The role of Lp-PLA2 and biochemistry parameters as potential biomarkers of coronary artery disease in Asian South-Indians: a case-control study
Introduction
Coronary artery disease (CAD) is the leading cause of death and disability worldwide (1,2). Inflammation plays a key role in CAD and the various phases of atherosclerotic plaque development and rupture (3,4). Lipoprotein associated phospholipase A2 (Lp-PLA2) is a proinflammatory enzyme that is expressed in tissues that contain large numbers of macrophages. While in circulation, Lp-PLA2 [also known as platelet activating factor-acetylhydrolase (PAF-AH)] associates with diverse lipoproteins including high and low density lipoproteins (HDL and LDL) (3). Both Lp-PLA2 activity and mass (concentration) are associated with CAD as well as cerebrovascular outcomes (4). Several large studies including ARIC, WOSCOPS, MONICA, and Rotterdam have demonstrated that Lp-PLA2 is an independent predictor of CAD (5).
South Asians make up 1/5th of the world population and Asian Indians are a majority in this group (6). India has an estimated 31.8 million people with CAD, and the incidence of CAD in this population is expected to grow. However, there is very little data on the association of Lp-PLA2 with CAD and atherogenic dyslipidemia prevalent in this population (7). The objective of our study was to evaluate the relationship between Lp-PLA2 levels and CAD; compare its utility with established and emerging biomarkers of cardiac risk in an Indian population.
Methods
Study subjects
We conducted a cross-sectional case-control study at three centers in South India encompassing both urban and rural populations. Two groups of subjects were enrolled: a CAD group and an age and gender matched normal control group. Subjects in the CAD group were enrolled if they had acute coronary syndrome (ACS), were ≥21 years of age and presented themselves within 12 hours from the onset of symptoms. The CAD group subjects were enrolled from the emergency rooms of each the three study centers. Subjects were excluded if they had used a statin drug >7 days prior to enrolment, had known familial dyslipidemia, renal insufficiency (creatinine clearance <50 mL/min), or significant obstructive or restrictive lung disease. Healthy controls were age and gender matched with the CAD group. Control subjects were excluded if they had a known history of heart disease, lung disease, stroke, diabetes mellitus, hypertension and current or prior tobacco/alcohol use. Over the 4-year follow-up period after initial enrollment, the subjects in the control group did not develop any symptoms of CAD. Written informed consents in the subject’s native language were obtained from all subjects. The Institutional Ethics Committee (IEC) at each enrolling center independently approved the study protocol.
Laboratory analysis
Blood samples were collected from CAD group subjects within an hour of the presentation at the emergency ward, prior to the administration of the loading dose of statins or antiplatelet agents. Fasting blood samples (12 hours fasting, morning sample) were collected from control group subjects. Samples were centrifuged at 4 °C for 10 minutes at 3,000 rpm and the blood serum was separated and aliquots were stored at −40 °C. Lp-PLA2 concentration was determined using FDA approved, PLAC Test ELISA kit (diaDexus Inc., San Francisco, CA, USA) using a sandwich ELISA method with monoclonal antibodies (2C10 & 4B4 tagged with HRP) raised against Lp-PLA2. Colorimetric detection of the enzymatic turnover of the substrate tetramethylbenzidine (TMB) was done at 450 nm. Lp-PLA2 activity was measured using FDA approved PLAC Test Activity assay (diaDexus, Inc.). This method follows the rate of formation of 4-nitrophenol that is coloured reaction product formed when Lp-PLA2 enzyme present in the serum hydrolyses the sn-2 position of the substrate 1-myristoyl-2-(4-nitrophenyl succinyl) phosphatidylcholine.
The lipid profile parameters, ApoA1, ApoB, Lipoprotein(a) [Lp(a)], high sensitivity C-reactive protein (hs-CRP) and FBS were evaluated in Beckman Coulter AU 480 biochemistry analyzer, using commercially available Diasys kits (DiaSys Diagnostic systems GmbH, Holzheim, Germany). The principle for detection was immunoturbidimetry for ApoA1 with intra-assay coefficient of variability (CV) 1.7–3.7% and inter-assay CV 1.6–2.4% and ApoB (CV 2.2–2.6% and 1.8–3.5%). Lp(a) (CV 1.0–2.0% and 2.0–3.0%) and hs-CRP (CV 1.3–2.7% and 1.0–1.3%) was detected using the principle of particle enhanced immunoturbidimetry (8). Fasting blood glucose (FBS) was analysed using an enzymatic photometric method using glucose oxidase-Peroxidase. Total cholesterol (TC) was analysed by enzymatic photometric method using cholesterol esterase, cholesterol oxidase, and peroxidase. HDL cholesterol (HDL-C) was measured by direct immunoenzymatic photometric method; LDL cholesterol (LDL-C) by direct selective enzymatic photometric method and triglycerides (TG) by photometric enzymatic method using glycerol-3-phosphate and oxidase. Creatinine was measured in CAD group using modified Jaffe’s method.
Echocardiogram
Transthoracic echocardiographic examinations were performed with commercially available systems (Vivid E9 XD Clear echo machine, GE Healthcare, Horton, Norway, equipped with M4S transducer and BT13 software or Philips iE33, Philips Medical Systems, Norway). The data were stored in a DICOM format and analysed offline (EchoPac version113, GE Healthcare, Horten, Norway).
Angiographic analysis
Invasive coronary angiography (CAG) was performed in the CAD group (Philips Allura clarity FD lab or Artis Zee Siemens lab) by experienced operators. Diameters of reference and stenotic coronary arteries were measured by a computer assisted quantitative method. CAD was defined as ≥70% stenosis in a major epicardial coronary artery, ≥50% in the left main coronary artery, and/or fractional flow reserve <0.8.
Statistical methods
Since the study had case-control matched pair design, continuous variables with skewed distribution (assessed by D’Agostino-Pearson test) were compared with Wilcoxon rank sum test, and those with normal distribution were compared by paired student t-test (9). To study the correlation between Lp-PLA2, lipids and other cardiac biochemistry parameters in subjects with CAD, Spearman’s Rho (ρ) or Pearson’s r correlation was computed depending on skewed or normal distribution of data respectively.
Association with risk for CAD
Cox regression method was used to determine crude univariate Hazard ratios of association between Lp-PLA2, lipid profile and other cardiac biochemistry parameters with the event of CAD [as also suggested by Pearce (10)]. All variables with P<0.25 in the univariate analysis, were then selected for multivariate analysis. After testing and accounting for variables with significant interactions, a final Cox proportionality hazards model using backward conditional method was created to provide final adjusted Hazard ratios while eliminating confounding variables. Receiver operating characteristic (ROC) analysis was performed to examine the discriminative ability of the finally selected variables in identifying cases from healthy controls and Youden index criterion was used to determine variable’s cut off with optimum balance of sensitivity and specificity. Two-tailed α<0.05 was set as significant beforehand. Continuous data with normal and skewed distribution was reported as mean (SD, standard deviation) and median (IQR, inter quartile range) respectively, and numbers reported as n (%). All the nonparametric tests were conducted using MedCalc version 15 (MedCalc software, Ostend, Belgium) and all other tests performed using SPSS statistical software version 24.0 (IBM Corporation, Armonk, New York, USA) and results have been reported with 95% confidence interval wherever appropriate.
Results
We enrolled a total of 200 subjects (mean age 50.7±9.6 years, 87.5% males), of which, 97 qualified the inclusion criteria in the CAD group and 92 in the control group. Finally, 83 subjects completed the study in the CAD group (mean age 51±8.9 years, 85% males) and 83 subjects in the control group (mean age 50±8.9) years, 86.5% males). Case-control pairs with complete data for both participants were identified for the statistical analysis. In the CAD group (n=83), 34 (41%) subjects were diabetic, 24 (29%) hypertensive, 45 (54%) current smokers and 39 (47%) had hyperlipidemia. Further, 71 (86%) subjects were non-vegetarians and 16 (19%) were consuming alcohol on a daily/weekly basis. Baseline demographic and laboratory parameters of subjects are presented in Table 1. Compared with the control group, the CAD group had significantly higher levels of Lp-PLA2 concentration (mean, 342 vs. 261.8 ng/mL; median, 253 vs. 246 ng/mL), hs-CRP (mean, 5.1 vs. 2.6 mg/L; median, 2.4 vs. 1.6 mg/L) and ApoB/ApoA1 (mean, 0.76 vs. 0.68; median, 0.77 vs. 0.68) but lower Lp-PLA2 activity (mean, 151.7 vs. 165.4 nmol/min/mL; median, 154 vs. 166 nmol/min/mL) and Apo A1 (mean, 113.9 vs. 136.2 mg/dL; median, 115 vs. 136 mg/dL).
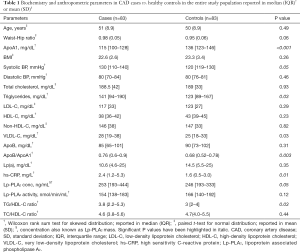
Full table
Univariate Cox regression analysis revealed that ApoB/ApoA1 ratio, hs-CRP, TG/HDL-C ratio and Lp-PLA2 concentration were associated (P≤0.05) with increased hazard of the event of CAD, while, ApoA1 was associated with increased survival (Table 2). Variables with P value <0.25 from the univariate analysis were considered for multivariate analysis. The final multivariate model included ApoA1 and Lp-PLA2 concentration (Table 3).
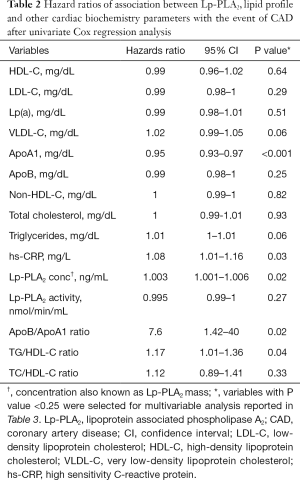
Full table
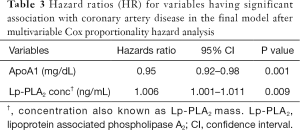
Full table
Lp-PLA2 and CAD
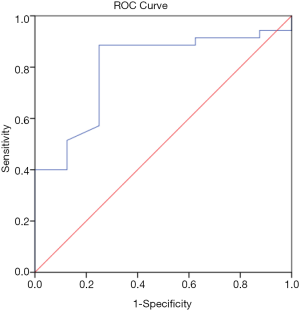
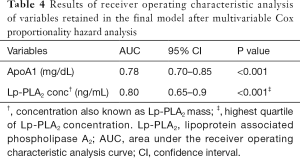
In the CAD group, Lp-PLA2 concentration positively correlated with TC (ρ=0.19, P=0.02), non-HDL-C (ρ=0.20, P=0.02), ApoA1 (ρ=0.23, P=0.03), Lp-PLA2 activity (ρ=0.27, P=0.001) and Lp(a) (r=0.25, P=0.02). Lp-PLA2 activity correlated positively with TC (ρ=0.28, P=0.001), LDL-C (ρ=0.30, P<0.001), non-HDL-C (ρ=0.35, P<0.001), ApoB (ρ=0.35, P<0.001) and negatively correlated to HDL-C (ρ=−0.24, P=0.004). There was no correlation between Lp-PLA2 concentration and LDL-C. Univariate Cox regression analysis showed Lp-PLA2 concentration to be associated with risk of CAD (HR 1.003; 95% CI, 1.001–1.006; P=0.02). ROC analysis revealed the highest quartile of Lp-PLA2 concentration to have good discriminative ability in distinguishing subjects with CAD vs. controls with area under curve (AUC) of 0.80 (95% CI, 0.65–0.9; P<0.001) (Figure 1). Cut off value for Lp-PLA2 concentration of >427 ng/mL was found to have the optimum balance of sensitivity (88.6%) and specificity (75%).
Other biomarkers and CAD
Univariate Cox regression analysis revealed ApoB/ApoA1, ApoA1 and TG/HDL-C ratio to be significantly associated with the event of CAD. The final Cox proportionality hazards model revealed Lp-PLA2 concentration (β=0.006, SE =0.002, P=0.009) to have positive association with the event of CAD, while negative association was observed for ApoA1 (β=−0.05, SE =0.02, P=0.001). Lp-PLA2 activity, ApoB, hs-CRP and TG/HDL-C ratio were found to not have any statistically significant association with the event of CAD on multivariable analysis.
ROC curve analysis demonstrated ApoA1 to have useful discriminative utility in identifying cases from healthy controls with AUC of 0.78 (95% CI, 0.70–0.85; P<0.001) (Figure 2). Cut off value for ApoA1 of ≤129.6 mg/dL was found to have the optimum balance of sensitivity (78.2%) and specificity (68.3%). The results of the ROC analysis are presented in Table 4.
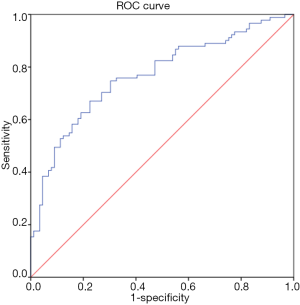
Full table
Discussion
We found that in a south Indian population, subjects with CAD had higher levels of systolic blood pressure, Lp-PLA2 concentration (Figure 3), TG, VLDL-C, hs-CRP, TG/HDL-C and ApoB/ApoA1 compared with normal healthy controls. Conversely, ApoA1 levels were significantly lower in CAD subjects (Figure 4). To our knowledge, this is the first study to evaluate the role of Lp-PLA2 concentration and activity in CAD among Asian Indians living in India. It is also one of the first studies to explore the relationship between Lp-PLA2 and important biomarkers for atherosclerosis in the Indian population.
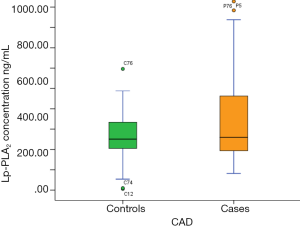
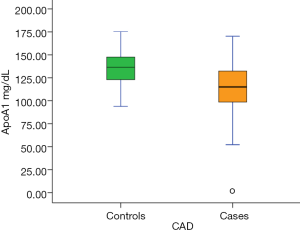
Role of Lp-PLA2 in CAD
Lp-PLA2 is also known as platelet activating factor-acetyl hydrolase (PAF-AH) or PLA2G7 since it belongs to the PLA2 family (3). We found that Lp-PLA2 concentrations were higher in subjects with CAD compared with normal healthy controls, corroborating findings of several population based studies which found elevated levels of circulating plasma Lp-PLA2 concentration in atherosclerotic disease (11-15). However, Lp-PLA2 activity in the CAD group was lower than normal controls, a finding that resonated with results of studies in other Asian populations, such as a South Korean population (16) with lower Lp-PLA2 activity in subjects with CAD attributed to a single nucleotide polymorphism (V279F) in the Lp-PLA2 gene and a Japanese population with complete loss of Lp-PLA2 activity in 27% of subjects with CAD which was attributed to a missense mutation of the Lp-PLA2 gene (17). In a north Indian population with type II diabetes mellitus, Lp-PLA2 activity was associated with oxidized LDL levels, with higher Lp-PLA2 activity among subjects with newly diagnosed diabetes. Although this study did not evaluate CAD prevalence, it was speculated that the increased Lp-PLA2 activity could lead to greater risk of CAD among subjects with type II diabetes mellitus (18). Taken together, these disparate population studies suggest that Lp-PLA2 concentration is associated with CAD, whereas the data on Lp-PLA2 activity is less strong.
Several drugs are known to reduce Lp-PLA2 concentration and activity, most importantly statins (19,20). Subjects in our study were excluded if they had been on statin therapy for more than a week prior to enrolment, which is much longer than the minimum period suggested for any plausible reduction in Lp-PLA2 activity due to statin use. Alterations of Lp-PLA2 or the biomarkers of interest by an acute phase reaction in the cast group is unlikely as the blood samples were drawn <12 hours of symptom onset. The role of Lp-PLA2 activity as a target for pharmaceutical intervention was cast in doubt after publication of the Integrated Biomarker and Imaging Study 2, a randomized placebo controlled phase II trial which found no benefit with the Lp-PLA2 inhibitor darapladib in reducing major adverse cardiac events in patients with an ACS (21). Ikonomidis et al. suggested a prognostic role for Lp-PLA2 in chronic CAD subjects and attributed the same to potentially detrimental effects of the enzyme on endothelial function and arterial wall properties (22). Yang et al. reported initial evidence of role of Lp-PLA2 in endothelial dysfunction in an in vitro model of atherosclerosis, by regulating the expression of AMP-activated protein kinase (AMPK) that is a potential therapeutic target for improving endothelial function (23). The results of our and other studies indicated that Lp-PLA2 concentration had diagnostic utility in CAD, whereas the potential role of this enzyme as a therapeutic target needs further corroboration.
Atherogenic dyslipidemia and ApoA1
The dyslipidemia pattern in the subjects of our study was similar to typical atherogenic dyslipidemia found in Asian Indians, which is quite different from Caucasian subjects. Characteristic lipid findings in Asian Indians include apparently normal or borderline LDL-C, low HDL-C, and elevated TG (7). In our study, there was no significant correlation between Lp-PLA2 concentration and LDL-C, as against what was reported in studies done in European and American populations (24). Instead, our study found significant correlation with the more atherogenic LDL-like particle-Lp(a) (25). Consistent with findings from previous studies, Lp-PLA2 concentration positively correlated with TC and non-HDL-C (4).
Circulating plasma Lp-PLA2 is also known to associate with HDL-C (3). HDL-C is said to have atheroprotective function and ApoA1 is the major protein of HDL-C (26). Interestingly, Lp-PLA2 concentration positively correlated with ApoA1 (ρ=0.23, P=0.03) in subjects with CAD, while earlier studies reported a negative relationship (4). Kujiraoka et al. had also reported positive correlation between HDL-C associated PLA2 and plasma ApoA1 and attributed it to higher HDL-C associated PLA2 among subjects with hyperlipidemia and diabetes (27). In our study, 41% of the CAD group subjects were exposed to diabetes and 29% to hyperlipidemia. This could be a contributing factor for the positive correlation between Lp-PLA2 and ApoA1.
The INTERHEART study had highlighted that, in Asian Indian populations, ApoA1 is a better marker of protection than HDL-C. Our results are consistent with the findings of the INTERHEART study wherein ApoB/ApoA1 ratio was found to be higher in CAD group than in control group (28), and on ROC analysis ApoA1 had higher significant AUC compared to Lp-PLA2 concentration and other risk factors for CAD. These findings suggest a good diagnostic utility for ApoA1 in this study population. However, in the highest quartile, Lp-PLA2 concentration showed better discriminative ability for CAD than ApoA1.
Limitations
There are several limitations of our study which must be noted. First, our study was a cross-sectional case-control study which limited our ability to understand the incremental impact of diabetes, dyslipidemia, hypertension, tobacco use, alcohol consumption in the CAD vs. control groups, since they formed the exclusion criteria for the control group in our study. The relatively small sample size also implies that the results are suggestive in nature and that further larger studies need to be done to assess the conclusions of our study.
Conclusions
Lp-PLA2 concentrations were found to be elevated in Asian (South) Indian subjects with CAD compared with normal subjects. Lp-PLA2 concentration and ApoA1 levels showed superior discriminative ability in identifying subjects with CAD when compared to biochemical risk factors for CAD such as hs-CRP, HDL-C and ApoB/ApoA1 ratio. However, in the highest quartile of Lp-PLA2 concentration, it had the best diagnostic utility in this study population. Elevated plasma Lp-PLA2 concentration; reduced ApoA1 were significantly associated with risk for CAD. Our results support the hypothesis that Lp-PLA2 may be a novel risk marker for CAD in Asian Indians. Further studies are needed to determine the effectiveness of these and other emerging markers for early detection of CAD in Asian Indians.
Acknowledgements
We would like to thank the Founder of the Sri Sathya Sai Institutions, Sri Sathya Sai Baba Ji for his vision, guidance and providing facilities totally free of cost for doing socially relevant medical research. We thank Dr. Cholenahally Nanjappa Manjunath, Director, Sri Jayadeva Institute of Cardiovascular Sciences and Research (SJICSR), for permitting us to recruit participants from the SJICSR, Bangalore. We also thank Dr. Vishwanath Mohan, Founder President, Madras Diabetes Research Foundation (MDRF), Chennai, for guiding us with the study topic. We thank Dr. Sasidhara Prasad, Dr. Ramnath Venkita Subramania Iyer, Dr. (Mrs.) Sai Mangala Divi, Mr. Ramalingam Vaithilingam and the team of nursing and biochemistry staff at SSSIHMS and SJICSR for their extraordinary support.
Funding: This work was supported by research grants from Department of Biochemistry, New Delhi, Government of India (BT/PR13010/MED/12/440/2009); and the 4S Foundation Inc., Maryland, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics board of Sri Sathya Sai Institute of Higher Learning (No. SSSIHL\IEC\PSN\CHE\2014\01).Written informed consents in the subject’s native language were obtained from all subjects.
References
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl 2005;6:21-9. [Crossref] [PubMed]
- Stafforini DM. Plasma PAF-AH (PLA2G7): Biochemical Properties, Association with LDLs and HDLs, and Regulation of Expression. Enzymes 2015;38:71-93. [Crossref] [PubMed]
- Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010;375:1536-44. [Crossref] [PubMed]
- Sakka S, Siahanidou T, Voyatzis C, et al. Elevated circulating levels of lipoprotein-associated phospholipase A2 in obese children. Clin Chem Lab Med 2015;53:1119-25. [Crossref] [PubMed]
- Bilen O, Kamal A, Virani SS. Lipoprotein abnormalities in South Asians and its association with cardiovascular disease: Current state and future directions. World J Cardiol 2016;8:247-57. [Crossref] [PubMed]
- Chandra KS, Bansal M, Nair T, et al. Consensus statement on management of dyslipidemia in Indian subjects. Indian Heart J 2014;66 Suppl 3:S1-51. [Crossref] [PubMed]
- Diasys. Lp(a) 21 FS Package Insert. Available online: http://www.diasys-deutschland.de/produkte/reagenzien/immunturbidimetrische-tests/reagent-details/17-lp-a-21-fs/reagent.show, accessed April 4, 2017.
- Fay MP, Proschan MA. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat Surv 2010;4:1-39. [Crossref] [PubMed]
- Pearce N. Analysis of matched case-control studies. BMJ 2016;352:i969. [Crossref] [PubMed]
- Stafforini DM, Zimmerman GA. Unraveling the PAF-AH/Lp-PLA2 controversy. J Lipid Res 2014;55:1811-4. [Crossref] [PubMed]
- Carlquist JF, Muhlestein JB, Anderson JL. Lipoprotein-associated phospholipase A2: a new biomarker for cardiovascular risk assessment and potential therapeutic target. Expert Rev Mol Diagn 2007;7:511-7. [Crossref] [PubMed]
- Persson M, Berglund G, Nelson JJ, et al. Lp-PLA2 activity and mass are associated with increased incidence of ischemic stroke: a population-based cohort study from Malmo, Sweden. Atherosclerosis 2008;200:191-8. [Crossref] [PubMed]
- Koenig W, Khuseyinova N, Lowel H, et al. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation 2004;110:1903-8. [Crossref] [PubMed]
- Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004;109:837-42. [Crossref] [PubMed]
- Paik JK, Chae JS, Jang Y, et al. Effects of V279F in the Lp-PLA(2) gene on markers of oxidative stress and inflammation in Koreans. Clin Chim Acta 2010;411:486-93. [Crossref] [PubMed]
- Stafforini DM, Satoh K, Atkinson DL, et al. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest 1996;97:2784-91. [Crossref] [PubMed]
- Garg S, Madhu SV, Suneja S. Lipoprotein associated phospholipase A2 activity & its correlation with oxidized LDL & glycaemic status in early stages of type-2 diabetes mellitus. Indian J Med Res 2015;141:107-14. [Crossref] [PubMed]
- O'Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation 2006;113:1745-52. [Crossref] [PubMed]
- Winkler K, Abletshauser C, Friedrich I, et al. Fluvastatin slow-release lowers platelet-activating factor acetyl hydrolase activity: a placebo-controlled trial in patients with type 2 diabetes. J Clin Endocrinol Metab 2004;89:1153-9. [Crossref] [PubMed]
- Hassan M. STABILITY and SOLID-TIMI 52: Lipoprotein associated phospholipase A2 (Lp-PLA2) as a biomarker or risk factor for cardiovascular diseases. Glob Cardiol Sci Pract 2015;2015:6. [Crossref] [PubMed]
- Ikonomidis I, Kadoglou NN, Tritakis V, et al. Association of Lp-PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis 2014;234:34-41. [Crossref] [PubMed]
- Yang L, Cong HL, Wang SF, et al. AMP-activated protein kinase mediates the effects of lipoprotein-associated phospholipase A2 on endothelial dysfunction in atherosclerosis. Exp Ther Med 2017;13:1622-9. [Crossref] [PubMed]
- Gregson J, Stirnadel-Farrant HA, Doobaree IU, et al. Variation of lipoprotein associated phospholipase A2 across demographic characteristics and cardiovascular risk factors: a systematic review of the literature. Atherosclerosis 2012;225:11-21. [Crossref] [PubMed]
- Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care 2008;31:811-22. [Crossref] [PubMed]
- Chhabra S, Narang R, Lakshmy R, et al. APOA1-75 G to A substitution associated with severe forms of CAD, lower levels of HDL and apoA-I among northern Indians. Dis Markers 2005;21:169-74. [Crossref] [PubMed]
- Kujiraoka T, Iwasaki T, Ishihara M, et al. Altered distribution of plasma PAF-AH between HDLs and other lipoproteins in hyperlipidemia and diabetes mellitus. J Lipid Res 2003;44:2006-14. [Crossref] [PubMed]
- McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet 2008;372:224-33. [Crossref] [PubMed]


