Recent updates on echocardiography and ultrasound for Kawasaki disease: beyond the coronary artery
Kawasaki disease (KD) is an acute inflammatory process affecting the arterial wall that results in panvasculitis in infants and young children. A recent review of autopsies of KD patients, mostly accompanied with coronary artery abnormalities (CAAs), revealed that there were the three-associated vasculopathic processes: acute necrotizing arteritis, subacute or chronic vasculitis, and luminal myofibroblastic proliferation (LMP) (1). Acute arteritis is predominantly characterized by neutrophilic infiltrate that can lead to necrosis in the vessel wall. Subacute vasculitis begins weeks after the onset of fever, can still be detected months to years later, and is closely associated with LMP. LMP is associated with the subacute process that occurs months to years after the disease onset and can result in luminal narrowing, which can lead to cardiac ischemia. These vascular remodeling processes have been provisionally designated as “KD vasculopathy”. In this review, we focused on the latest updates on echocardiography and ultrasound for KD according to the processes of KD vasculopathy.
Acute phase of KD
Coronary z-score
CAAs develop in 20% to 25% of untreated KD patients and in about 5% of patients treated with intravenous immunoglobulin (2). Although recent progress in the treatment of KD has decreased the incidence of echocardiographically detectable CAAs to <3% at 28 days after onset, KD remains the most common cause of pediatric acquired heart disease in developed countries (3).
Commonly used definitions of CAAs have relied on the Japanese Ministry of Health (JMH) criteria, which dichotomously define abnormalities established in 1984 (4) (Table 1). The JMH criteria were widely used because of their simple and quick assessment of CAAs; however, it has been recognized that these criteria are arbitrary without considering the size of patients, reflect only the time-point of maximum dimension, and lack a standard specific for each major coronary artery (CA). Previously, de Zorzi et al. investigated the distribution of CA dimensions, adjusted for the body surface area (BSA) as z-scores using linear regression equations derived from a normal non-febrile control population, in 125 patients with KD whose arteries were classified as “normal” based on the JMH criteria. They noted that 27% of patients with no CA involvement using the JMH criteria had at least 1 CA z-score >2, or 2 SDs from normal based on the BSA. This study indicated that coronary dilatation in KD patients was more prevalent than previously reported, highlighting the need for the systemic long-term surveillance of this population (5). This was recognized in the latest American Heart Association (AHA) guidelines published in 2004, which defined CAAs as any coronary segment with an internal diameter z-score ≥2.5 (6). In this statement, CAAs were further subdivided into small (<5 mm), large (≥5 to <8 mm), and giant (≥8 mm) CAAs based on their absolute dimensions, and not on their body-surface-area-adjusted z-scores (Table 1). Z-scores are increasingly being used to help guide the initial diagnosis and identify patients who may need increased surveillance or more aggressive therapy (i.e., additional immunoglobulin therapy or adjunctive anti-inflammatory therapy). Although the AHA criteria recognize a greater spectrum of CAAs, the classification does not optimally discriminate between the different severities of CAAs and incompletely adjusts for the body size. Recently, Manlhiot et al. proposed a revision of the AHA criteria to account for differences in the body size and dimensions of CA branches. They reported that the AHA classification underestimated the severity of CAAs in 19–32% of small CAAs and 35–78% of medium CAAs, particularly in infants and small children. Then, they determined the definition of CAA to be small if the z-score was ≥2.5 to <5, large if it was ≥5 to <10, and giant if it was ≥10 (7). This method is, however, prone to significant variation in the calculated z-score with minor variation in the measurement of the coronary size.

Full table
There are limited studies in the literature that provide z-score equations and calculators for the CA in children. Z-score equations were published using a linear function with the BSA by Kurotobi et al. (8) and Tan et al. (9). However, these studies were limited by small numbers of patients, homogenous patient populations, and non-digital data sets. Subsequently, z-score equations using an exponential function with the BSA were proposed by McCrindle et al. (10) and Olivieri et al. (11). More recently, Dallaire and Dahdah published a KD z-score equation based on a large pediatric cohort (n=1,033) using regression with the square root of BSA (12). They also analyzed z-score boundaries for the left main CA (mm) against the BSA on comparison with those previously published by McCrindle et al. and Olivieri et al. They found that their greater representation of small children and infants allowed the identification of a steeper slope at a low BSA than those observed by others. Kobayashi et al. generated the sex-specific z-score of each internal CA diameter using lambda-mu-sigma (LMS) models in a large Japanese pediatric population (≤18 years old, n=3,851) (13).
As for the reproducibility of CA measurement and variability on calculating z-scores among these recently proposed and easily accessible formulas, those of McCrindle et al., Olivieri et al., and Dallaire and Dahdah, Ronai et al. demonstrated that CA measurements had high inter- and intra-observer agreements. However, the CA z-score varied significantly on the basis of the z-score formula with larger dimensions (14). Discrepancies in z-scores among the three formulas may affect clinical decision making regarding the recommendation of anticoagulation in patients with a z-score ≥10.
The predictive value of z-scores to assess the long-term outcome and CA pathology in adult life is unknown. Specifically, the significance of having a z-score between 2.5 and 5.0 that resolves over the first 2 months after disease onset can only be answered by long-term follow-up studies of this population into adulthood. It is recommended that clinicians should consider the sources of variation in z-scores before beginning anticoagulation in children whose maximum z-score are approaching a threshold of 10 (Figure 1).
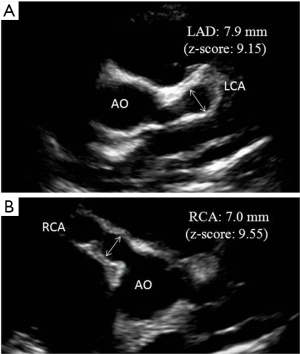
Carotid intima-media thickness (CIMT)
Measurement of the CIMT is a widely applied and validated noninvasive imaging modality for the evaluation of early structural changes in the arterial wall in multiple populations (15,16). A greater CIMT can not only be a result of atherosclerotic changes, but can also be caused by other processes involving injury and inflammation. The inflammation of vessel walls precedes morphological changes and is believed to be the initial step in many rheumatic diseases, including Takayasu’s arteritis (17), systemic lupus erythematosus (18), rheumatoid arthritis (19), and Behçet’s disease (20).
Wu et al. investigated CIMT measurements in children aged 3–60 months old with acute KD (more than four principle KD diagnostic criteria and a fever lasting >5 days) and 14 febrile patients (controls) aged 3–194 months old with acute infection and similar characteristics to those of KD patients. They demonstrated that children with KD had a significantly greater CIMT compared with controls (0.550±0.081 vs. 0.483±0.046 mm, respectively, P=0.01), suggesting that the CIMT could be a useful diagnostic tool in the early diagnosis of acute KD (21).
The CIMT may be increased early after the marked inflammation associated with acute KD, but subsides in most patients over time, making the elapsed time from the onset of acute illness a particularly relevant factor.
Subacute or chronic phase
Dobutamine stress echocardiography (DSE)
The progression of CAAs to a stenotic lesion is an important determinant of the prognosis of KD and CAAs, however, little is known about the incidence of coronary stenotic lesions during follow-up in KD patients with CAAs. It is generally accepted that an initial aneurysm exceeding 8.0 mm (giant aneurysm) evolves into CA disease (CAD). Moreover, it was shown that KD patients with an initial coronary aneurysm exceeding 6.0 mm (large aneurysm) had a greater than 50% chance of developing a clinically significant stenotic CA during a follow-up of 15 years (22). However, it is questionable whether the level of initial coronary involvement alone will determine the fate of CAAs, especially in KD patients with risk levels III (small-to-medium coronary aneurysm) and IV (large or giant coronary aneurysm), because assigned coronary risk level is not permanently fixed for given patients and may change over the course of follow-up.
DSE is a widely accepted and useful noninvasive test for the diagnosis, risk stratification, and prognosis of adult patients with suspected or known CAD. DSE has also been shown to provide valuable information for the detection of CA stenosis in children and adolescents with KD (23,24). Although DSE is an established technique for the detection and prognostic stratification of CAD, the prognostic value of DSE in adolescents and young adults with KD and CAAs for predicting cardiac events is unknown. Recently, we reviewed 58 adolescent KD patients, including 36 patients with CAAs documented by coronary angiography, and 22 patients with normal coronary arteries documented by echocardiography who underwent DSE at initial testing (between 1996–1998, mean age: 13.6 years) and at 15 years follow-up. During a mean follow-up of 14.7 years, there were 16 patients with major adverse cardiac events (MACEs) (acute myocardial infarction: n=1; old myocardial infarction: n=7; CA bypass grafting: n=4; percutaneous coronary intervention: n=4). Significant CAD (>70% coronary stenosis) was detected in 31.0% of patients at initial testing and 42.1% at follow-up. Cumulative event-free survival rate to 15 years was 25.0% in patients with wall motion score index (WMSI) ≥1.25 and 91.7% in patients with WMSI <1.25. Cox regression analysis showed the grade of peak WMSI at initial testing to be the only independent predictor of MACEs (relative risk: 3.28; 95% confidence interval: 1.73–6.20) (25).
Although the existence of large to giant aneurysms at the acute phase alone may enhance the risk level of MACEs, the higher WMSI (≥1.25, e.g., over two segments with akinesia induced by DSE) in KD patients identified a subset of patients at higher risk of MACEs. For these patients, an aggressive approach must be undertaken according to AHA guidelines to prevent MACEs during follow-up. In contrast, the incidence of MACEs in KD patients with a negative DSE or lower WMSI (<1.25) is so low that no additional intervention could be indicated.
CIMT
The CIMT is associated with traditional cardiovascular risk factors, as well as atherosclerosis in other vascular beds (including the coronary arteries), and future cardiovascular disease (CVD) events (26). In our previous study, we reported that adolescent and young adult KD patients with CAAs had a greater CIMT than matched controls (27). Therefore, concerns have been raised as to whether KD patients are really at risk for premature atherosclerosis later in adulthood. Since then, the CIMT was compared in several studies between patients with a history of KD and controls. Assessment of studies of the CIMT after KD is complicated by significant study heterogeneity, including differences in subjects’ age, the elapsed time from acute KD, ethnicity, size of the study, and methodology. Dietz et al. recently published a systematic review and meta-analysis of the CVD risk in patients with KD, including a total of 15 published CIMT studies (28). Some found an increased CIMT in all KD patients or in patients with CAAs compared with controls, whereas others did not find any difference; therefore, the results were conflicting. As a whole, there was no significant difference in the mean CIMT between KD patients and controls on statistical meta-analysis,, although there was a trend toward a greater CIMT in KD patients. In addition, they also observed that patients (n=161) with a history of KD (aged 7–20 years) had a significantly increased CIMT following early disease compared with age-matched siblings (n=82) in their cross-sectional study. On plotting the CIMTs of KD patients and controls against the age, an increased CIMT was observed over the entire age range in only KD patients with CAAs. Although the CIMT of patients without CCAs initially increases, the values normalize at a later age, suggesting vascular repair of a generalized vasculopathy. Patients with giant or medium-sized CAAs show a trend toward a continually increasing CIMT, suggesting a more marked impact on the arterial wall (29). Moreover, this trend was validated by their subsequent longitudinal cohort study (KD: n=319, aged 5–43 years; matched control: n=150) over a 15-year period that evaluated the long-term impact of a history of KD on the CVD risk (30). Although these studies have major flaw on the lack of the standardization of the CIMT using the z-score of CIMT (31), these highlight the importance of prospective tracking of this subset of KD patients with CAAs and an increased CIMT for the stratification of future CVD risk. In contrast, there was no evidence of major vascular structural changes in KD patients in whom CAAs had never been detected.
Textural parameters of the intima-media complex (IMC) on B-mode echocardiography
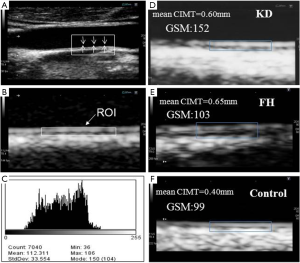
Although it is suggestive that an increased CIMT found long after KD indicates an increased risk of atherosclerosis, this inference must be interpreted with caution because of the different vascular pathophysiology that occurs in KD and that in traditional atherosclerosis. Suzuki et al. examined patients who died 3 to 12 years after KD and found active remodeling of the aneurysmal walls, characterized by intimal proliferation and neoangiogenesis, but there was no evidence of atherosclerosis. Such vascular remodeling was accompanied by the strong expression of vascular growth factors (GFs), including platelet-derived GF, vascular endothelial GF, and transforming GF (TGF)-β1, but no fatty streaks or the accumulation of macrophages, as is seen in atherosclerosis (32). Although we cannot extrapolate readily these findings to other non-coronary vascular structures such as the carotid artery, the finding of GFs in arterial remodeling could explain the thickened CIMT in patients long after KD with CAAs. These histopathological findings were consistent with our results based on the textural parameter of the carotid IMC using the gray scale median (GSM) in KD patients with CAAs compared with controls, in which we demonstrated a higher GSM in KD patients that may indicate the alteration of tissue components and heterogeneity of the carotid IMC, suggesting the development of arteriosclerotic vascular remodeling after vasculitis. This is distinct from that of atherosclerosis with lower GSM often observed in familial hypercholesterolemia (33) (Figure 2).
Flow-mediated dilatation (FMD)
Endothelial dysfunction is one of the earliest changes identified to date during various types of vascular remodeling (34). This modality is based on the concept of reactive hyperemia caused by increased shear stress that results in the endothelial release of nitric oxide and correlates with the coronary endothelial function. Decreased percentage of flow-mediated dilatation (%FMD) reflects endothelial dysfunction (35).
Since the 1st report of FMD on KD by Celermajer et al. (36), the endothelial function has been studied in patients with a history of KD by many investigators. There is some debate about whether endothelial dysfunction is pervasive in the KD population, as prior studies have shown discrepant findings. A recent meta-analysis analyzed 18 studies on the endothelial function of patients at various time point years after KD, and found extensive heterogeneity in the results. In general, most studies found a reduced endothelial function (lower %FMD) in KD patients with CAAs compared with controls; however, the results for subjects without CAAs were more variable (28). In the articles by Silva et al. (37) and Sabri et al. (38), mean values of %FMD in the control group ranged from 6.2% to 8.0%, which are lower than those in other reports. Therefore, discrepant results for KD patients might be partially caused to the differences in %FMD values of the control group (39). Whether this is related to differences in ethnicity, diet, or methodology for the measurement of FMD remains unclear.
As for the analysis of relevant factors for a reduced %FMD, Ishikawa et al. investigated %FMD in younger children who were within 5 years after the onset of KD. They reported that %FMD was inversely correlated with the total duration of fever during the acute phase of KD (40). Recently, Mori et al. also reported that %FMD of children with a history of KD was significantly lower than that in the control subjects, and a febrile period >10 days during the acute phase of KD was an independent risk factor for endothelial dysfunction regardless of the presence of CCAs (41).
These studies suggest that prolonged inflammatory process during the acute phase of KD may cause remaining vascular injury for several years. However, none of the studies analyzed the implications for management, and the outcomes in patients long after KD are unknown.
Carotid contrast-enhanced ultrasonography (CEUS)
Although direct visualization of the carotid adventitial vasa vasorum (VV) and intraplaque neovascularization (IPN) using carotid CEUS has emerged as a new surrogate marker for the early detection of atherosclerosis and vulnerability (42,43), its clinical implications for KD patients remain unclear.
Previous experimental studies showed that the adventitia may become the primary early site for the vessel wall response to arterial injury, which includes inflammatory cell accumulation, myofibroblast migration, and VV expansion (44). In fact, Takahashi et al. demonstrated that the inflammatory changes in the adventitia occur prior to the changes in the intima in various animal models of KD vasculitis (45). Furthermore, Hamaoka-Okamoto et al. revealed that the proliferation of adventitial VV was accompanied by an increase in the inflammation confirmed by a scanning electron microscope (SEM) in a murine model of KD vasculitis (46). Additionally, in specimens of coronary arteries from KD patients with CAAs, Suzuki et al. showed that the overexpression of GFs was observed in the microvessels of the thick intima and there was an expanded VV network in the adventitia (32). These results support the hypothesis that VV proliferation might contribute to the progression of arteriosclerotic vascular remodeling, acting as a conduit for the entry of various cytokines, GFs, and blood cells in KD patients with CAAs (47).
To investigate whether carotid CEUS enables the direct visualization and quantitative evaluation of the VV, we measured the CIMT in conjunction with CEUS after a bolus injection of Sonazoid (perflubutane, GE Healthcare, Oslo, Norway) in adult KD patients with giant CAAs (worst-ever z-score ≥10) and matched controls. We set the regions of interest (ROIs) in the vessel lumen (L) and wall of the common carotid artery (W), and a time-intensity curve (TIC) was generated for each area. The enhanced intensity (EI) was calculated by subtracting the baseline intensity (BI) from the peak intensity (PI) in the vessel lumen (EIL) and wall (EIW), and the ratio of EIW and EIL (EIW/EIL) as a quantitative marker of VV, and they were compared between KD patients and controls.
Carotid CEUS enabled direct visualization by tracing the microbubbles moving into the carotid wall from the adventitial VV and quantitative assessment of the carotid VV network using TIC analysis in all KD patients and controls (Figures 3,4). CEUS demonstrated a significantly increased mean CIMT and significantly increased density of the VV network in KD patients with CAAs. Previous studies demonstrated that semi-quantification of the VV on CEUS (increased density of the adventitial VV) correlated with VV quantification on histology confirmed by human specimens obtained from endarterectomy (48,49). Thus, carotid CEUS may be useful for assessing the degree of VV proliferation in association with arteriosclerotic remodeling in KD patients with CAAs (unpublished data).
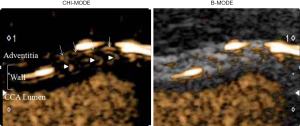
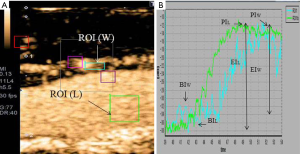
Conclusions
We reviewed the latest updates on echocardiography and ultrasound for KD. The use of multiple novel approaches of echocardiography would provide valuable information for vascular health and may serve as a method to stratify the risk of CV events in KD patients with CAAs. Until further longitudinal data become available, KD patients should be followed long-term and assessed for known CV risks according to the AHA guidelines (6), with particular attention paid to the degree of initial CA involvement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Orenstein JM, Shulman ST, Fox LM, et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One 2012;7:e38998. [Crossref] [PubMed]
- Son MB, Newberger JW. Kawasaki disease. Pediatr Rev 2013;34:151-62. [Crossref] [PubMed]
- Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol 2012;22:216-21. [Crossref] [PubMed]
- JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2008)--digest version. Circ J 2010;74:1989-2020. [Crossref] [PubMed]
- de Zorzi A, Colan SD, Gauvreau K, et al. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr 1998;133:254-8. [Crossref] [PubMed]
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease:a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747-71. [Crossref] [PubMed]
- Manlhiot C, Millar K, Golding F, et al. Improved classification of coronary artery abnormalities based only on coronary artery z-score after Kawasaki disease. Pediatr Cardiol 2010;31:242-9. [Crossref] [PubMed]
- Kurotobi S, Nagai T, Kawakami N, et al. Coronary diameter in normal infants, children and patients with Kawasaki disease. Pediatr Int 2002;44:1-4. [Crossref] [PubMed]
- Tan TH, Wong KY, Cheng TK, et al. Coronary normograms and the coronary-aorta index: objective determinants of coronary artery dilatation. Pediatr Cardiol 2003;24:328-35. [Crossref] [PubMed]
- McCrindle BW, Li JS, Minich LL, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation 2007;116:174-9. [Crossref] [PubMed]
- Olivieri L, Arling B, Friberg M, et al. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiogr 2009;22:159-64. [Crossref] [PubMed]
- Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr 2011;24:60-74. [Crossref] [PubMed]
- Kobayashi T, Fuse S, Sakamoto N, et al. A new z-score curve of the coronary arterial internal diameter using the lambda-mu-sigma method in a pediatric population. J Am Soc Echocardiogr 2016;29:794-801.e29. [Crossref] [PubMed]
- Ronai C, Hamaoka-Okamoto A, Baker AL, et al. Coronary artery aneurysm measurement and z-score variability in Kawasaki disease. J Am Soc Echocardiogr 2016;29:150-7. [Crossref] [PubMed]
- Heiss G, Sharrett AR, Barnes R, et al. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol 1991;134:250-6. [Crossref] [PubMed]
- Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007;115:459-67. [Crossref] [PubMed]
- Schmidt WA, Nerenheim A, Seipelt E, et al. Diagnosis of early Takayasu arteritis with sonography. Rheumatology (Oxford) 2002;41:496-502. [Crossref] [PubMed]
- Lima DS, Sato EI, Lima VC, et al. Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J Rheumatol 2002;29:292-7. [PubMed]
- Van Doornum S, McColl G, Jenkins A, et al. Screening for atherosclerosis in patients with rheumatoid arthritis: comparison of two in vivo tests of vascular function. Arthritis Rheum 2003;48:72-80. [Crossref] [PubMed]
- Oflaz H, Mercanoglu F, Karaman O, et al. Impaired endothelium-dependent flow-mediated dilation in Behcet’s disease: more prominent endothelial dysfunction in patients with vascular involvement. Int J Clin Pract 2005;59:777-81. [Crossref] [PubMed]
- Wu TH, Kuo HC, Tain YL, et al. Common carotid artery intima-media thickness is useful for diagnosis of the acute stage of Kawasaki disease. BMC Pediatr 2014;14:98. [Crossref] [PubMed]
- Tsuda E, Kamiya T, Ono Y, et al. Incidence of stenotic lesions predicted by acute phase changes in coronary arterial diameter during Kawasaki disease. Pediatr Cardiol 2005;26:73-9. [Crossref] [PubMed]
- Noto N, Ayusawa M, Karasawa K, et al. Dobutamine stress echocardiography for detection of coronary artery stenosis in children with Kawasaki disease. J Am Coll Cardiol 1996;27:1251-6. [Crossref] [PubMed]
- Kimball TR, Witt SA, Daniels SR. Dobutamine stress echocardiography in the assessment of suspected myocardial ischemia in children and young adults. Am J Cardiol 1997;79:380-4. [Crossref] [PubMed]
- Noto N, Kamiyama H, Karasawa K, et al. Lomg-term prognostic impact of dobutamine stress echocardiography in patients with Kawasaki disease and coronary artery lesions. J Am Coll Cardiol 2014;63:337-44. [Crossref] [PubMed]
- Eikendal AL, Groenewegen KA, Anderson TJ, et al. Common carotid intima-media thickness relates to cardiovascular events in adults aged <45 years. Hypertension 2015;65:707-13. [Crossref] [PubMed]
- Noto N, Okada T, Yamasuge M, et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with Kawasaki disease and coronary artery lesions. Pediatrics 2001;107:1095-9. [Crossref] [PubMed]
- Dietz SM, Tacke CE, Hutten BA, et al. Peripheral Endothelial (Dys)Function, Arterial Stiffness and Carotid Intima-Media Thickness in Patients after Kawasaki Disease: A Systematic Review and Meta-Analyses. PLoS One 2015;10:e0130913. [Crossref] [PubMed]
- Dietz SM, Tacke CE, Gort J, et al. Carotid intima-media thickness in patients with a history of Kawasaki disease. Circ J 2015;79:2682-7. [Crossref] [PubMed]
- Dietz SM, Tacke CE, de Groot E, et al. Extracardial Vasculopathy After Kawasaki Disease: A Long-Term Follow-up Study. J Am Heart Assoc 2016;5:e003414. [Crossref] [PubMed]
- Noto N, Kato M, Abe Y, et al. Reassessment of carotid intima-media thickness by standard deviation score in children and adolescents after Kawasaki disease. Springerplus 2015;4:479. [Crossref] [PubMed]
- Suzuki A, Miyagawa-Tomita S, Komatsu K, et al. Active remodeling of the coronary arterial lesions in the late phase of Kawasaki disease: immunohistochemical study. Circulation 2000;101:2935-41. [Crossref] [PubMed]
- Noto N, Okada T, Abe Y, et al. Characteristics of earlier atherosclerotic involvement in adolescent patients with Kawasaki disease and coronary artery lesions: Significance of gray scale median on B-mode ultrasound. Atherosclerosis 2012;222:106-9. [Crossref] [PubMed]
- Ross R.. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801-9. [Crossref] [PubMed]
- Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2-12. [Crossref] [PubMed]
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992;340:1111-5. [Crossref] [PubMed]
- Silva AA, Maeno Y, Hashmi A, et al. Cardiovascular risk factors after Kawasaki disease: A case control study. J Pediatr 2001;138:400-5. [Crossref] [PubMed]
- Sabri MR, Tavana EN, Ahmadi A, et al. Does Vitamin C improve endothelial function in patients with Kawasaki disease? J Res Med Sci 2015;20:32-6. [PubMed]
- Katayama H.. Analysis of Arterial Endothelial Function Assessed by the Noninvasive Method of Flow-Mediated Dilatation in Patients with a History of Kawasaki Disease: A Review of the Literature. J Pediatric Infect Dis 2016;1:2.
- Ishikawa T, Iwashima S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J Pediatr 2013;163:1117-21. [Crossref] [PubMed]
- Mori Y, Katayama H, Kishi K, et al. Persistent high fever for more than 10 days during acute phase is a risk factor for endothelial dysfunction in children with a history of Kawasaki disease. J Cardiol 2016;68:71-5. [Crossref] [PubMed]
- Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients:early and late signs of symptomatic atherosclerosis. Circulation 2004;110:2843-50. [Crossref] [PubMed]
- Moreno PR, Purushothaman KR, Sirol M, et al. NeovascularizationNeovascularization in human atherosclerosis. Circulation 2006;113:2245-52. [Crossref] [PubMed]
- Moos MPW, John N, Gräbner R, et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2005;25:2386-91. [Crossref] [PubMed]
- Takahashi K, Oharaseki T, Wakayama M, et al. Histopathological features of murine systemic vasculitis caused by Candida albicans extract—an animal model of Kawasaki disease. Inflamm Res 2004;53:72-7. [Crossref] [PubMed]
- Hamaoka-Okamoto A, Suzuki C, Yahata T, et al. The involvement of the vasa vasorum in the development of vasculitis in animal model of Kawasaki disease. Pediatr Rheumatol Online J 2014;12:12. [Crossref] [PubMed]
- Moulton KS, Vakili K, Zurakowski D, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci 2003;100:4736-41. [Crossref] [PubMed]
- Magnoni M, Coli S, Marrocco-Trischitta M, et al. Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur J Echocardiogr 2009;10:260-4. [Crossref] [PubMed]
- Staub D, Patel MB, Tibrewala A, et al. Vasa vasorum and plaque neovascularization on contrast-enhanced carotid ultrasound imaging correlates with cardiovascular disease and past cardiovascular events. Stroke 2010;41:41-7. [Crossref] [PubMed]


