurysms. Careful study of the preoperative imaging is crucial in planning these procedures and anticipating these potential difficulties. Evaluation of the proximal neck for length, diameter, calcifications, thrombus, and degree of angulation is often the first step in the planning process. Assessment of the access vessels is an absolute necessity to determine if the patient’s arteries will accommodate the large sheaths and devices. This will also determine whether a purely percutaneous approach is possible or whether femoral cut-down, or surgical conduit, is needed to deliver the device. Recently, the development of simulators and 3D printing has allowed specialists to rehearse procedures using the patient’s uploaded imaging studies (8). Moreover, one potentially challenging step in endovascular aortic aneurysm repairs is the cannulation of the contralateral gate. Knowledge of the patient’s aneurysm anatomy and planning the placement of the contralateral gate within the aneurysm lumen will facilitate cannulation. At times, a variety of wires and catheters may be necessary to successfully cannulate the gate. Rarely, a wire may need to be snared either from the ipsilateral access or from a brachial access site.
Snorkeling and chimney technique
“Snorkeling” or “chimney” techniques (so called Ch-EVAR) describe the placement of a covered stent into a branch vessel with the proximal part of the stent extending above the proximal edge of the main aortic stent graft (Figure 1). This technique allows for more proximal placement of the aortic stent graft in short necked thoracic or AAAs while maintaining perfusion to the viscera and kidneys. Ch-EVAR was initially described as a bail-out procedure when the renal arteries were inadvertently covered during conventional EVAR (9). Limitations of the Ch-EVAR technique include loss of wall apposition with resultant gutters increasing the chance of type 1 endoleaks. Furthermore, chimney stents may become kinked, compressed, or occluded.
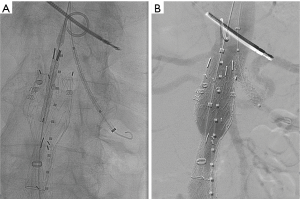
However, the procedure has evolved into a safe and effective adjunctive technique to repair abdominal and thoracoabdominal aneurysms with unsuitable anatomies. The procedure involves gaining access for both the main aortic device as well as the target renal or visceral arteries for the chimney device. This often requires bilateral femoral artery access for the main aortic device, unilateral or bilateral arm access, or surgical conduit to facilitate catheterization of the renal and/or visceral arteries. Therefore, accurate pre-procedural planning is crucial to determine the planned seal zone and identify the branch vessels to be covered by the main stent graft.
There are multiple variations by which Ch-EVAR is performed but the overall strategy remains the same. The branch vessel to be stented is cannulated from the arm access and a 6 to 8 F sheath advanced into the vessel. Through this, a balloon expandable or self-expanding covered stent is advanced. Then, the main aortic device is delivered from the conventional femoral approach and deployed across the visceral vessel sheaths. The chimney stent graft can then be adjusted as needed and then deployed. Molding balloons are simultaneously inflated in the main aortic stent graft and the chimney stent in a kissing balloon fashion. Guidewire access should be maintained until the remainder of the aortic stent graft is deployed and completion angiograms performed.
The “hypogastric snorkel” technique similarly involves placement of a covered stent parallel to an iliac limb device to preserve perfusion to the hypogastric artery in order to extend the distal seal zone in short common iliac arteries or in cases of common iliac artery aneurysms (Figure 2). This technique can be performed bilaterally or after embolization of one of the hypogastric arteries. The goal of this technique is to reduce the risk of complications from bilateral hypogastric artery embolization such as buttock necrosis, bowel ischemia, and neurological deficits.
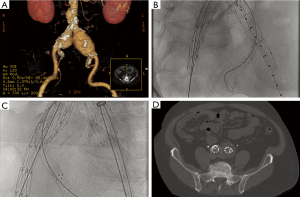
Whether the use of balloon expandable chimney stents achieves greater patency compared with self-expanding chimney stents remains a point of debate. While balloon expandable stents provide greater radial force and radiographic visibility, self-expanding stents offer increased flexibility. One small study did show an increased tendency for type I endoleak with the use of self-expanding stents compared with balloon expandable stents, but no difference in technical success or patency during follow-up (10). Further, the use of bare metal stents can be adequate when a minimal sealing segment just below the chimney stent is available such as in a conical-shaped neck. However, in all other cases, covered stents seem superior from a sealing standpoint (11).
The Ch-EVAR technique has been shown to be feasible in treating aneurysms with challenging neck anatomy with good results (12-14). The Pericles registry retrospectively reviewed and analyzed 898 chimney grafts in 517 patients treated by Ch-EVAR from 2008 to 2014. At a mean follow-up of 17.1 months, primary patency was 94%, secondary patency was 95.3%, and overall survival of patients was 79%. Intraoperative type Ia endoleak was noted in 41 (7.9%) of patients with follow up imaging showing resolution in all but 2 of these patients. The registry also suggested that type Ia endoleaks can be minimized by utilizing at least 20 mm of landing zone. Additionally, a majority of intraoperatively detected type Ia endoleaks were resolved with prolonged kissing balloon inflation or additional cuff placement (15).
Periscope or reverse chimney technique
A “periscope” or “reverse chimney” technique describes placement of the covered stent below the distal edge of the main aortic stent graft. This facilitates the extension of the distal seal zone in thoracoabdominal or abdominal aneurysms.
Sandwich technique
The “sandwich” technique involves placement of a covered stent positioned between two aortic main body components in order to maintain side branch perfusion in mid-graft position. Novel approaches have been described utilizing a combination of chimney grafts and periscopes with and without sandwiching the grafts with a bridging graft in the treatment of thoracoabdominal aneurysms (TAAA) (16).
Fenestrated stent grafts
Fenestrated stent graft devices offer a unique solution to juxtarenal aortic aneurysms to prevent proximal endoleaks, stent graft migration, and subsequent aneurysm rupture. Radiopaque markers outline the fenestrations, scallops, and mark the anterior and posterior positions of the stent graft to facilitate accurate positioning and cannulation. Pre-cannulation of the aortic branch vessels with wires prior to device delivery may be done to align the graft before deploying the stent. After the fenestrated device is deployed, the renal and visceral branches are cannulated in order to deliver covered stents into the branch arteries. To achieve this, a large sheath is advanced in the contralateral groin access and parallel smaller sheaths advanced into the branch vessels. At our institutions, we utilize the Gore® Dryseal sheath (W. L. Gore, Flagstaff, AZ, USA) to facilitate delivery of parallel Cook® Ansel II sheaths (Cook Medical, Bloomington, IN, USA), which are advanced into the renal arteries. Either the Atrium iCastTM (Atrium Medical, Hudson, NH, USA) or the Gore® VBX (W. L. Gore, Flagstaff AZ, USA) covered balloon expandable stents are delivered into the renal arteries and post-dilated. This is followed by delivery of the distal bifurcated stent graft device and iliac extensions as needed.
An advantage of fenestrated endografts is the lack of the gutters created by chimney grafts, lessening the likelihood of type 1 endoleaks. Currently, customized fenestrated devices require several weeks to be manufactured and thus are not immediately available in the acute setting. “Off-the-shelf” fenestrated grafts are currently in development and will help mitigate this disadvantage. As an interim solution, many physicians have adopted creating fenestrations and scallop themselves on main body grafts (Figure 3).
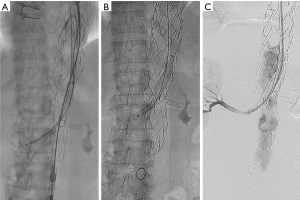
The data for FEVAR is encouraging. A multicenter trial in the United States evaluated 30 patients and a total of 77 visceral vessels treated with FEVAR. There was a 100% technical success rate with 20% overall mortality at 24 month follow-up (17). A more recent review comparing Ch-EVAR and FEVAR consisted of 542 patients who underwent FEVAR and demonstrated an advantage over the Ch-EVAR cohort with respect to 30-day mortality, late mortality, and renal adverse events. However, the FEVAR group demonstrated longer mean operative time and greater blood loss. The FEVAR cohort exhibited 1.1% 30-day mortality, 98.8% technical success rate, and 5.35% late mortality with a mean follow-up of 12.8 months. A total of 58 secondary interventions were performed in this FEVAR cohort, some of which were related to endoleaks, target vessel occlusion or stenosis, limb occlusion or stenosis, and excess bleeding or hematoma. The rates of endoleaks were 5.35%, 12.7%, and 2.4% for type I, type II, and type III respectively (18).
At the moment, there is limited data evaluating the safety and efficacy of physician modified fenestrated devices. An interim analysis from an investigational device exemption trial published by Starnes et al evaluated the results of 59 patients over a 50-month time period. The study showed a 95% rate of sac stability or regression and minimal rate of endoleak. Furthermore, there was 94% freedom from aneurysm related mortality at the 4-year mark. The midterm data are favorable and suggestive that treatment with physician modified fenestrated endografts are a safe and efficacious option (19).
Branched stent grafts
For aortic aneurysm anatomies not amenable to fenestrated repair, a physician modified branched endograft may be a suitable option. The pre-procedure planning for a branched stent graft is imperative for technical success. Branched devices require an aneurysm diameter large enough to accommodate the total diameters of the main body graft as well as the branches. On the day of the procedure, the stent graft is unsheathed on a sterile field on a back table and fenestrations are cut into the stent graft followed by attachment of a covered stent at each fenestration to serve as branches to the renal and visceral arteries. Radiopaque markers are sewn in at the origins of the branches and the anterior and posterior position of the stent graft to facilitate in-situ positioning. The modified device is re-sheathed and deployed in the patient after appropriate positioning is confirmed. The branched components and target vessels are then cannulated, followed by deployment of a bridging covered stent, extending into the target vessel (Figure 4).
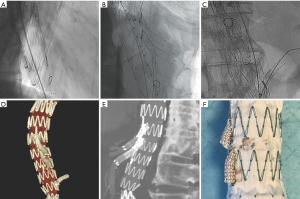
Similar to fenestrated endografts, branched endografts maintain visceral, renal and hypogastric artery preservation without using adjunctive techniques such as placing chimney or snorkel stents. This gives the interventionalist additional options which allow them to treat increasingly complex aortic anatomy and pathology.
Directional branched devices
Some of the more novel options are “off-the-shelf” branched devices also known as directional branched devices. Like their earlier physician modified counterparts, the directional branched devices, used mainly for treatment of TAAAs, have a main body device with small visceral and renal branches that are extended with covered stents for flow preservation. Depending on the stent graft type, there are a variety of orientations and attachment methods for the branch components.
Cook® Zenith® t-Branch® TAAA Endograft device has caudally orientated branches. The Cook® Zenith® t-Branch® device is currently approved for use in Europe in the treatment of TAAA (Figure 5). In contrast, Gore’s® EXCLUDER® Thoracoabdominal Branch Endoprosthesis (TAMBE) is designed with cranial orientation for its renal branches. These branched directional devices are currently under investigational use in the United States. Gore’s TAAA directional branch is under clinical trial in Brazil and the United states with early cases reports showing 100% technical success (Clinicaltrials.gov ID: NCT02528500) (20). Similarly, Medtronic® and Sanford Vascular Innovations® are conducting trials for their directional branch graft within the United States (Clinincaltrails.gov ID: NCT02294435). The Medtronic® thoracoabdominal device has a thoracic flow divider component with one limb used to extend the graft to the iliacs and the other limb made up of smaller branches that are extended to their respective target branch vessels.
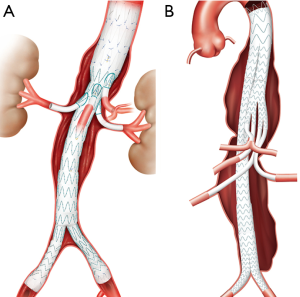
The potential advantage of these off the shelf branched devices is easy availability and shorter procedure times as the back table modification is no longer required. Additionally, it allows treating a longer aneurysmal aortic segment with preservation of key visceral and renal branches. However, since these devices result in increased aortic coverage there is a theoretical increase in the risk of spinal cord ischemia (21).
Similarly, endografts with iliac branches help in maintaining flow within the hypogastric arteries, precluding the need for embolization prior to stent placement or utilization of the snorkel technique (Figure 5). Furthermore, branched iliac devices allow for a better distal seal in the setting diseased common iliac arteries. Several companies have developed branched devices that allow stent graft deployment into both branches of the common iliac artery. Currently the Gore® Excluder® Iliac Branch Endoprosthesis (Figure 6) is approved for use in the United States. COOK® Zenith® Branch Endovascular Graft—Iliac Bifurcation, is approved for use in Europe but is still under investigation in the United States.
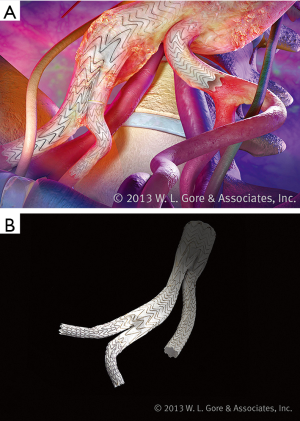
The benefit of the off the shelf iliac branched devices is it mitigates the risks associated with hypogastric embolization and gutter leaks associated with snorkel. However, since it represents another modular component there is theoretically an additional site for a type Ib or III endoleak.
Early data regarding the branched iliac devices has been promising. The US GORE Pivotal trial showed >95% patency, 100% freedom from type I and III endoleaks, and a 3% reintervention rate at 6 months. No growth of the common iliac artery aneurysm, aneurysm related death, or aneurysm rupture was demonstrated (22). Similar results were obtained in the Global Registry for Endovascular Aortic Treatment (GREAT) (23). In a single-center prospective investigational device exemption study of 130 patients, The Zenith branched device demonstrated a 94% rate of technical success and a branch patency of 94.6% at 30 days and 81.8% at 5 years. The same study quoted a freedom from device related endoleak of 96% at 5 years and a 9% reintervention rate. The study noted 67% iliac sac regression with no cases of sac growth (24). Short term data are reassuring and have allowed the availability of yet another tool within the armamentarium of the treating physician.
Endovascular aneurysm sealing (EVAS)
In addition to previously described EVAR techniques, the Nellix® EVAS (Endologix, Irvine, CA, USA) system uses two balloon-expandable stainless steel stents surrounded by a non-porous polyfluoroethylene (PTFE) endobag designed to fill the aortic aneurysm sac to exclude the aneurysm. A polyethylene glycol (PEG)-based biocompatible and BioStable solution fills the endobag to seal the aortic aneurysm, with curing times less than 10 minutes. The multicenter Nellix FORWARD-IDE Study which included 150 patients revealed a 100% technical success rate, a 2.7% 30-day major adverse event rate, a 1-year 94% treatment success rate at 1 year, and a 1-year 3.1% endoleak rate (25).
Furthermore, in cases where proximal landing zones are inadequate, EVAS can be combined with chimney stents, the so-called chEVAS procedure. Youssef et al. presented 15 patients with failed EVAR who underwent EVAS for progressive aortic aneurysm sac growth due to endoleak. Ten of these 15 patients underwent chEVAS due to inadequate landing zones. The procedure was technically successful in 100% of the cases. No reinterventions, aneurysm-related mortalities, graft thrombosis, endoleaks, or chimney graft occlusions were observed during a median follow-up of 8 months (26).
Conclusions
The treatment of aortic aneurysms continues to evolve, further expanding the population of patients that can be treated with an endovascular approach. As the technology grows so do the number of challenging aortic anatomies that endovascular specialists take on, further pushing the envelope in the arena of aortic repair.
Acknowledgements
Thank you to Drs. Wesley Lew and Kevin Patel for providing back table pictures of a modified branched device for Figure 4.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1881-9. [Crossref] [PubMed]
- United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. [Crossref] [PubMed]
- Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. [Crossref] [PubMed]
- Abbruzzese TA, Kwolek CJ, Brewster DC, et al. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg 2008;48:19-28. [Crossref] [PubMed]
- Leurs LJ, Kievit J, Dagnelie PC, et al. Influence of infrarenal neck length on outcome of endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2006;13:640-8. [Crossref] [PubMed]
- AbuRahma AF, Campbell J, Stone PA, et al. The correlation of aortic neck length to early and late outcomes in endovascular aneurysm repair patients. J Vasc Surg 2009;50:738-48. [Crossref] [PubMed]
- AbuRahma AF, Campbell J, Stone PA, et al. Early and late clinical outcomes of endovascular aneurysm repair in patients with an angulated neck. Vascular 2010;18:93-101. [Crossref] [PubMed]
- Itagaki MW. Using 3D printed models for planning and guidance during endovascular intervention: a technical advance. Diagn Interv Radiol 2015;21:338-41. [Crossref] [PubMed]
- Greenberg RK, Clair D, Srivastava S, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg 2003;38:990-6. [Crossref] [PubMed]
- Donas KP, Pecoraro F, Torsello G, et al. Use of covered chimney stents for pararenal aortic pathologies is safe and feasible with excellent patency and low incidence of endoleaks. J Vasc Surg 2012;55:659-65. [Crossref] [PubMed]
- Bruen KJ, Feezor RJ, Daniels MJ, et al. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg 2011;53:895-904; discussion 904-5. [Crossref] [PubMed]
- Ohrlander T, Sonesson B, Ivancev K, et al. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther 2008;15:427-32. [Crossref] [PubMed]
- Sugiura K, Sonesson B, Akesson M, et al. The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg (Torino) 2009;50:475-81. [PubMed]
- Moulakakis KG, Mylonas SN, Avgerinos E, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg 2012;55:1497-503. [Crossref] [PubMed]
- Donas KP, Lee JT, Lachat M, et al. Collected world experience about the performance of the snorkel/chimney endovascular technique in the treatment of complex aortic pathologies: the PERICLES registry. Ann Surg 2015;262:546-53; discussion 552-3. [Crossref] [PubMed]
- Lobato AC, Camacho-Lobato L. Endovascular treatment of complex aortic aneurysms using the sandwich technique. J Endovasc Ther 2012;19:691-706. [Crossref] [PubMed]
- Greenberg RK, Sternbergh WC 3rd, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg 2009;50:730-7.e1. [Crossref] [PubMed]
- Li Y, Hu Z, Bai C, et al. Fenestrated and Chimney Technique for Juxtarenal Aortic Aneurysm: A Systematic Review and Pooled Data Analysis. Sci Rep 2016;6:20497. [Crossref] [PubMed]
- Starnes BW, Heneghan RE, Tatum B. Midterm results from a physician-sponsored investigational device exemption clinical trial evaluating physician-modified endovascular grafts for the treatment of juxtarenal aortic aneurysms. J Vasc Surg 2017;65:294-302. [Crossref] [PubMed]
- Oderich GS, Silveira PG. Initial Experience With the GORE® EXCLUDER® Thoracoabdominal Branch Endoprosthesis. Endovascular Today 2016;15:12-6.
- Resch TA. Current and Future Endovascular Treatment Options for TAAAs. Endovascular Today 2016;15:59-62.
- Schneider DB, Matsumura JS, Lee JT, et al. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the Gore Iliac Branch Endoprosthesis. J Vasc Surg 2017;66:775-85. [Crossref] [PubMed]
- Ellozy S, Schneider D, Mastumura J. Outcomes at One Year With the Gore Excluder Iliac Branch Endoprosthesis. J Vasc Surg 2016;65:832. [Crossref]
- Wong S, Greenberg RK, Brown CR, et al. Endovascular repair of aortoiliac aneurysmal disease with the helical iliac bifurcation device and the bifurcated-bifurcated iliac bifurcation device. J Vasc Surg 2013;58:861-9. [Crossref] [PubMed]
- Holden A. Clinical outcomes after Nellix Endovascular Aneurysm Sealing. Semin Vasc Surg 2016;29:102-5. [Crossref] [PubMed]
- Youssef M, Zerwes S, Jakob R, et al. Endovascular Aneurysm Sealing (EVAS) and Chimney EVAS in the Treatment of Failed Endovascular Aneurysm Repairs. J Endovasc Ther 2017;24:115-20. [Crossref] [PubMed]


