Echocardiographic evaluation and guidance for MitraClip procedure
Introduction
Mitral regurgitation is a major contributor to congestive heart failure. Those with advanced age and multiple comorbidities may not be a candidate for traditional mitral valve surgery to correct mitral regurgitation. The MitraClip® Delivery System (Abbott, Abbott Park, IL, USA) was originally intended to mimic the Alfieri surgical approach to mitral repair by clipping together the A2 and P2 scallops (1). This minimally invasive catheter based approach has become a major breakthrough in the treatment of patients with symptomatic severe mitral regurgitation and high surgical risk. True collaborative spirit amongst heart team members, imaging researchers and device manufacturer research and development This manuscript provides an overview of the important echocardiographic aspects related to adequate patient selection and procedural guidance for successful implantation of the MitraClip, review of potential complications of the procedure, and a glimpse towards future directions.
Patient selection
Patients considered for MitraClip implantation should be first optimally medically managed for their congestive heart failure. In addition, management may also include percutaneous revascularization and cardiac resynchronization therapy, if appropriate. If after optimization, mitral regurgitation is still considered significant enough to be contributing to ongoing heart failure, a heart team consisting of a mitral valve surgeon, an interventional cardiologist and an advanced echocardiographer with expertise in 3-dimensional mitral valve imaging will discuss therapeutic strategies. Patients deemed not good surgical candidates can be considered for MitraClip after review of the pathoanatomic mechanism of the mitral regurgitation and discussion about the potential feasibility of the procedure. The MitraClip has been implanted now in over 50,000 patients worldwide. Patients with prohibitive surgical risk and degenerative mitral valve disease are currently FDA approved for commercial use of MitraClip therapy and have a class IIB indication (2). Patients with functional mitral regurgitation are currently being randomized into the COAPT trial for mitral surgery vs. MitraClip therapy (ClinicalTrials.Gov ID# NCT01626079). It is also important to mention that patients with severe tricuspid regurgitation, severe pulmonary hypertension or right ventricular dysfunction can also represent an important subgroup of patients where post-procedural outcomes might be more limited despite reduction of MR severity with MitraClip device.
Pre-procedural echocardiographic evaluation
Echocardiography is a well-established method for evaluating mitral regurgitation. Transthoracic echocardiography is the initial technique that typically identifies the pathoanatomic mechanism and severity of the mitral regurgitation. Additional findings important to the discussion for candidacy for MitraClip include right and left ventricular size and function, left atrial size, pulmonary hypertension, concomitant aortic valve disease, and severity of tricuspid regurgitation.
Etiology of mitral regurgitation and implications for MitraClip procedure
The mitral apparatus should be examined carefully to determine the mechanism of the regurgitation as from either degenerative or functional cause. Degenerative mitral valve disease is from either Barlow’s disease (Figures 1,2) or fibroelastic deficiency with prolapse or flail leaflets (Figures 3,4). Flail mitral leaflets should be evaluated for the degree of mobility and the maximal flail gap distance measured to determine if successful grasping will be possible (ideally flail width <15 mm and flail gap <10 mm). Functional mitral regurgitation could be either from ischemia/infarct or from a dilated cardiomyopathy with subsequent leaflet and papillary muscle tethering displacing the leaflet coaptation below the annular level (Figures 5,6). Adequate leaflet grasping and procedural success have been traditionally associated with a coaptation depth <11 mm and coaptation length of >2 mm.
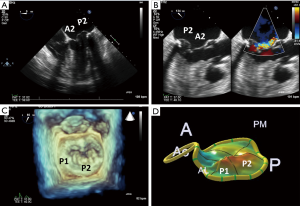

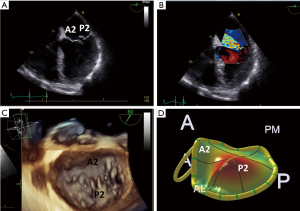

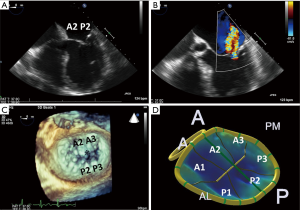

In addition, ideal candidates for MitraClip procedural would also have: central mitral valve pathology between the A2/P2 scallops, mitral valve area ≥4.0 cm2, normal leaflet thickness and mobility, no/minimal leaflet calcification, posterior leaflet mobile length ≥10 mm or classic isolated mitral valve prolapse. Since there are the few chordae in the middle portions of the A2 and P2 scallops this is the ideal location for grasping the leaflets and to avoid complications of entangling chordae or even tearing these structures as the MitraClip is brought into the left ventricle. The mitral valve area should be planimetered in the parasternal short axis 2D view, or preferably by postprocessing with multiplanar reformatting of a dedicated 3D dataset (Figure 7). Mitral valve area can decrease by 50% with the first clip and further by 30–40% with the 2nd clip (6). A mitral valve area <4.0 cm2 is considered a relative contraindication to the procedure as the MitraClip will likely result in potentially significant stenosis. Mitral gradients should also be established as a baseline, however, overestimation of stenosis can be occur with severe mitral regurgitation from associated increased transmitral flow. The individual scallops and chordae should be evaluated to determine where the MitraClip would need to be placed. Presence of leaflet and/or annular calcification will require detailed assessment to ascertain whether the MitraClip will be able to adequately grasp and successfully hold the mitral leaflets. There should ideally be at least 5 mm of distal mitral leaflet tip without calcification.
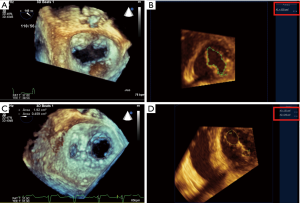
Mitral valve clefts are better seen by 3D TEE imaging. Congenital or more commonly acquired mitral valve clefts can often be associated with asymmetric leaflet tethering in patients with secondary MR due to dilated/ischemic cardiomyopathy. Although more challenging, isolated case reports have been published demonstrating success in experienced centers (7,8). Therefore, caution is needed before tackling such pathology which may not be easily approachable. Center expertise is required to optimize reduction of mitral regurgitation after one or multiple MitraClip devices are implanted (Figure 8).
Assessing mitral regurgitation severity pre-MitraClip
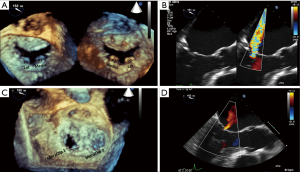
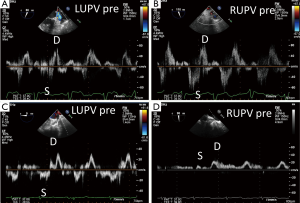
The severity of the mitral regurgitation should be assessed both by qualitatively as well as with quantitative methods (9,10). The patient’s blood pressure should be noted at the same time because high afterload can increase the severity of mitral regurgitation. Color Doppler jet size is very useful for determining mitral regurgitation severity; however, underestimation is possible with eccentric jets that hug the left atrial wall. Assessing pulmonary venous flow pattern for systolic blunting or flow reversal in left and right sided pulmonary veins is supportive of the severity of mitral regurgitation, however, caution is needed when the left atrium is severely enlarged and with eccentric wall-hugging jets as these findings might be less pronounced or only significant in either the left or right sided pulmonary veins that an eccentric jet is directed towards (Figure 9).
Quantitation of mitral regurgitation severity should be performed using 2D or 3D proximal isovelocity surface area (PISA) method or preferably 3D vena contracta area (9-11) (Figures 10,11).
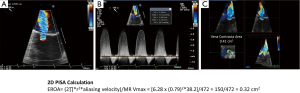

There is great debate whether there should be different thresholds for mitral regurgitation severity for different types of MR (i.e., primary vs. secondary) (2,9,13,14). Using 2D PISA, the effective regurgitant orifice area (EROA) criteria for severe primary mitral regurgitation is ≥0.4 cm2 and regurgitant volume is 60 mL/beat; for secondary mitral regurgitation, European valvular guidelines propose EROA ≥0.2 cm2 and regurgitant volume is 30 mL/beat (15). Recent 2017 ACC/AHA Valvular guidelines update considers that for secondary MR, an EROA >0.2 cm2 is more sensitive, whereas >0.4 cm2 is more specific (16). 2D PISA in secondary MR may underestimate EROA due to the crescentic shape of the MR regurgitant orifice.
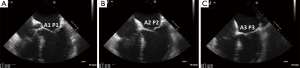
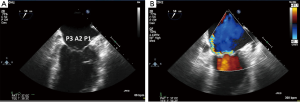
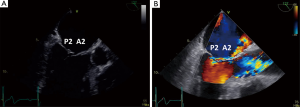
Since the mitral valve is viewed optimally through the left atrium with TEE, and closer proximity to the TEE probe, better visualization increases confidence of precisely defining the mitral pathoanatomy and quantification of MR severity. Foster and colleagues sweep and image the mitral valve for 3 different angles 0, 60–90 and 120 degrees (17). In the mid-esophageal 0 degrees view, the corresponding pairs of scallops are imaged with A1/P1 with the probe withdrawn and the aortic valve in view. With deeper probe insertion, A2/P2 are imaged and deeper still, A3/P3 (Figures 12,13). In the 60 degrees plane, P1/A2/P3 scallops are visualized; clockwise medial rotation will visualize A1/A2/A3 and counterclockwise lateral rotation will visualize P1/P2/P3 (Figure 14). At 120 degrees, A2/P2 are typically imaged (Figure 15).

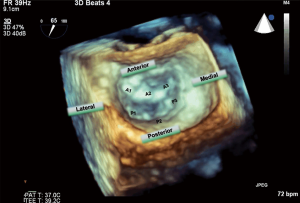
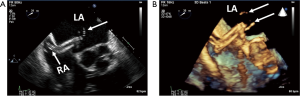
3D TEE of the mitral valve is extraordinarily able to give a comprehensive image of the entire mitral valve with all of the scallops visualized simultaneously. An en-face view of the atrial side of the entire mitral valve (surgeon’s view) and adjacent structures like the left atrial appendage and aortic valve is possible with real time live 3D imaging (Figures 16,17). Flail segments can more precisely be identified. In addition, the location of clefts and perforations may be more apparent and easier to visualize. Significant mitral regurgitation related to those abnormalities may be difficult to eliminate entirely just with the MitraClip.

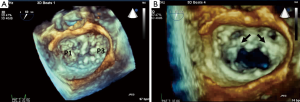
Both TTE and TEE should be reviewed by the heart team to confirm the proper approach to mitral repair and other associated pathologies. Agreement should be made as to the precise location for MitraClip placement and the possible need for multiple MitraClips (particularly in cases with flail width >10 mm or tall flail gap). In EVEREST I intervention was limited to the A2–P2 with central jets (20). In EVEREST II, the majority of patients had primary mitral disease (21) and was compared to open mitral valve surgery. Despite patients receiving the MitraClip having more residual mitral regurgitation, they had similar outcomes. As the continued experience builds with the MitraClip system, expanding treatment beyond A2-P2 related mitral regurgitation has been reported (22). Patients with highly mobile leaflets, large flail width and gap are likely to need more than a single MitraClip device (23). If there are multiple mitral regurgitation jets then the order of MitraClipping will be determined in the order of MR jet severity, taking into consideration patient’s mitral valve area and gradients (Figures 18-20).


Patients with severe concomitant aortic stenosis, severe tricuspid regurgitation, right ventricular dilatation and/or dysfunction represent a challenging group for which heart team should discuss the risks and expected benefits of MitraClip. Patients with severe aortic stenosis who are not surgical candidates may benefit from transaortic valve replacement prior to MitraClip (26). Patients with severe tricuspid regurgitation, severe pulmonary hypertension or right ventricular dysfunction can also represent an important subgroup of patients where MitraClip outcomes might be more limited despite reduction of MR severity. In addition, the residual atrial septal defect (ASD) with usually a left to right shunt may increase the risk of right sided failure and consideration for ASD closure should factored in (27).
Procedural echocardiographic guidance
Transseptal puncture
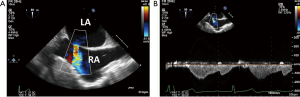
The interatrial septum is evaluated in the zero degree view apical four chamber view, and a measurement perpendicular to the mitral annulus at mid systole is made to determine the ideal site for transseptal puncture. Tenting of the atrial septum with simultaneous biplane or x-plane views including the aortic valve in short axis as an anterior landmark and the bicaval view 110 degrees view to determine the superior height is very useful for this purpose. A superior and posterior septal puncture site >4 cm from the mitral annulus will be needed for a degenerative valve and a puncture site between 3–4 cm will be adequate for a functional valve with apical tethering of mitral valve leaflets (Figure 21A-C). Once the septum is punctured, the guidewire is positioned into the left upper pulmonary vein with care to avoid entering the left atrial appendage and the risk of perforation.
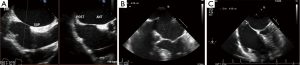
Introduction of delivery sheath and clip delivery system
Once the delivery sheath is in the left atrium, TEE should be used to help avoid contact with the left atrial wall. It can be positioned with echo visualization of the mitral valve using the long axis view (~120 degrees), the commissural view (~60 degrees) and the 3D view of the mitral valve (surgeon’ s view). The Clip Delivery System is then advanced into the left atrium with TEE guidance to avoid injury to the left atrial wall.
MitraClip positioning above the mitral valve
2D with color Doppler and real time 3D are used to pinpoint the location of the mitral pathology that is being targeted for the MitraClip. Real time 3D with color is generally not useful because of the current very low frame rate. The arms of the MitraClip are opened in the left atrium to verify that they are aligned perpendicular to the coaptation of the anterior and posterior scallops that are going to be grasped prior to being passed into the left ventricle. This can best be shown with the 120 degrees view or the en-face live 3D view of the mitral valve (28) (Figure 22).
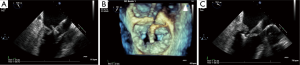
Entering the MitraClip into the left ventricle
The MitraClip arms are closed when passing through the mitral leaflets and reopened when below the coaptation of the leaflets those are being clipped. Verification that the arms are still perpendicular is important, but significant manipulation should be avoided to minimize chordal and subchondral entanglement. For A2/P2 regurgitation, the left ventricular outflow tract view at ~120 degrees is an excellent view for showing the grasping of the A2/P2 scallops. For A1/P1 or A3/P3 regurgitation, adjusting the intercommissural view angle to show the mitral clip perpendicular to the leaflets is necessary or to use the en-face 3D view.
Grasping the mitral leaflets
Echocardiography with zoom and high resolution imaging and fluoroscopy can confirm that the mitral leaflets were successfully grasped (Figure 23). Assessment of adequate leaflet grasping is key to ensure procedural success. This has to be done carefully and usually a leaflet grasp length of approximately 5 mm is considered adequate. It is important to visualize continuous leaflet insertion while grasping to avoid rolling leaflets/chordae. Clip stability both by TEE and fluoroscopy should be assessed.
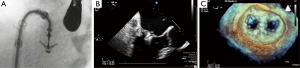
In addition, residual mitral regurgitation with color Doppler is performed by rotating the TEE probe medially (clockwise) and laterally (counterclockwise) relative to the view that shows the MitraClip. The pulmonary vein flow pattern is assessed with the goal of achieving a systolic dominant pattern (Figure 24). Redeployment of a MitraClip is sometimes necessary if there is still significant mitral regurgitation near the site the MitraClip was placed. Mean mitral gradients should be assessed and if significant, the MitraClip should not be deployed. Mitral stenosis should be evaluated before after each MitraClip placement and if the mean gradient exceeds 5 mmHg the MitraClip should probably not be deployed (29). 3D planimetry can also be used to assess the residual mitral valve area (Figure 25) (30). Intraprocedural monitoring of the left atrial pressure and the presence of V waves is also commonly done to assess the success of reducing or eliminating significant mitral regurgitation (31).

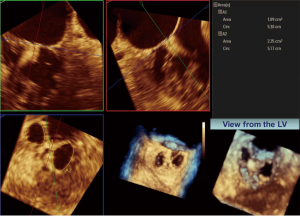
Releasing and deploying additional MitraClips
After the MitraClip system has been deployed and released from the guide catheter, 2D, 3D echocardiography and importantly fluoroscopy are used to observe MitraClip stability and adequate creation of a double orifice mitral valve anatomy. In the presence of residual significant mitral regurgitation, additional MitraClip(s) may be deployed as necessary to minimize residual mitral regurgitation while avoid stenosis.
With degenerative mitral valve pathology, in particular with Barlow’s disease and highly mobile leaflets, excessive MitraClip movement is often seen despite adequate grasping and reduction of mitral regurgitation. In such cases, a second MitraClip placed immediately adjacent to the first MitraClip can stabilize and reduce the leaflet tension of the prior MitraClip and minimize the risk of subsequent leaflet detachment (32). It is obviously important to reassess residual regurgitation and stenosis.
Removing the delivery system across the atrial septum
The delivery sheath is withdrawn back across the atrial septum and out the femoral vein access. The size and direction of shunting of the residual ASD is documented (Figure 26). It appears that persistent interatrial shunting is associated with worse clinical outcomes and mortality (33). In our experience, in cases of significant right ventricular dilation, dysfunction or severe pulmonary hypertension, it may be appropriate to place an occluder device in the created ASD, particularly if MitraClip results were satisfactory.
Procedural complications
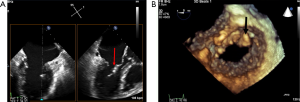
Potential complications related to the nature of an intracardiac procedure can occur such as new intracardiac thrombus on guidewires and/or delivery sheaths (34) (Figures 27,28). MitraClip deployment out of the chordal free area between A2/P2 increases risk of entangling chordae and the risk of rupturing chordae (36). Multiple grasping attempts in addition to MitraClip potentially can lead to leaflet perforation (37). Single leaflet device attachment has been reported in up to 5% of large European registry (38), but recently lower incidence was reported in large commercial US experience (1.1%) (39) (Figures 29,30).


Follow up echocardiography
Transthoracic echocardiography is often performed the day following the MitraClip deployment to evaluate the position and stability of the MitraClips and to assess the severity of residual mitral regurgitation. Because of the residual MR jet eccentricity, comprehensive evaluation by both color and spectral Doppler is again important, in particular acquiring a sweep during multiple heart beats. Mitral gradients should be assessed and if possible planimetry of the mitral valve openings. Pulmonary vein pulse wave Doppler should be assessed to help determine the severity of residual mitral regurgitation. Left ventricular size and function, estimation of pulmonary artery systolic pressure and presence of pericardial effusion should be done. If there is doubt about the position and stability of the MitraClip and residual mitral regurgitation, a repeat TEE may be indicated. The patient could be a candidate for additional MitraClips if needed and if there is not significant mitral stenosis.
Future directions
Technological advances with the recent release of MitraClip NT have improved delivery catheter steering control and response. In addition, there was a gripper material change from Elgilot to Nitinol, which allows for improved leaflet engagement by widening the gripper drop angle up to 120 degrees, improved grasping and deeper leaflet insertion. Taken together, these additions will facilitate operator’s ability to achieve adequate procedural results including more challenging cases such as non A2–P2 pathology, significant leaflet tethering, cleft/perforation, large flail/prolapse.
Despite these improvements, one still needs to be respectful of the anatomy. In particular, patients with concomitant mitral stenosis with reduced mitral valve area (i.e., <4 cm2 with reduced leaflet mobility and resting mitral valve gradients >5 mmHg), represent a challenging group. This is commonly seen with patients being evaluated for transcatheter aortic valve replacement (TAVR) where mitral annular calcification is frequently present.
As mentioned previously, comprehensive evaluation of the right ventricular size and function, pulmonary hypertension and severity of tricuspid regurgitation need to be also carefully factored into the decision-making process. The residual left to right shunting from the procedural ASD, can facilitate worsening right-sided dysfunction and potentially procedural outcomes (33). In addition, in the presence of significant pulmonary hypertension and increased right atrial pressure, right to left shunting with hypoxemia can occur (27). As such, particularly after adequate MitraClip results, percutaneous ASD closure should be considered.
Use of patient-specific 3D printing mitral valve models, derived from computed tomography (CT) datasets, might be helpful in selected cases with challenging anatomy where multiple procedures might be considered in the same patient (41).
As mentioned before, mitral leaflet and annular calcification can limit the suitability for Mitraclip therapy given the risk for mitral stenosis. Although TEE can visualize and identify mitral leaflet and annular calcification, cardiac CT scan quantify the extent and severity of mitral leaflet and annular calcification. This is typically done using gated, non-contrast prospective acquisitions with low radiation exposure. If intravenous contrast is given, evaluation of the residual mitral valve area can also be performed.
Lastly, quantification of residual mitral regurgitation post-MitraClip is challenging due to artifacts created by the clip limiting visualization of the jet origin, eccentricity of mitral regurgitation jet, altered native mitral valve anatomy and presence of atrial arrhythmia. Cardiac magnetic resonance (CMR) might be a potential role in evaluating the severity of residual mitral regurgitation in selected patients post-MitraClip implant. In a small, single-center study including 25 patients post-MitraClip, CMR was shown to have excellent reproducibility and lower interobserver variability in comparison with echocardiography (42). In addition, CMR might have also a complementary role to evaluation of LV remodeling post-transcatheter intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr João L. Cavalcante received investigator-initiated grant support from Medtronic Inc.; the other authors have no conflicts of interest to disclosure.
References
- Feldman T, Foster E, Glower DD, et al. PPercutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-406. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. Degenerative mitral valve—Barlow’s disease. Asvide 2017;4:551. Available online: http://www.asvide.com/articles/1870
- Katz WE, Conrad Smith AJ, Crock FW, et al. Degenerative mitral valve—fibroelastic deficiency. Asvide 2017;4:552. Available online: http://www.asvide.com/articles/1871
- Katz WE, Conrad Smith AJ, Crock FW, et al. Functional mitral regurgitation. Asvide 2017;4:553. Available online: http://www.asvide.com/articles/1872
- Tamburino C, Ussia GP, Maisano F, et al. Percutaneous mitral valve repair with the MitraClip system: acute results from a real world setting. Eur Heart J 2010;31:1382-9. [Crossref] [PubMed]
- Freixa X, Hayami D, Basmadjian A, et al. MitraClip repair of a "trileaflet" regurgitant mitral valve. EuroIntervention 2015;11:355. [Crossref] [PubMed]
- Öztürk C, Schueler R, Werner N, et al. MitraClip procedure for the treatment of a pseudo-cleft in the posterior mitral leaflet. Eur Heart J Cardiovasc Imaging 2015;16:112. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Thavendiranathan P, Phelan D, Collier P, et al. Quantitative assessment of mitral regurgitation: how best to do it. JACC Cardiovasc Imaging 2012;5:1161-75. [Crossref] [PubMed]
- Sugeng L, Weinert L, Lang RM. Real-time 3-dimensional color Doppler flow of mitral and tricuspid regurgitation: feasibility and initial quantitative comparison with 2-dimensional methods. J Am Soc Echocardiogr 2007;20:1050-7. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. 3D quantification of mitral regurgitation using vena contracta area method. Asvide 2017;4:554. Available online: http://www.asvide.com/articles/1873
- Grayburn PA, Carabello B, Hung J, et al. Defining "severe" secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol 2014;64:2792-801. [Crossref] [PubMed]
- Marwick TH, Zoghbi WA, Narula J. Redrawing the borders: considering guideline revision in functional mitral regurgitation. JACC Cardiovasc Imaging 2014;7:333-5. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44. [Crossref] [PubMed]
- Writing Committee Members, Boris JR, Béland MJ, et al. 2017 AHA/ACC Key Data Elements and Definitions for Ambulatory Electronic Health Records in Pediatric and Congenital Cardiology: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards. J Am Coll Cardiol 2017;pii: S0735-1097(17)37850-6.
- Foster GP, Isselbacher EM, Rose GA, et al. Accurate localization of mitral regurgitant defects using multiplane transesophageal echocardiography. Ann Thorac Surg 1998;65:1025-31. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. Transesophageal mid esophageal 4 chamber views at 0 degrees. Asvide 2017;4:555. Available online: http://www.asvide.com/articles/1874
- Katz WE, Conrad Smith AJ, Crock FW, et al. The left atrium with an enface view of the mitral valve in a patient with ischemic mitral regurgitation. Asvide 2017;4:556. Available online: http://www.asvide.com/articles/1875
- Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686-94. [Crossref] [PubMed]
- Whitlow PL, Feldman T, Pedersen WR, et al. Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) High Risk Study. J Am Coll Cardiol 2012;59:130-9. [Crossref] [PubMed]
- Estévez-Loureiro R, Franzen O, Winter R, et al. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. J Am Coll Cardiol 2013;62:2370-7. [Crossref] [PubMed]
- Armstrong EJ, Rogers JH, Swan CH, et al. Echocardiographic predictors of single versus dual MitraClip device implantation and long-term reduction of mitral regurgitation after percutaneous repair. Catheter Cardiovasc Interv 2013;82:673-9. [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. The mitral valve showing prolapse of the P1 and P3 scallops with a small flail portion of the P1 scallop. Asvide 2017;4:557. Available online: http://www.asvide.com/articles/1876
- Katz WE, Conrad Smith AJ, Crock FW, et al. The mitral valve after 2 MitraClips deployed between A1 and P1 and A3 and P3 scallops resulting in a triple orifice mitral valve inflow and significant reduction of mitral regurgitation. Asvide 2017;4:558. Available online: http://www.asvide.com/articles/1877
- Kische S, D'Ancona G, Paranskaya L, et al. Staged total percutaneous treatment of aortic valve pathology and mitral regurgitation: institutional experience. Catheter Cardiovasc Interv 2013;82:E552-63. [PubMed]
- Chandraprakasam S, Satpathy R. When to close iatrogenic atrial septal defect after percutaneous edge to edge repair of mitral valve regurgitation. Cardiovasc Revasc Med 2016;17:421-3. [Crossref] [PubMed]
- Faletra FF, Pedrazzini G, Pasotti E, et al. Role of real-time three dimensional transoesophageal echocardiography as guidance imaging modality during catheter based edge-to-edge mitral valve repair. Heart 2013;99:1204-15. [Crossref] [PubMed]
- Cockburn J, Fragkou P, Hildick-Smith D. Development of mitral stenosis after single MitraClip insertion for severe mitral regurgitation. Catheter Cardiovasc Interv 2014;83:297-302. [Crossref] [PubMed]
- Biaggi P, Felix C, Gruner C, et al. Assessment of mitral valve area during percutaneous mitral valve repair using the MitraClip system: comparison of different echocardiographic methods. Circ Cardiovasc Imaging 2013;6:1032-40. [Crossref] [PubMed]
- Eleid MF, Sanon S, Reeder GS, et al. Continuous Left Atrial Pressure Monitoring During MitraClip: Assessing the Immediate Hemodynamic Response. JACC Cardiovasc Interv 2015;8:e117-9. [Crossref] [PubMed]
- Singh GD, Smith TW, Rogers JH. Multi-MitraClip therapy for severe degenerative mitral regurgitation: "anchor" technique for extremely flail segments. Catheter Cardiovasc Interv 2015;86:339-46. [Crossref] [PubMed]
- Schueler R, Öztürk C, Wedekind JA, et al. Persistence of iatrogenic atrial septal defect after interventional mitral valve repair with the MitraClip system: a note of caution. JACC Cardiovasc Interv 2015;8:450-9. [Crossref] [PubMed]
- Huntgeburth M, Müller-Ehmsen J, Brase C, et al. Thrombus formation at the MitraClip system during percutaneous mitral valve repair. JACC Cardiovasc Interv 2014;7:e111-2. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. Mobile thrombus on the delivery catheter tip seen within the right atrium and in the left atrium. Asvide 2017;4:559. Available online: http://www.asvide.com/articles/1878
- Benito-González T, Estévez-Loureiro R, Gualis J. Chordal Rupture Following MitraClip Implantation Resulting in Massive Mitral Regurgitation. J Invasive Cardiol 2015;27:E224-5. [PubMed]
- Citro R, Baldi C, Mastrogiovanni G, et al. Partial clip detachment and posterior mitral leaflet perforation after mitraclip implantation. Int J Cardiol 2014;171:e113-6. [Crossref] [PubMed]
- Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61. [Crossref] [PubMed]
- Sorajja P, Mack M, Vemulapalli S, et al. Initial Experience With Commercial Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol 2016;67:1129-40. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Crock FW, et al. Transesophageal echo X-plane view of the mitral valve post MitraClip placement showing a single leaflet device attachment of MitraClip to the anterior mitral leaflet. Asvide 2017;4:560. Available online: http://www.asvide.com/articles/1879
- Little SH, Vukicevic M, Avenatti E, et al. 3D Printed Modeling for Patient-Specific Mitral Valve Intervention: Repair With a Clip and a Plug. JACC Cardiovasc Interv 2016;9:973-5. [Crossref] [PubMed]
- Hamilton-Craig C, Strugnell W, Gaikwad N, et al. Quantitation of mitral regurgitation after percutaneous MitraClip repair: comparison of Doppler echocardiography and cardiac magnetic resonance imaging. Ann Cardiothorac Surg 2015;4:341-51. [PubMed]


