Left atrial function: evaluation by strain analysis
The left atrium has an important role in modulating left ventricular filling, contributing up to a third of cardiac output (1). The left atrium has additionally been identified as an important biomarker of cardiovascular disease and adverse cardiovascular outcomes (2,3). While previously left atrial (LA) size was utilised, the role of LA function as a biomarker is increasingly being evaluated (4), both independently and also in combination with LA size (5-7). However, LA function is complex, comprising of three main components: reservoir function in systole when blood fills the left atrium, as a conduit in early diastole corresponding to passive left ventricular filling and as an active contractile chamber in late diastole (8,9).
Current techniques to evaluate LA function
There is no single parameter that best defines LA function and a variety of parameters have been previously defined (4,10). Transmitral peak A wave velocity (11), its velocity time integral and atrial fraction (12) are well described measures of LA contractile function. The LA ejection force, based on Newtonian principles, incorporates peak A velocity and was used as a marker of LA function (13). Subsequently, tissue Doppler derived A' velocity was utilised as a less load dependent measure of LA contractile function (14,15), demonstrating good correlation with traditional Doppler and LA volumetric measurements. Colour tissue Doppler analysis was able to evaluate segmental LA function (15), demonstrating temporal changes with improved LA function following cardioversion (16). However, using these measures mandates the presence of sinus rhythm (SR). The LA function index (LAFI) was derived to evaluate LA function even in atrial fibrillation (AF) (17). Additionally, volumetric measures including the LA ejection fraction (LAEF) and LA expansion index (LAEI) have been utilised, both in SR and AF (5,6,18).
More recently, strain analysis has been utilised for evaluation of LA function (19,20). Strain evaluates myocardial deformation while strain rate examines the rate of change in strain, and can be measured throughout the cardiac cycle, thereby enabling the evaluation of LA reservoir function (in systole) and conduit and contractile function (in diastole) (5). This review will focus on the various types of strain analysis for evaluation of LA function, alterations in LA strain in physiological and pathologic states that alter LA function and finally evaluate its utility as a prognostic marker.
Strain and strain rate imaging of the LA
Strain and strain-rate imaging have several advantages over conventional echocardiography in evaluation of LA function. Firstly, strain imaging is not evaluated relative to the transducer position, thus allowing discrimination between active and passive myocardial tissue movement (21-24). Strain parameters are relatively independent of tethering effects and is less load dependent compared to traditional parameters of LA function (25,26). Additionally, strain and strain rate parameters permit evaluation of phasic atrial function throughout the cardiac cycle (27). There are as yet no validated strain algorithms that have been developed exclusively for evaluation of LA function. However, several studies have utilised strain software that was developed for the left ventricle, with adjustments to the width of the ‘region of interest’ (ROI) to evaluate LA strain (28, 29).
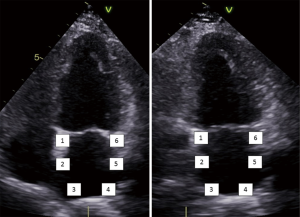
LA strain measurements can be obtained by tissue Doppler imaging (TDI), two dimensional (2D) speckle tracking echocardiography (STE) and velocity vector imaging (VVI). For the latter two techniques, longitudinal strain and strain rate curves are generated for each of six atrial segments, obtained from the apical four and two chamber views (Figure 1) (5). Heterogeneous segmental deformation of the LA has also been observed, with higher values noted in the regions adjacent to the mitral annulus (4,5).
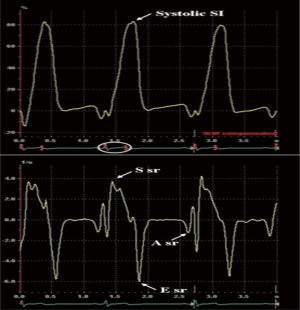
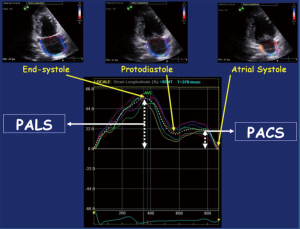
In the reservoir phase, as the LA fills and stretches, there is positive atrial strain that reaches its peak in systole at the end of LA filling, prior to opening of the mitral valve. Following this, passive LA emptying ensues with opening of the mitral valve resulting in decreased atrial strain with negative deflection of the strain curve up to a plateau period which is analogous to diastasis. A second deflection in the strain curve is then observed corresponding to atrial systole. Peak atrial longitudinal strain (PALS) or LA systolic strain is measured at the end of the reservoir phase. Peak atrial contraction strain (PACS) or late diastolic strain, is measured following the P wave and corresponds to active atrial contraction (Figure 2) (30).
LA strain curves have two patterns that differ based on the time in the cardiac cycle from which the software processing begins i.e., either at the onset of the P wave (atrial cycle/diastolic gating) or the onset of the QRS complex (ventricular cycle/systolic gating) (4,5). If the strain processing begins at onset of QRS, ventricular end diastole is the zero reference and peak positive longitudinal strain corresponds to atrial reservoir function, strain during early diastole reflects atrial conduit function and strain during late diastole corresponds to atrial contractile function (Figure 3). Conversely, if software processing begins at onset of P wave, atrial end diastole becomes the zero reference and the first negative peak strain represents the atrial contractile function, positive peak strain corresponds to conduit function, and their sum (strain total) represents reservoir function (Figure 3). Strain rate in ventricular systole (S sr), early diastole (E sr) and late diastole (A sr) correspond to reservoir, conduit, and booster pump functions in both methods (5,30).
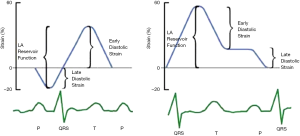
A recent study by Hayashi et al. showed that the degree of correlation between LA strain and parameters of LA function determined by three-dimensional echocardiography were stronger when the onset of P wave was used as the reference than when the QRS onset was used (31). Nevertheless, as timing of reference point selection has not been uniform in studies to date, no standardised method for LA strain analysis has yet been proposed.
Reference values for LA strain and strain rate have been reported (4). In a multicentre study involving 329 healthy subjects, Morris et al. reported LA systolic strain (i.e., PALS) to be 45.5%±11.4% and LA strain rate during late diastole (i.e., PACS) to be −2.11±0.61 s-1. The lowest expected values (using mean −2 SD) was 23.1% for LA systolic strain and −0.91 s-1 for A sr in late diastole (32,33).
LA strain—association with age and gender
Studies have shown a small yet significant relationship between age and LA strain and strain rate. Using tissue Doppler-derived strain, Boyd et al. showed significant reductions in global LA systolic strain and strain rates with ageing. A reduction in S sr and E sr was noted from the 6th decade with a corresponding compensatory increase in A sr. Moreover, these changes in strain rate were observed almost a decade prior to similar changes in traditional atrial phasic volume parameters (34).
Using volumetric assessments, Meel et al. demonstrated a decreasing LA conduit function with ageing while reservoir function remained unchanged and contractile function augmented. Of interest however, the authors also established a trend of declining LA systolic strain by 2D STE in the older age groups, while LA contractile strain remained unaffected (35). Similar findings were reported by Morris et al. using 2D STE in healthy controls (32).
Recent work by Yoshida et al. (36) have alluded to a significant interaction between gender and LA function, an association which had not been demonstrated in previous studies utilising volumetric assessments. The study which investigated the impact of gender differences on the relationship between stroke risk and LA mechanics, demonstrated lower values of LA systolic strain amongst women in a cohort of 414 subjects with paroxysmal and/or persistent AF. Female gender was found to have a significant interaction with stroke risk and LA function but not LA size (36).
Correlation of LA strain with diastolic dysfunction
Although a myriad of risk factors have been shown to contribute to diastolic dysfunction, the specific pathophysiological mechanisms for the transition from impaired diastolic function to a clinical state of symptomatic diastolic heart failure (HF) has not been well defined (37-39). LA strain has been shown to correlate with the degree of diastolic dysfunction. In a study comparing 329 normal adults to 377 adults with diastolic dysfunction, Morris et al. demonstrated that LA function derived both volumetrically and by 2D STE strain, was inversely related to LV filling pressure (mitral E/E' ratio) and to the degree of LV diastolic dysfunction (32). LA systolic strain was also found to correlate with invasively determined LV end diastolic pressure as well as levels of N-terminal pro-B-type natriuretic peptide as reported by Kurt et al. (40). Using Doppler-derived strain, Guan et al. demonstrated significant changes in LA strain rate parameters between different grades of LV diastolic dysfunction in subjects with preserved LVEF, despite no significant difference in LA volume between the groups (41).
Compared with patients with normal diastolic function, patients with mild diastolic dysfunction had significantly reduced E sr and S sr but increased A sr. Another study (42) demonstrated that LA systolic strain (measured using STE) inversely correlated with LV end diastolic pressure. Similar to the E/E' ratio, LA systolic strain correlates with LV end diastolic pressure in patients with preserved or mildly reduced LVEF, whereas in patients with moderate or severe reduction of EF, E/E' correlated poorly and LA systolic strain provided a better estimation of LV filling pressures. In diastolic dysfunction, LA strain is likely altered secondary to increased LV filling pressures, i.e., LA afterload, with consequent mechanical stress on the LA leading to reduced reservoir function (43,44).
LA strain by 2D STE
STE is a newer echocardiographic technique for strain and strain rate analyses that tracks ‘speckles’ or natural acoustic markers in the 2D ultrasound image. The geometric shift of each speckle position is traced throughout the cardiac cycle (4,5,45).
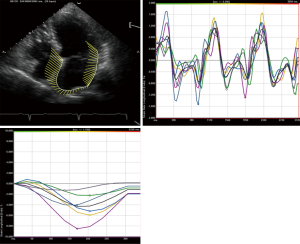
STE strain is increasingly applied in the study of LA mechanics. Apical four and two chamber view images of the LA are obtained using conventional 2 dimensional echocardiography, at relatively high frame rates (60–80 fps). The LA endocardium is traced in both four and two chamber views and the ROI adjusted to the thinner wall of the atrium. In regions of discontinuities of the LA wall, such as areas corresponding to pulmonary veins and LA appendage, extrapolation of the LA endocardial and epicardial surfaces at the junction of these structures are performed to obtain the ROI. The ROI is divided into six segments and the total of 12 segments (Figure 1) is analysed with the software generating the individual segmental longitudinal strain curves together with global strain in each view (Figure 4) (4,20,30).
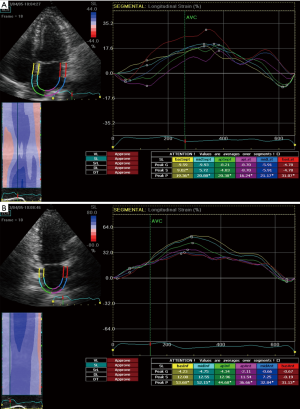
The feasibility and reproducibility of STE for the study of LA mechanics have been validated in several studies (28,46-49), and can be obtained in ~90% of cases. The major limitation is the necessity for adequate 2 dimensional image quality and acquisition at a relatively high frame rate (4,5). Though its utility is presently still limited to research settings, there is rapidly emerging data regarding the role of LA strain in various physiological and disease states which will be discussed below.
Hypertension
Hypertension is associated with morphologic and functional abnormalities of the LA. An increase in LA size in hypertensive patients is a common finding with alterations in LA strain using tissue Doppler-derived strain imaging (50).
In a study by Mondillo et al. investigating 2D STE LA strain indices in hypertensive patients with normal LA size, LA strain was reduced despite normal volumetric measures of LA function, suggesting that strain abnormalities precede structural LA changes in hypertension (51). These findings were confirmed by Sahebjam et al. in a similar study of hypertensive patients relative to healthy controls (52).
2D STE is also useful in determining the effects of medications on LA function in patients with hypertension. Degirmenci et al. showed that LA reservoir, conduit, and booster pump functions improved after treatment with renin-angiotensin receptor blockers and beta blockers for 12 months in patients with mild to moderate hypertension. The improvement of LA systolic strain occurred in conjunction with blood pressure lowering, with a corresponding decrease in LA volumes. No significant difference was found between the angiotensin receptor blockers and the beta blocker treatment groups with respect to the LA changes suggesting that improvement of LA function was primarily dependent on blood pressure control rather than the class of antihypertensive agents (53).
Diabetes mellitus
A common coexistent cardiac risk factor, diabetes mellitus is similarly associated with structural and functional changes of the left atrium. The prevalence of LV diastolic dysfunction is significantly greater in diabetic patients than in the general population. (54-57).
Though relatively less studied than hypertension, diabetes is an independent contributor to LA enlargement and dysfunction. Earlier studies based on LA phasic volume assessment and tissue Doppler derived strain rate imaging demonstrated impairment in LA reservoir and conduit function in diabetes, occurring in association with increased LV mass, abnormal LV geometry and LV diastolic dysfunction with an associated increase in LA contractile function (58-61). In a recent study comparing 73 type 2 diabetic patients to age and gender matched normal controls Kadappu et al. demonstrated larger indexed LA volumes in diabetics, independent of the effect of hypertension and diastolic dysfunction. A significant reduction in 2D STE LA global strain compared to the normal controls, with no significant alteration in LA strain with increasing grades of diastolic dysfunction was observed, suggesting the independent effect of diabetes on the LA function (62). These findings were mirrored by Muranaka et al. in a study assessing LV and LA function by strain rate imaging in diabetics. Impairment of LA reservoir and conduit function by STE strain rate was demonstrated in diabetic patients even in the absence of LV hypertrophy, hypertension or diastolic dysfunction (63).
In a study involving diabetic and hypertensive patients with normal LA size, Mondillo et al. demonstrated impairment in LA strain that preceded changes in volumetric measures of LA function (51).
Chronic kidney disease (CKD)
LA enlargement has been well described in end stage renal disease and maximum LA volume demonstrated to be an independent prognostic factor in this population (64). The cause for LA changes are multifactorial and have traditionally correlated with presence of LV hypertrophy, degree of diastolic dysfunction as well as common co-existing cardiovascular risk factors such as hypertension and diabetes mellitus (65-67).
There is emerging data that CKD is an independent factor affecting changes in LA function. In a study comparing stage 3 CKD patients to age and risk factor matched controls as well as healthy controls, Kadappu et al. reported PALS to be reduced in both CKD group and hypertensive control group compared to healthy controls, with a significant difference among the three groups, pointing to an independent and incremental effect of CKD on LA function (28). These findings were further corroborated in a larger study involving 228 participants whereby PALS and ASr were noted to be significantly altered in the CKD group, with LA strain being the most sensitive parameter of myocardial involvement with alterations in LA strain proceeding changes to LA volume (66). Similar findings were demonstrated by Ohara et al. in their study investigating LA function in CKD patients with normal LA size; LA strain was impaired in CKD patients despite normal LA size, and in comparison to the control group (68).
Ischaemic heart disease
LA strain provides useful information in patients with ischaemic heart disease. PALS has been noted to decrease with reducing LV systolic and diastolic function in patients with myocardial infarction treated with percutaneous coronary intervention; interestingly, PALS demonstrated improvement in patients who underwent cardiac rehabilitation (69-70). Additionally, reduced PALS was shown to predict increased risk of new-onset AF after coronary artery bypass graft surgery (4,5).
PALS is also a predictor of cardiovascular events in patients who have suffered acute myocardial infarction. In a study evaluating 2D STE PALS in 320 patients post AMI, Antoni et al. demonstrated that PALS was an independent predictor of all-cause mortality, re-infarction and HF hospitalisation after adjustment for clinical and other echocardiographic parameters (71). The findings of this study were however contradicted by Ersbøll et al. in a similar study investigating 2D STE LA strain in 843 post infarct patients. Ersbøll et al. demonstrated PALS was associated with the composite outcome for HF and death, but not after adjustment for clinical and echocardiographic parameters, bringing into question the independent prognostic value of LA strain in patients post myocardial infarction (72).
HF
2D STE LA strain has had an increasing role in the management of patients with HF. LA strain provides an accurate surrogate estimate of LV filling pressure, an important factor in the diagnosis of HF as well as for guiding HF therapy. In a study of 36 patients with advanced systolic HF (EF <35%) undergoing right heart catheterisation, Cameli et al. demonstrated that LA systolic strain had the highest diagnostic accuracy, sensitivity and specificity in predicting elevated LV filling pressure and was the best determinant of pulmonary capillary wedge pressure (58). This was corroborated in a further study of 80 patients undergoing left heart catheterisation. LA systolic strain had a better correlation to invasively obtained LV filling pressures compared to Doppler indices, across patient groups with varying LV ejection fraction. Mean E/E' ratio provided good correlations with LVEDP in patients with preserved or mildly reduced LV ejection fraction but correlated poorly with patients with moderate to severe reductions of LV ejection fraction (42). Similar findings were shown by Kurt et al. in 62 patients undergoing cardiac catheterisation whereby LA systolic strain was noted to correlate strongly with LVEDP and N-terminal pro-B-type natriuretic peptide levels (40).
In HF patients with preserved ejection fraction (HFpEF), 2D STE LA strain could differentiate between patients with diastolic dysfunction and those with clinical HFpEF. In 64 patients undergoing simultaneous right heart catheterisation and echocardiographic imaging, Kurt et al. demonstrated that LA systolic strain was significantly lower in patients with HFpEF than in patients who had LV diastolic dysfunction without HF. The LA stiffness index, which was calculated as the ratio of LA systolic strain to E/E', was noted to be the most accurate index to differentiate patients with HFpEF from diastolic dysfunction (39).
In patients with HF with reduced ejection fraction (HFrEF), 2D STE LA strain was significantly lower in idiopathic cardiomyopathies when compared with ischemic cardiomyopathy (73). In cardiac amyloidosis, Modesto et al. highlighted the presence of LA dysfunction as detected by LA strain, above and beyond that due to diastolic impairment, suggesting an independent effect of amyloidosis on the LA (74). In hypertrophic cardiomyopathy, LA strain was reduced compared to healthy controls and patients with secondary LVH resulting from hypertension (75,76).
2D STE LA strain has also been shown to be an independent predictor of exercise capacity in both HFrEF and HFpEF. Kusunose et al. demonstrated that PALS was an independent predictor of estimated metabolic equivalents in patients with HFpEF (77). These findings were further affirmed by D’Andrea et al. in both HFpEF and HFrEF (73).
2D STE LA strain has also demonstrated prognostic value in HF and can additionally be utilised as an indicator of treatment response for HF therapies. LA systolic strain was an independent predictor of death and need for heart transplantation in addition to age, LV ejection fraction and brain natriuretic peptide in a study by Helle-Valle et al. involving 143 patients with symptomatic HFrEF (78). Response to cardiac resynchronisation therapy has been associated with improvements in PALS in patients with ischaemic and non-ischaemic cardiomyopathies (79-81).
Valvular heart disease
2D STE LA strain is decreased in patients with mitral and aortic valve disease and useful in prediction of clinical outcomes. In mitral regurgitation, PALS by STE was shown to decrease with increasing severity of mitral regurgitation (82) and was an independent predictor of mitral valve surgery and post-operative outcomes. The study by Debonnaire et al. investigating 121 patients with severe primary mitral regurgitation demonstrated PALS to have the highest accuracy in identifying patients who proceeded to mitral valve surgery amongst all indices of LA function. Participants with LA systolic strain of ≤24% showed worse survival during follow up regardless of symptom status (83). These findings were mirrored by Yang et al. in a similar study involving 104 patients with asymptomatic severe mitral regurgitation (84). Moreover, Borg et al. demonstrated that LA systolic strain was an independent predictor of postoperative AF in those undergoing mitral valve surgery for severe mitral regurgitation (85).
In mitral stenosis, LA systolic strain is impaired even in asymptomatic patients and can predict development of AF and cardiovascular events. In 101 patients with rheumatic mitral stenosis, Ancona et al. demonstrated reduced LA systolic strain, the degree of which correlated with worse cardiovascular outcomes during a 3-year follow-up, irrespective of LA volume, age and mitral valve area. LA systolic strain was also the most powerful predictor of new onset AF at 4-year follow-up (86).
In patients with aortic stenosis (AS), O’Connor et al. and Lisi et al. demonstrated that LA systolic strain closely correlated with LA reverse remodelling in patients post aortic valve replacement (87,88). Similar to mitral valve disease, LA systolic strain had predictive value for adverse outcomes in patients with AS. In a study by Galli et al. involving 128 patients with severe AS, reduced LA systolic strain was associated with an increase in all-cause mortality, worsening HF and cardiac hospitalization (89). LA systolic strain was also a predictor of postoperative AF in patients with severe AS undergoing surgery (90).
AF
AF is the commonest clinical arrhythmia and is associated with increased morbidity and mortality, with thromboembolic stroke being a major associated risk (91,92). LA remodelling and dysfunction is associated with AF (93). LA strain measurements are more sensitive than volumetric measures (5) and recent studies using 2D STE have shown an association between reduced LA reservoir and contractile function and paroxysmal AF that precedes LA enlargement (20,94). Yoon et al. (29) demonstrated that reduced LA systolic strain was the strongest independent echocardiographic predictor of progression from paroxysmal to persistent AF. Another study demonstrated that LA strain was predictive of paroxysmal AF in patients with cryptogenic stroke, even in patients with normal LA size (95).
Catheter ablation pulmonary vein isolation can be an effective treatment for AF; however AF recurrence is still a major issue (96). Known predictors of AF recurrence post catheter ablation include LA dilatation, advanced age, hypertension and persistent as opposed to paroxysmal AF (97,98). Studies in AF patients who have undergone catheter ablation with restoration of SR have shown a relationship between LA systolic strain and AF recurrence (44,99-102). In particular, this relationship also applies when the assessment of the LA function by LA systolic strain is performed irrespective of rhythm (i.e., in both AF and SR post-procedure) (101). Studies have additionally demonstrated differing relationships between regional LA strain (in particular LA lateral strain) (100,101) and LA global strain (44,99,102) in predicting recurrence of AF. Predicting the likelihood of AF recurrence using LA strain analysis would be very valuable in candidate selection for catheter ablation.
LA strain and stroke
Understanding the relationship between LA strain and ischemic stroke could be of importance, especially for risk-stratification in AF and decisions regarding anticoagulation. Obokata et al. (103) demonstrated that global LA systolic strain was independently associated with acute embolism in patients with paroxysmal or persistent AF, and provided incremental diagnostic value over the CHA2DS2-VASc score. In another retrospective case-control study (104), reduced LA systolic strain was associated with increased risk of stroke and transient ischemic attacks in patients with paroxysmal AF and low CHADS2 scores (≤1 prior to stroke). In a small retrospective study of 66 patients with permanent AF, Shih et al. (105) demonstrated that LA systolic strain and peak systolic strain rate were independently associated with previous stroke. Furthermore, Hsu et al. demonstrated that an increased ratio of transmitral E-velocity to LA strain and reduced LA strain were associated with cerebrovascular events and provided incremental value to a model containing CHA2DS2-VASc score and LV function for predicting subsequent stroke (106).
Several studies have also demonstrated that LA strain is predictive of a subsequent diagnosis of AF in patients with ischemic stroke (95,107,108). These studies support the hypothesis that atrial functional remodelling and reduced atrial contraction can result in subsequent thromboembolism (8). Therefore, LA strain analysis may provide an invaluable prognostic tool for AF; albeit larger prospective studies in stroke patients are required to confirm these early reports.
LA strain by TDI
TDI depicts myocardial motion (measured as tissue velocity) at specific locations in the heart. Integration of velocity over time yields displacement or the absolute distance moved by that point.
Tissue-Doppler LA strain and strain rate are measured offline from colour tissue Doppler images of the atria obtained in the apical four and two chamber views, at high frame rates (>100 fps). A narrow sample volume (9 mm × 1 mm) is selected due to the thin atrial walls, as compared with the sample volume used for LV strain measurements (9 mm × 9 mm). The sample volume is placed superiorly in each of the four LA walls; septal and lateral walls in the apical four chamber view and the inferior and anterior walls in the apical two chamber view (109). The sample volume is then tracked frame by frame, within this position in the LA wall, to prevent sampling of blood pool. The superior location in the LA walls is selected to avoid interference from mitral annular motion. Peak LA systolic strain was measured by adjusting the electrocardiogram gating to the start of the QRS complex (systolic gating) (Figure 5). Atrial strain rate was measured in S sr, E sr and A sr (Figure 6).
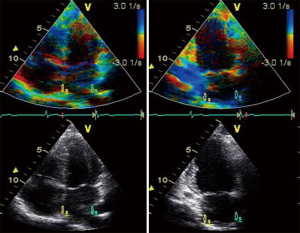
From a physiological precept, healthy ageing is associated with alterations in LV systolic and diastolic strain, with corresponding changes in atrial strain. Doppler LA strain parameters demonstrate age related changes earlier than corresponding volumetric measurements of phasic LA function (34).
Tissue Doppler assessment of atrial strain has been utilised in a variety of clinical settings to quantitate atrial function, remodelling and changes in phasic atrial function. Evaluations have included patients with hypertension (19), hypertrophic cardiomyopathy (79,110,111), Fabry disease (112), atrial septal defects (113), valvular stenosis (114,115) and AF (109,116).
TDI LA strain also has demonstrated prognostic value. Decreased LA systolic strain has been associated with increased LV end-diastolic pressure (58,117) and is thus, a predictor of diastolic HF (40). LA TDI strain has also been shown to be an important predictor of maintenance of SR following both cardioversion (118) and ablation for AF (119). In the only outcome based study using LA TDI strain, Paraskevaidis et al. showed that total TDI atrial strain in patients with hypertrophic cardiomyopathy was the strongest predictor of 12-month outcomes (110).
More recently TDI LA strain has been used to demonstrate reduced reservoir and conduit phasic function with preserved active contractile function in patients with metabolic syndrome (120). The use of tissue Doppler techniques in this cohort may have been of particular importance in the setting of potential suboptimal 2D image quality.
LA strain by VVI
VVI is a novel echocardiographic method that combines speckle tracking and endocardial border detection. Similar to 2D STE strain imaging, VVI is angle independent but has additional advantages of simpler and faster tracking/processing times compared to conventional STE with the use of a continuously self-updating software and requires only a single frame tracing of the endocardial border (121,122).
With VVI analysis, 2D images of apical four and two chamber views are obtained with recommended frame rates between 70–100 Hz. The endocardium of the LA is manually traced in the four and two chamber views and velocity vectors are generated in cine loop format. The ROI is delineated and tracked. The displacement of LA endocardial pixels of the ROI and the velocity of deformation in every frame with the elongation or shortening of myocardium throughout the cardiac cycle, are the strain and SR measures which are calculated automatically (Figure 7). Special reference settings are applied, including valve annulus, chamber borders and tissue motion (122,123).
VVI has been shown to be feasible and less time consuming in assessing LA volumes and function. In a study by Valocik et al. retrospectively assessing 100 transthoracic echocardiograms, LA volumes derived from VVI time volume curves had a good correlation with conventional LA volume assessment. A moderate level of correlation was noted with respect to LAEF. VVI led to a 62% reduction in measurement time in comparison to conventional 2D assessment (123). These findings were corroborated by Motoki et al. in a separate study involving 127 patients with AF. Measurement of LA strain and SR by VVI and 2D STE was noted to be feasible in a large proportion of patients with comparable strain and strain rate measurements using the two techniques (124).
LA strain assessed by VVI has shown clinical utility in patients with HF. Esmaeilzadeh et al. demonstrated that LA strain by VVI was significantly lower in patients with HFrEF compared to healthy subjects in a study involving 35 patients with LVEF <35% in SR. On multivariable analysis of diastolic parameters, a significant inverse relationship was identified between pulmonary arterial pressure and LA strain suggesting that systolic pulmonary artery pressure in HFrEF may be related to LA contractile dysfunction (125).
In diabetic patients, LA strain by VVI was useful in characterising diastolic function. In a study involving 121 patients with type 2 diabetes mellitus, Jarnert et al. demonstrated that. LA strain by VVI was impaired in type 2 diabetes mellitus patients with mild or moderate LV diastolic dysfunction compared to those without diastolic dysfunction (126).
LA strain and fibrosis vs. MRI vs. histology
A number of reports have established a link between atrial dysfunction and fibrosis especially in the context of AF. Several factors have been implicated in the development of LA fibrosis (127). LA fibrosis is associated with LA functional remodelling. LA fibrosis in the context of AF has been extensively evaluated (128,129). While delayed-enhancement cardiac magnetic resonance imaging (DE-CMR) is considered the ‘gold standard’, and has been used in patients with paroxysmal and persistent AF (130,131), the cost and availability of DE-CMR is a limitation.
Echocardiographic evaluation of LA remodelling (i.e., alteration in size and/or function) has been used as a surrogate for LA fibrosis. More recently, 2D STE LA strain analysis was shown to inversely correlate with the extent of fibrosis detected by DE-CMR in patients with persistent AF (130); additionally persistent AF patients had increased fibrosis as compared with paroxysmal AF, with reduced mid septal and mid lateral segmental LA strain. The same group also evaluated AF recurrence following catheter ablation. AF recurrence was lower in the subgroup with decreased structural remodelling, and this subgroup also demonstrated an increase in LA strain (132). Cameli and colleagues correlated LA strain with histological fibrosis in a group of patients with mitral valve disease (133). A negative correlation was observed between the extent of fibrosis on histology and LA strain (133).
The reversal of LA fibrosis has been demonstrated in animal studies with therapeutic agents including spironolactone, angiotensin converting enzyme inhibitors and angiotensin II receptor blockers (134-136). There are no similar reports in human studies. Kokubu and colleagues reported an improvement in LA strain in a group of hypertensive patients treated with ACEI and ARB, but only in the subgroup with normal LA volume (137). Thus, while there are limited reports, it appears that LA strain may be a surrogate measure of LA fibrosis and may be useful in evaluating therapeutic agents that may reverse fibrosis.
Conclusions
LA function is an important emerging entity and carries significant clinical and prognostic implications. Assessment of LA strain represents a simple, accurate and reproducible technique to evaluate LA function. LA strain and strain rate parameters are more sensitive than conventional parameters of atrial function. Strain parameters demonstrate alterations prior to alterations in LA volumes with new data regarding its prognostic relevance emerging rapidly. However, what is lacking is specific guidelines regarding its measurement (i.e., gating on QRS vs. P wave), standardization of methodology and development of LA specific software algorithms, that is essential for advancement of both future research and clinical application.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Matsuda Y, Toma Y, Ogawa H, et al. Importance of left atrial function in patients with myocardial infarction. Circulation 1983;67:566-71. [Crossref] [PubMed]
- Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006;47:2357-63. [Crossref] [PubMed]
- Tsang TS, Barnes ME, Gersh BJ, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol 2003;42:1199-205. [Crossref] [PubMed]
- Vieira MJ, Teixeira R, Goncalves L, et al. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr 2014;27:463-78. [Crossref] [PubMed]
- Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol 2014;63:493-505. [Crossref] [PubMed]
- Abhayaratna WP, Fatema K, Barnes ME, et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol 2008;101:1626-9. [Crossref] [PubMed]
- Tops LF, Delgado V, Bertini M, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol 2011;57:324-31. [Crossref] [PubMed]
- Hoit BD. Assessing atrial mechanical remodeling and its consequences. Circulation 2005;112:304-6. [Crossref] [PubMed]
- Barbier P, Solomon SB, Schiller NB, et al. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999;100:427-36. [Crossref] [PubMed]
- Leung DY, Boyd A, Ng AA, et al. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J 2008;156:1056-64. [Crossref] [PubMed]
- Manning WJ, Silverman DI, Katz SE, et al. Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol 1995;75:624-6. [Crossref] [PubMed]
- Manning WJ, Silverman DI, Katz SE, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol 1994;23:1535-40. [Crossref] [PubMed]
- Manning WJ, Silverman DI, Katz SE, et al. Atrial ejection force: a noninvasive assessment of atrial systolic function. J Am Coll Cardiol 1993;22:221-5. [Crossref] [PubMed]
- Hesse B, Schuele SU, Thamilasaran M, et al. A rapid method to quantify left atrial contractile function: Doppler tissue imaging of the mitral annulus during atrial systole. Eur J Echocardiogr 2004;5:86-92. [Crossref] [PubMed]
- Thomas L, Levett K, Boyd A, et al. Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr 2003;4:92-100. [Crossref] [PubMed]
- Boyd AC, Schiller NB, Ross DL, et al. Segmental atrial contraction in patients restored to sinus rhythm after cardioversion for chronic atrial fibrillation: a colour Doppler tissue imaging study. Eur J Echocardiogr 2008;9:12-17. [PubMed]
- Thomas L, Hoy M, Byth K, et al. The left atrial function index: a rhythm independent marker of atrial function. Eur J Echocardiogr 2008;9:356-62. [PubMed]
- Lee A, See VA, Lim TW, et al. Atrial fibrillation ablation by single ring isolation versus wide antral isolation: Effects on left atrial size and function. Int J Cardiol 2016;206:1-6. [Crossref] [PubMed]
- Eshoo S, Boyd AC, Ross DL, et al. Strain rate evaluation of phasic atrial function in hypertension. Heart 2009;95:1184-91. [Crossref] [PubMed]
- Yuda S, Muranaka A, Miura T. Clinical implications of left atrial function assessed by speckle tracking echocardiography. J Echocardiogr 2016;14:104-12. [Crossref] [PubMed]
- Sutherland GR, Stewart MJ, Groundstroem KW, et al. Color Doppler myocardial imaging: a new technique for the assessment of myocardial function. J Am Soc Echocardiogr 1994;7:441-58. [Crossref] [PubMed]
- Heimdal A, Stoylen A, Torp H, et al. Real time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013-9. [Crossref] [PubMed]
- Stoylen A, Heimdal A, Bjornstad K, et al. Strain rate imaging by ultrasound in the diagnosis of regional dysfunction of the left ventricle. Echocardiography 1999;16:321-9. [Crossref] [PubMed]
- Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation 2000;102:1158-64. [Crossref] [PubMed]
- Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474-80. [Crossref] [PubMed]
- Marwick TH. Measurement of strain and strain rate by echocardiography: Ready for prime time? J Am Coll Cardiol 2006;47:1313-27. [Crossref] [PubMed]
- Rosca M, Lancellotti P, Popescu BA, et al. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011;97:1982-9. [Crossref] [PubMed]
- Kadappu KK, Abhayaratna K, Boyd A, et al. Independent Echocardiographic Markers of Cardiovascular Involvement in Chronic Kidney Disease: The Value of Left Atrial Function and Volume. J Am Soc Echocardiogr 2016;29:359-67. [Crossref] [PubMed]
- Yoon YE, Oh IY, Kim SA, et al. Echocardiographic Predictors of Progression to Persistent or Permanent Atrial Fibrillation in Patients with Paroxysmal Atrial Fibrillation (E6P Study). J Am Soc Echocardiogr 2015;28:709-17. [Crossref] [PubMed]
- Cameli M, Lisi M, Righini FM, et al. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound 2012;10:4. [Crossref] [PubMed]
- Hayashi S, Yamada H, Bando M, et al. Optimal Analysis of Left Atrial Strain by Speckle Tracking Echocardiography: P-wave versus R-wave Trigger. Echocardiography 2015;32:1241-9. [Crossref] [PubMed]
- Morris DA, Takeuchi M, Krisper M, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 2015;16:364-72. [Crossref] [PubMed]
- Pathan F, D'Elia N, Nolan MT, et al. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 2017;30:59-70.e8. [Crossref] [PubMed]
- Boyd AC, Richards DA, Marwick T, et al. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart 2011;97:1513-9. [Crossref] [PubMed]
- Meel R, Khandheria BK, Peters F, et al. Effects of age on left atrial volume and strain parameters using echocardiography in a normal black population. Echo Res Pract 2016;3:115-23. [Crossref] [PubMed]
- Yoshida K, Obokata M, Kurosawa K, et al. Effect of Sex Differences on the Association Between Stroke Risk and Left Atrial Anatomy or Mechanics in Patients With Atrial Fibrillation. Circ Cardiovasc Imaging 2016;9:e004999. [Crossref] [PubMed]
- Gottdiener JS, Kitzman DW, Aurigemma GP, et al. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons ≥ 65 years of age (the cardiovascular health study). Am J Cardiol 2006;97:83-9. [Crossref] [PubMed]
- Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 2007;49:198-207. [Crossref] [PubMed]
- Kurt M, Wang J, Torre-Amione G, et al. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging 2009;2:10-15. [Crossref] [PubMed]
- Kurt M, Tanboga IH, Aksakal E, et al. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging 2012;13:524-30. [Crossref] [PubMed]
- Guan Z, Zhang D, Huang R, et al. Association of left atrial myocardial function with left ventricular diastolic dysfunction in subjects with preserved systolic function: a strain rate imaging study. Clin Cardiol 2010;33:643-9. [Crossref] [PubMed]
- Cameli M, Sparla S, Losito M, et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016;33:398-405. [Crossref] [PubMed]
- Cameli M, Mandoli GE, Loiacono F, et al. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev 2016;21:65-76. [Crossref] [PubMed]
- Machino-Ohtsuka T, Seo Y, Tada H, et al. Left atrial stiffness relates to left ventricular diastolic dysfunction and recurrence after pulmonary vein isolation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:999-1006. [Crossref] [PubMed]
- Perk G, Tunick PA, Kronzon I, et al. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr 2007;20:234-43. [Crossref] [PubMed]
- Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two dimensional speckle tracking. Cardiovasc Ultrasound 2009;7:6. [Crossref] [PubMed]
- Saraiva RM, Demirkol S, Buakhamsri A, et al. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr 2010;23:172-80. [Crossref] [PubMed]
- Kim DG, Lee KJ, Lee S, et al. Feasibility of twodimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity. Echocardiography 2009;26:1179-87. [Crossref] [PubMed]
- Di Salvo G, Pacileo G, Castaldi B, et al. Two-dimensional strain and atrial function: a study on patients after percutaneous closure of atrial septal defect. Eur J Echocardiogr 2009;10:256-9. [Crossref] [PubMed]
- Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278-85. [Crossref] [PubMed]
- Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr 2011;24:898-908. [Crossref] [PubMed]
- Sahebjam M, Mazareei A, Lotfi-Tokaldany M, et al. Comparison of left atrial function between hypertensive patients with normal atrial size and normotensive subjects using strain rate imaging technique. Arch Cardiovasc Imaging 2014;2:e16081. [Crossref]
- Degirmenci H, Duman H, Demirelli S, et al. Assessment of effect of irbesartan and nebivolol on the left atrium volume and deformation in the patients with mild-moderate hypertension. Eur Rev Med Pharmacol Sci 2014;18:781-9. [PubMed]
- Poulsen MK, Henriksen JE, Dahl J, et al. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging 2010;3:24-31. [Crossref] [PubMed]
- Nichols GA, Hillier TA, Erbey JR, et al. Congestive heart failure in type 2 diabetes: prevalence, incidence and risk factors. Diabetes Care 2001;24:1614-9. [Crossref] [PubMed]
- Boyer JK, Thanigaraj S, Schechtman KB, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004;93:870-5. [Crossref] [PubMed]
- Andersen NH, Poulsen SH, Poulsen PL, et al. Left ventricular dysfunction in hypertensive patients with type 2 diabetes mellitus. Diabet Med 2005;22:1218-25. [Crossref] [PubMed]
- Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound 2010;8:14. [Crossref] [PubMed]
- Huang G, Zhang L, Xie M, et al. Assessment of left atrial function in diabetes mellitus by left atrial volume tracking method. J Huazhong Univ Sci Technolog Med Sci 2010;30:819-23. [Crossref] [PubMed]
- Ernande L, Rietzschel ER, Bergerot C, et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr 2010;23:1266-72. [Crossref] [PubMed]
- Liu Y, Wang K, Su D, et al. Noninvasive assessment of left atrial phasic function in patients with hypertension and diabetes using two-dimensional speckle tracking and volumetric parameters. Echocardiography 2014;31:727-35. [Crossref] [PubMed]
- Kadappu KK, Boyd A, Eshoo S, et al. Changes in left atrial volume in diabetes mellitus: more than diastolic dysfunction?. Eur Heart J Cardiovasc Imaging 2012;13:1016-23. [Crossref] [PubMed]
- Muranaka A, Yuda S, Tsuchihashi K, et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 2009;26:262-71. [Crossref] [PubMed]
- Tripepi G, Benedetto FA, Mallamaci F, et al. Left atrial volume in end-stage renal disease: A prospective cohort study. J Hypertens 2006;24:1173-80. [Crossref] [PubMed]
- Essig M, Escoubet B, de Zuttere D, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant 2008;23:239-48. [Crossref] [PubMed]
- Kadappu KK, Kuncoro AS, Hee L, et al. Chronic kidney disease is independently associated with alterations in left atrial function. Echocardiography 2014;31:956-64. [Crossref] [PubMed]
- Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging 2009;2:93-9. [Crossref] [PubMed]
- Ohara Y, Yoshimura Y, Fukuoka Y, et al. Early detection of left atrial strain abnormalities by speckle-tracking in patients with chronic kidney disease and normal left atrial size. Eur Heart J 2013;34:2914. [Crossref]
- Değirmenci H, Bakırcı EM, Demirtaş L, et al. Relationship of Left Atrial Global Peak Systolic Strain with Left Ventricular Diastolic Dysfunction and Brain Natriuretic Peptide Level in Patients Presenting with Non-ST Elevation Myocardial Infarction. Med Sci Monit 2014;20:2013-9. [Crossref] [PubMed]
- Deniz Acar R, Bulut M, Ergün S, et al. Effect of cardiac rehabilitation on left atrial functions in patients with acute myocardial infarction. Ann Phys Rehabil Med 2014;57:105-13. [Crossref] [PubMed]
- Antoni ML, ten Brinke EA, Atary JZ, et al. Left atrial strain is related to adverse events in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Heart 2011;97:1332-7. [Crossref] [PubMed]
- Ersbøll M, Andersen MJ, Valeur N, et al. The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging 2013;6:26-33. [Crossref] [PubMed]
- D’Andrea A, Caso P, Romano S, et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: A two-dimensional speckle strain study. Int J Cardiol 2009;132:354-63. [Crossref] [PubMed]
- Modesto KM, Dispenzieri A, Cauduro SA, et al. Left atrial myopathy in cardiac amyloidosis: implications of novel echocardiographic techniques. Eur Heart J 2005;26:173-9. [Crossref] [PubMed]
- Anwar AM, Soliman OI, Nemes A, et al. An integrated approach to determine left atrial volume, mass and function in hypertrophic cardiomyopathy by two-dimensional echocardiography. Int J Cardiovasc Imaging 2008;24:45-52. [Crossref] [PubMed]
- Matsumoto K, Tanaka H, Imanishi J, et al. Preliminary observations of prognostic value of left atrial functional reserve during dobutamine infusion in patients with dilated cardiomyopathy. J Am Soc Echocardiogr 2014;27:430-9. [Crossref] [PubMed]
- Kusunose K, Motoki H, Popovic ZB, et al. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012;98:1311-7. [Crossref] [PubMed]
- Helle-Valle T, Opdahl A, Broch K, et al. Left atrial strain by speckle tracking echocardiography in patients with heart failure—an independent and incremental predictor of cardiac death or need of heart transplantation. Circulation 2011;124:A8551.
- D’Andrea A, Caso P, Romano S, et al. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: a two-dimensional speckle strain study. Eur Heart J 2007;28:2738-48. [Crossref] [PubMed]
- Valzania C, Gadler F, Boriani G, et al. Effect of Cardiac Resynchronization Therapy on Left Atrial Size and Function as Expressed by Speckle Tracking 2-Dimensional Strain. Am J Cardiol 2016;118:237-43. [Crossref] [PubMed]
- Teixeira R, Moreira N, Soares F, et al. Left atrial total longitudinal strain improves after cardiac resynchronization therapy. Eur Heart J Cardiovasc Imaging 2012;13:i166.
- Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: left atrial deformation analysis by twodimensional speckle tracking echocardiography. Echocardiography 2011;28:327-34. [Crossref] [PubMed]
- Debonnaire P, Leong DP, Witkowski TG, et al. Left atrial function by two-dimensional speckle-tracking echocardiography in patients with severe organic mitral regurgitation: association with guidelines-based surgical indication and postoperative (long-term) survival. J Am Soc Echocardiogr 2013;26:1053-62. [Crossref] [PubMed]
- Yang LT, Liu YW, Shih JY, et al. Predictive value of left atrial deformation on prognosis in severe primary mitral regurgitation. J Am Soc Echocardiogr 2015;28:1309-17.e4. [Crossref] [PubMed]
- Borg AN, Pearce KA, Williams SG, et al. Left atrial function and deformation in chronic primary mitral regurgitation. Eur J Echocardiogr 2009;10:833-40. [Crossref] [PubMed]
- Ancona R, Comenale Pinto S, Caso P, et al. Two-dimensional atrial systolic strain imaging predicts atrial fibrillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J Am Soc Echocardiogr 2013;26:270-7. [Crossref] [PubMed]
- O'Connor K, Magne J, Rosca M, et al. Impact of aortic valve stenosis on left atrial phasic function. Am J Cardiol 2010;106:1157-62. [Crossref] [PubMed]
- Lisi M, Henein MY, Cameli M, et al. Severity of aortic stenosis predicts early post-operative normalization of left atrial size and function detected by myocardial strain. Int J Cardiol 2013;167:1450-5. [Crossref] [PubMed]
- Galli E, Fournet M, Chabanne C, et al. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging 2016;17:533-41. [Crossref] [PubMed]
- Imanishi J, Tanaka H, Sawa T, et al. Left atrial booster-pump function as a predictive parameter for new-onset postoperative atrial fibrillation in patients with severe aortic stenosis. Int J Cardiovasc Imaging 2014;30:295-304. [Crossref] [PubMed]
- Prystowsky EN, Benson DW, Fuster V, et al. Management of patients with atrial fibrillation. A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation 1996;93:1262-77. [Crossref] [PubMed]
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8. [Crossref] [PubMed]
- Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol 2008;51:1-11. [Crossref] [PubMed]
- Kojima T, Kawasaki M, Tanaka R, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging 2012;13:227-34. [Crossref] [PubMed]
- Pagola J, González-Alujas T, Flores A, et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke 2014;45:e164-6. [Crossref] [PubMed]
- Tzou WS, Marchlinski FE, Zado ES, et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:237-42. [Crossref] [PubMed]
- Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077-81. [Crossref] [PubMed]
- Berruezo A, Tamborero D, Mont L, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007;28:836-41. [Crossref] [PubMed]
- Hwang HJ, Choi EY, Rhee SJ, et al. Left atrial strain as predictor of successful outcomes in catheter ablation for atrial fibrillation: a two-dimensional myocardial imaging study. J Interv Card Electrophysiol 2009;26:127-32. [Crossref] [PubMed]
- Mirza M, Caracciolo G, Khan U, et al. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two-dimensional speckle strain study. J Interv Card Electrophysiol 2011;31:197-206. [Crossref] [PubMed]
- Yasuda R, Murata M, Roberts R, et al. Left atrial strain is a powerful predictor of atrial fibrillation recurrence after catheter ablation: study of a heterogeneous population with sinus rhythm or atrial fibrillation. Eur Heart J Cardiovasc Imaging 2015;16:1008-14. [PubMed]
- Hammerstingl C, Schwekendiek M, Momcilovic D, et al. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol 2012;23:247-55. [Crossref] [PubMed]
- Obokata M, Negishi K, Kurosawa K, et al. Left atrial strain provides incremental value for embolism risk stratification over CHA2DS2-VASc score and indicates prognostic impact in patients with atrial fibrillation. J Am Soc Echocardiogr 2014;27:709-16.e4. [Crossref] [PubMed]
- Azemi T, Rabdiya VM, Ayirala SR, et al. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J Am Soc Echocardiogr 2012;25:1327-32. [Crossref] [PubMed]
- Shih JY, Tsai WC, Huang YY, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr 2011;24:513-9. [Crossref] [PubMed]
- Hsu PC, Lee WH, Chu CY, et al. Prognostic role of left atrial strain and its combination index with transmitral E-wave velocity in patients with atrial fibrillation. Sci Rep 2016;6:17318. [Crossref] [PubMed]
- Olsen FJ, Jørgensen PG, Møgelvang R, et al. Predicting Paroxysmal Atrial Fibrillation in Cerebrovascular Ischemia Using Tissue Doppler Imaging and Speckle Tracking Echocardiography J Stroke. J Stroke Cerebrovasc Dis 2016;25:350-9. [Crossref] [PubMed]
- Kim D, Shim CY, Cho IJ, et al. Incremental Value of Left Atrial Global Longitudinal Strain for Prediction of Post Stroke Atrial Fibrillation in Patients with Acute Ischemic Stroke. J Cardiovasc Ultrasound 2016;24:20-7. [Crossref] [PubMed]
- Boyd AC, Schiller NB, Ross DL, et al. Differential recovery of regional atrial contraction after restoration of sinus rhythm after intraoperative linear radiofrequency ablation for atrial fibrillation. Am J Cardiol 2009;103:528-34. [Crossref] [PubMed]
- Paraskevaidis IA, Farmakis D, Papadopoulos C, et al. Two-dimensional strain analysis in patients with hypertrophic cardiomyopathy and normal systolic function: A 12-month follow-up study. Am Heart J 2009;158:444-50. [Crossref] [PubMed]
- Roşca M, Popescu BA, Beladan CC, et al. Left atrial dysfunction as a correlate of heart failure symptoms in hypertrophic cardiomyopathy. J Am Soc Echocardiogr 2010;23:1090-8. [Crossref] [PubMed]
- Boyd AC, Lo Q, Devine K, et al. Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J Am Soc Echocardiogr 2013;26:1415-23. [Crossref] [PubMed]
- Boyd AC, Cooper M, Thomas L. Segmental atrial function following percutaneous closure of atrial septum using occluder device. J Am Soc Echocardiogr 2009;22:508-16. [Crossref] [PubMed]
- Caso P, Ancona R, Di Salvo G, et al. Atrial reservoir function by strain rate imaging in asymptomatic mitral stenosis: Prognostic value at 3 year follow-up. Eur J Echocardiogr 2009;10:753-9. [Crossref] [PubMed]
- O'Connor K, Magne J, Rosca M, et al. Left atrial function and remodelling in aortic stenosis. Eur J Echocardiogr 2011;12:299-305. [Crossref] [PubMed]
- Thomas L, Mckay T, Byth K, et al. Abnormalities of left atrial function after cardioversion: an atrial strain rate study. Heart 2007;93:89-95. [Crossref] [PubMed]
- Wakami K, Ohte N, Asada K, et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr 2009;22:847-51. [Crossref] [PubMed]
- Di Salvo G, Caso P, Lo Piccolo R, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation 2005;112:387-95. [Crossref] [PubMed]
- Schneider C, Malisius R, Krause K, et al. Strain rate imaging for functional quantification of the left atrium: Atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J 2008;29:1397-409. [Crossref] [PubMed]
- Fang NN, Sui DX, Yu JG, et al. Strain/strain rate imaging of impaired left atrial function in patients with metabolic syndrome. Hypertens Res 2015;38:758-64. [Crossref] [PubMed]
- Chen J, Cao T, Duan Y, et al. Velocity Vector Imaging in Assessing the Regional Systolic Function of Patients with Post Myocardial Infarction. Echocardiography 2007;24:940-5. [Crossref] [PubMed]
- Vannan MA, Pedrizzetti G, Li P, et al. Effect of cardiac resynchronization therapy on longitudinal and circumferential left ventricular mechanics by velocity vector imaging: description and initial clinical application of a novel method using high-frame rate B-mode echocardiographic images. Echocardiography 2005;22:826-30. [Crossref] [PubMed]
- Valocik G, Druzbacká L, Valocikova I, et al. Velocity vector imaging to quantify left atrial function. Int J Cardiovasc Imaging 2010;26:641-9. [Crossref] [PubMed]
- Motoki H, Dahiya A, Bhargava M, et al. Assessment of left atrial mechanics in patients with atrial fibrillation: comparison between two-dimensional speckle-based strain and velocity vector imaging. J Am Soc Echocardiogr 2012;25:428-35. [Crossref] [PubMed]
- Esmaeilzadeh M, Nikparvar M, Maleki M, et al. Assessment of Inter and Intra-atrial Asynchrony in Patients with Systolic Heart Failure Using Velocity Vector Imaging. Res Cardiovasc Med 2013;2:114-20. [Crossref] [PubMed]
- Jarnert C, Melcher A, Caidahl K, et al. Left atrial velocity vector imaging for the detection and quantification of left ventricular diastolic function in type 2 diabetes. Eur J Heart Fail 2008;10:1080-7. [Crossref] [PubMed]
- Thomas L, Abhayaratna WP. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc Imaging 2017;10:65-77. [Crossref] [PubMed]
- Frustaci A, Chimenti C, Bellocci F, et al. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96:1180-4. [Crossref] [PubMed]
- Boldt A, Wetzel U, Lauschke J, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004;90:400-5. [Crossref] [PubMed]
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231-9. [Crossref] [PubMed]
- Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498-506. [Crossref] [PubMed]
- Kuppahally SS, Akoum N, Badger TJ, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 2010;160:877-84. [Crossref] [PubMed]
- Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol 2013;111:595-601. [Crossref] [PubMed]
- Milliez P, Deangelis N, Rucker-Martin C, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J 2005;26:2193-9. [Crossref] [PubMed]
- Li Y, Li WM, Gong YT, et al. The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol 2007;102:245-56. [Crossref] [PubMed]
- Yoon N, Cho JG, Kim KH, et al. Beneficial effects of an angiotensin-II receptor blocker on structural atrial reverse-remodeling in a rat model of ischemic heart failure. Exp Ther Med 2013;5:1009-16. [Crossref] [PubMed]
- Kokubu N, Yuda S, Tsuchihashi K, et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res 2007;30:13-21. [Crossref] [PubMed]