Role of echocardiography for takotsubo cardiomyopathy: clinical and prognostic implications
Introduction
Takotsubo cardiomyopathy (TTC) was first described in the Japanese population by Sato et al. in the 1990s (1). Since then, its pathophysiology has been widely known in the field of cardiology. The American Heart Association has categorized TTC as a type of secondary cardiomyopathy (2). TTC occurs in approximately 2% of the patients with acute coronary syndrome (3). Its onset is rare; however, its specific features play an important role in diagnosing the chest pain in clinical practice. Transient left ventricular (LV) wall motion abnormalities are peculiar in TTC and these abnormalities are not triggered by obstructive coronary artery disease (3-7). Post menopausal patients under emotional and physical stress predominantly suffer from this syndrome. TTC has generally favorable outcomes and LV wall motion abnormalities are rapidly improved. Meanwhile, according to the results of the earlier studies, 52% of the TTC patients have various complications (8-14); thus, TTC should be regarded as a more serious cardiovascular disease.
The pathophysiology of TTC is complicated, including the systemic physiological responses to severe stress and the cardiovascular responses to a sudden surge in administered catecholamines. Several hypotheses have been presented to explain this unique cardiac disorder in TTC (15-19) and broadly divided into a vascular or myocardial cause. These may not be mutually exclusive because the entire cardiovascular system is exposed to the same catecholamine storm. Since no pathophysiological mechanisms have elucidated TTC, many of these hypotheses are still being investigated. Epicardial coronary artery vasospasm and consequently regionally stunned myocardium may induce reversible LV wall motion abnormalities (1). Several studies investigating vasospastic provocation in the acute phase of TTC demonstrated positive results (10–43%) but not in all TTC patients (4,20). Various studies have reported that the endothelin levels are higher in patients with TTC than those with AMI (21). The coronary risk factors, including smoking, are also associated with endothelin function. Although the prevalence of smokers in TTC is varied (6–49%), the largest series have reported 22% which is higher than that in general population and similar to that in AMI (22). In fact, patients with TTC have the greater number of other comorbid cardiovascular risk factors than the general population, which is similar to those with AMI. It seems that the susceptibility to TTC may partially be related to a high prevalence of risk factors leading to endothelial dysfunction, which, in turn, might be a predisposing factor of TTC (23). Endothelin is a potential vasoconstrictor and mechanisms to affect multivessel vasospasm. It is quite difficult to distinguish a causation from an association because generalized impaired endothelial function secondary to oxidative stress appears after any stress and surge in catecholamines. If no spontaneous coronary spasm is identified, impaired coronary artery flow due to vulnerable plaque rupture should be assumed as a cause of TTC. However, a vascular cause has a low possibility to be a primary trigger for TTC due to the following reasons: (I) the discrepancies between severe LV dysfunction and cardiac enzyme elevation; (II) ECG findings; and (III) spontaneous or triggered coronary arterial spasm has not been found in all TTC cases. Several factors may synergistically influence TCC; some mechanistic studies have reported the conflicting results. Dobutamine or epinephrine, which has a dominant coronary vasodilatory effect, induces clinical cases (24): likewise, the high dose of β-adrenoceptor agonist isoproterenol induces acute apical dysfunction (25). These findings do not support vasospasm as a primary cause of TTC (5,7). Some authors have also concluded that vasospasm has no casual role in TTC (3,5). Accordingly, the primary vascular cause seems unlikely. A recent study has suggested that α-lipoic acid (ALA) treatment may improve cardiac dysfunction as compared with placebo because sympathetic activity dysfunction is closely associated with its pathophysiology (26).
Although invasive coronary angiography is indispensable to distinguish acute coronary syndrome from TTC, non-invasive imaging modalities are becoming useful for TTC assessment (14,27-29). Even in emergent situations, echocardiography provides the accurate evaluation of TTC and contributes to the increased number of detection in daily clinical practice. Transthoracic echocardiography depicts LV geometry, LV function and anatomic variants, detects complications, and monitors recovery. Here, we review the role of echocardiography for TTC assessment and its clinical and prognostic implications.
LV wall motion and systolic function
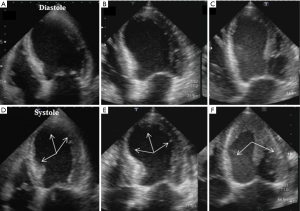
In the acute phase, an echocardiographic assessment of LV wall motion abnormalities (WMA) and systolic function are indispensable for diagnosing TTC (Figure 1). Key echocardiographic features in the acute phase is the large area of dysfunctional myocardium extending beyond the territory of a single coronary artery; which presents by symmetrical regional abnormalities, including the mid-ventricular segments of the anterior, inferior, and lateral walls (a circumferential pattern) (28-30). TTC is originally described as apical ballooning due to apical (with or without midventricular segment) akinesis or hypokinesis with preserved or hypercontractile basal segments (the classic or apical type, Figure 1B). LV myocardial dysfunction of TTC characterized by symmetric WMAs including the mid-ventricular segments of the anterior, inferior, and lateral walls (segment 7–12 based on the ASE guideline) over the apical segment (segment 13–17 based on the ASE guideline) should be considered peculiar to TTC and included in the differential diagnosis of TTC and acute coronary syndromes (31). These findings support the hypothesis of diffuse ventricular dysfunction secondary to myocardial stunning underlying the pathogenesis of TTC (32). Some earlier studies reported that advanced echocardiographic techniques, such as tissue Doppler imaging and speckle tracking imaging, could provide new insights into LV wall motion and systolic function assessment (33-36). Myocardial deformation imaging with the speckle tracking method evaluates symmetrical patterns of WMS and demonstrates transient circular impairments of longitudinal, circumferential and radial LV functions as well as LV twist mechanics deficiency (34-37). These techniques may help to differentiate TTC from anterior AMI. Furthermore, the peak systolic strain rate is also useful for assessing LV mechanics in this situation because the strain rate seems to be more useful than tissue Doppler velocities in evaluating the LV dynamics during volume loading in patients with depressed LV function (37). Nowadays, following three patterns have been defined: (I) inverted (reverse; Figure 1C); (II) mid-ventricular; and (III) localized types (38-40). In the majority of cases, WMA typically involve the apical and mid-ventricular segments (defined as apical ballooning) in contrast to the basal segments, which are often hyperkinetic (Figure 1). Several variant forms of TTC have been also identified; mid-ventricular TTC, the second most observed category, is classified as atypical and rarely reported as inverted TTC. This type of TTC was first reported by Haghi et al. in 2006 and the recognition of this pathophysiology became widely known (40). In a large series of patients with TTC in the Italian network, mid-ventricular and inverted TTC variants were detected in 18.1% and 4.8% of TTC patients, respectively (11). Although the causes of these differences are still controversial, there are tendencies towards patients with inverted TTC as follows: (I) younger than those with the other types of TTC (41); and (II) lower prevalence of dyspnea, pulmonary edema, cardiogenic shock, T-wave inversion, and acute reversible mitral regurgitation (11). The assessment of LV ejection fraction (EF) is also important in the management of TTC because, according to the results of a large series of studies, LVEF and WMA are closely associated with major adverse events (11). Although serum troponin or creatinine kinase is disproportionately low relative to the extent of WMA and cardiac dysfunction in TTC, the levels of serum cardiac natriuretic peptides [brain natriuretic peptide (BNP) or NT-proBNP] are extremely high and significantly correlate more closely with the degree of ventricular WMA (41-44). The limited evidence to date indicates that BNP and NT-proBNP are more useful diagnostic biomarkers compared with troponin, and its levels should be measured in all suspected cases. The use of invasive coronary angiography is often discussed because of many elderly TTC patients with different complications; thus, more noninvasive methods using echocardiography are required.
LV diastolic function
LV diastolic dysfunction of TTC evidenced by LV untwisting and increased E/e' ratio has recently been reported (11,45). E/e' ratio may be a simple and reproducible echocardiographic parameter for the assessment of LV filling pressure (11,28,45-49). The most common clinical complication in TTC is heart failure with or without pulmonary edema (50) and E/e' ratio is an independent predictor of heart failure and in-hospital mortality in the multivariate analyses (11,47). LV diastolic function should be early and systematically assessed in patients with high risk of cardiovascular events during follow-up. Since LV diastolic dysfunction is transient and reversible, echocardiography is indispensable for the management of TTC because it can be performed anytime during follow-up.
Right ventricular function
The right ventricle (RV) has been a forgotten chamber for a long time. Recent studies have reported that RV function has prognostic implications in several cardiovascular diseases (50-52). Of all cases of TCC, 13% involve RV (51). RV wall abnormalities in TTC lead to poor prognosis, and hospital death is also reported in some cases (52-54). Accordingly, not only LV but also RV wall assessment should be performed for predicting the prognosis. In addition, multiple echocardiographic windows are required to overcome the intrinsic limitations of conventional parameters in the evaluation of complex RV morphology. Citro et al. has reported that in patients with biventricular ballooning, the pattern of RV contraction mirrors that of LV WMA (55). RV dysfunction provides additional information, leading to differentiation of TTC from acute myocardial infarction.
Complications
TTC is a relatively benign disease because LV function is favorably improved, although, the accumulated data demonstrate that 52% of TTC patients suffer from various complications as follows (3,8-11).
Heart failure
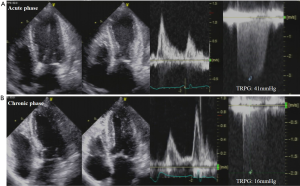
Heart failure with reduced EF is the most common complication in TTC observed in 12–45% of cases (56). Systolic heart failure is independently associated with older age, lower LVEF at presentation, higher admission rate and peak troponin levels, and a physical stressor preceding the onset of symptoms (11). In our study, old age and MR are independently associated with secondary pulmonary hypertension (57). Echocardiography evaluates cardiac function, pulmonary hypertension, and hemodynamics even in the chronic phase; that is, echocardiography is more useful than the other modalities (Figure 2). Therefore, the echocardiographic assessments of LV systolic and diastolic functions and the presence of pulmonary hypertension are crucial for the clinical management of TTC in the acute phase.
Left ventricular outflow tract obstruction (LVOTO)
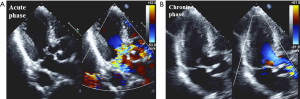
In the acute phase, LVOTO (defined as an intraventricular gradient ≥25 mmHg) caused by mitral valve systolic anterior motion may be trigger of myocardial stunning of the apical segments and hypercontraction of the basal LV myocardium (58). Significant LVOTO with gradients of 20–140 mmHg is reported in 10–25% of TTC patients, who often reveals MR (Figure 3) (59-61). This phenomenon is important for decision making in clinical practice, particularly for prescribing drugs. The use of inotropic drugs and nitrates probably exacerbate LVOTO with subsequent hemodynamic instability, meanwhile beta-blockers with propranolol decrease the gradient.

Acute MR
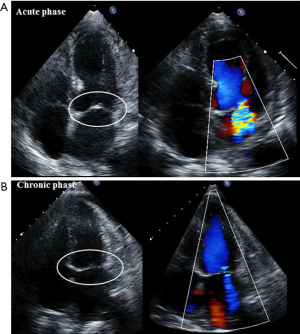
Acute MR is one of the serious complications and is detected in 14–25% of TTC patients (61,62). Those with significant MR reveal lower LVEF and higher pulmonary artery pressure, which may lead to acute heart failure and cardiogenic shock (61). Therefore, early detection by using echocardiography is important for providing appropriate management. The following two entirely different mechanisms cause acute MR (61): (I) systolic anterior motion of the mitral valve is associated with dynamic LVOTO (Figure 4); and (II) apical tethering of the mitral valve (Figure 5). In most cases, MR decreases as LV function returns to normal (Figures 4 and 5). The current American College of Cardiology/American Heart Association guidelines recommend mitral valve surgery in symptomatic patients with severe acute MR (63); whereas one study described their theory that an aggressive medical treatment of TTC should be the first priority because acute MR in TTC is reversible (61).
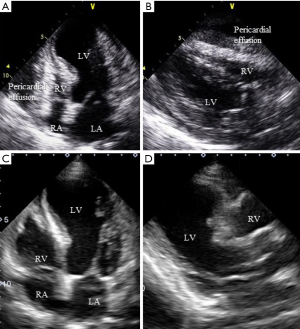
Pericardial effusion
Cardiovascular magnetic resonance imaging performed right after admission detects small pericardial effusion in 43% of TTC patients and most cases are recovered in the chronic phase (64). In the recovery phase, some patients have acute pericarditis with recurrent chest pain, reappearance of ST-segment elevation, and a small amount of pericardial effusion. Ventricular wall rupture is also serious, although, it rarely occurs 2–8 days after TTC onset (9,65,66). The echocardiographic assessment for not only pericardial effusion but also for cardiac tamponade is vital in the clinical practice (Figure 6).
Thrombus formation
Thrombus in the akinetic ventricular apex is observed in 2–8% of TTC patients, occasionally leading to the occurrence of stroke or arterial embolism (65,67,68). It takes 2–5 days to develop thrombi after symptom onset, which is evaluated by using cardiac magnetic resonance imaging in early post-contrast acquisition sequences when LV function is still depressed. In addition, new thrombus formation and subsequent embolism possibly identified 14 days after onset even though LV systolic function has already returned to normal. Cardiac magnetic resonance imaging clearly visualizes the thrombus; although, echocardiography is still required for checking thrombus formation during anticoagulation therapy. LV thrombi may be improved within 2 weeks of therapeutic anticoagulation; however, anticoagulation therapy should be provided for approximately 3 months. Follow-up imaging is advisable to confirm the recovery of apical contractile function. Therapeutic use of prophylactic anticoagulants in TTC patients with high risk has not been fully established because of insufficient evidence; however, it might be considered as a medical alternative.
Recurrence
A meta-analysis published in 2014 has described that in a 2-year follow-up, 3–8% of TTC patients had recurring its symptoms; requiring particular attention for this fact (7,8,69-72). Different LV wall motion disorders in the same patient are also reported; that is, wall motion disorder patterns are not always the same (73,74).
Conclusions
This review summarizes the significant points to be kept in mind while using echocardiography in the assessment of TTC. Since an echocardiographic examination is not enough to evaluate sympathetic tone activation and coronary flow, in TTC, echocardiography should be the first non-invasive imaging technique in a diagnosis of suspected TTC, as it provides sufficient evaluations even in an emergent case. TTC drastically changes cardiac function and hemodynamics, and echocardiography precisely depicts its time course. Further use of echocardiography should be considered for non-invasive assessment in patients with TTC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sato H, Tateishi H, Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Kodama K, Haze K, Hon M. editors. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo, Japan: Kagakuhyouronsha, 1990:56-64.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:e240-327. [Crossref] [PubMed]
- Akashi YJ, Goldstein DS, Barbaro G, et al. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 2008;118:2754-62. [Crossref] [PubMed]
- Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction: Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11-8. [Crossref] [PubMed]
- Kurisu S, Sato H, Kawagoe T, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002;143:448-55. [Crossref] [PubMed]
- Akashi YJ, Musha H, Kida K, et al. Reversible ventricular dysfunction takotsubo cardiomyopathy. Eur J Heart Fail 2005;7:1171-6. [Crossref] [PubMed]
- Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol 2015;12:387-97. [Crossref] [PubMed]
- Sharkey SW, Lesser JR, Zenovich AG, et al. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472-9. [Crossref] [PubMed]
- Akashi YJ, Tejima T, Sakurada H, et al. Left ventricular rupture associated with Takotsubo cardiomyopathy. Mayo Clin Proc 2004;79:821-4. [Crossref] [PubMed]
- Vidi V, Rajesh V, Singh PP, et al. Clinical characteristics of tako-tsubo cardiomyopathy. Am J Cardiol 2009;104:578-82. [Crossref] [PubMed]
- Citro R, Rigo F, D’Andrea A, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in tako-tsubo cardiomyopathy. JACC Cardiovasc Imaging 2014;7:119-29. [Crossref] [PubMed]
- Redfors B, Vedad R, Angerås O, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—AreportfromtheSWEDEHEART1registry. Int J Cardiol 2015;185:282-9. [Crossref] [PubMed]
- Schneider B, Athanasiadis A, Schwab J, et al. Complications in the clinical course of tako-tsubo cardiomyopathy. Int J Cardiol 2014;176:199-205. [Crossref] [PubMed]
- Lyon AR, Bossone E, Scheider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the task force on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8-27. [Crossref] [PubMed]
- Lyon AR, Rees PS, Prasad S, et al. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med 2008;5:22-9. [Crossref] [PubMed]
- Merli E, Sutcliffe S, Gori M, et al. Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006;7:53-61. [Crossref] [PubMed]
- Tranter MH, Wright PT, Sikkel MB, et al. Takotsubo cardiomyopathy: the pathophysiology. Heart Fail Clin 2013;9:187-96. [Crossref] [PubMed]
- Redfors B, Shao Y, Ali A, et al. Are the different patterns of stress-induced (Takotsubo) cardiomyopathy explained by regional mechanical overload and demand:supply mismatch in selected ventricular regions? Med Hypotheses 2013;81:954-60. [Crossref] [PubMed]
- Nef HM, Mollmann H, Akashi YJ, et al. Mechanismsofstress(Takotsubo) cardiomyopathy. Nat Rev Cardiol 2010;7:187-93. [Crossref] [PubMed]
- Patel SM, Lerman A, Lennon RJ, et al. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Eur Heart J Acute Cardiovasc Care 2013;2:147-52. [Crossref] [PubMed]
- Jaguszewski M, Osipova J, Ghadri JR, et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999-1006. [Crossref] [PubMed]
- Pelliccia F, Parodi G, Greco C, et al. Comorbidities Frequency in Takotsubo Syndrome: An International Collaborative Systematic Review Including 1,109 Patients. Am J Med 2015;128:654.e11-9. [Crossref]
- Martin EA, Prasad A, Rihal CS, et al. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol 2010;56:1840-6. [Crossref] [PubMed]
- Shao Y, Redfors B, Scharin Täng M, et al. Novel rat model reveals important roles of beta-adrenoreceptors in stress-induced cardiomyopathy. Int J Cardiol 2013;168:1943-50. [Crossref] [PubMed]
- Brouri F, Hanoun N, Mediani O, et al. Blockade of beta 1- and desensitization of beta 2-adrenoceptors reduce isoprenaline-induced cardiac fibrosis. Eur J Pharmacol 2004;485:227-34. [Crossref] [PubMed]
- Marfella R, Barbieri M, Sardu C, et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J Cardiol 2016;67:153-61. [Crossref] [PubMed]
- Bossone E, Lyon AR, Citro R, et al. Takotsubo cardiomyopathy: an integrated multi-imaging approach. Eur Heart J Cardiovasc Imaging 2014;15:366-77. [Crossref] [PubMed]
- Citro R, Lyon R, Meimoun P, et al. Standard and Advanced Echocardiography in Takotsubo (Stress) Cardiomyopathy: Clinical and Prognostic Implications. J Am Soc Echocardiogr 2015;28:57-74. [Crossref] [PubMed]
- Citro R, Rigo F, Ciampi Q, et al. Echocardiographic assessment of regional left ventricular wall motion abnormalities in patients with tako-tsubo cardiomyopathy: comparison with anterior myocardial infarction. Eur J Echocardiogr 2011;12:542-9. [Crossref] [PubMed]
- Patel SM, Lenon RJ, Prasad A, et al. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int J Cardiovasc Imaging 2012;28:687-94. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Wittstein IS. Stress cardiomyopathy: a syndrome of catecholaminemediated myocardial stunning? Cell Mol Neurobiol 2012;32:847-57. [Crossref] [PubMed]
- Mansencal N, Abbou N, Pillière R, et al. Usefulness of two-dimensional speckle tracking echocardiography for assessment of Tako-Tsubo cardiomyopathy. Am J Cardiol 2009;103:1020-4. [Crossref] [PubMed]
- Heggemann F, Hamm K, Kaelsch T, et al. Global and regional myocardial function quantification in Takotsubo cardiomyopathy in comparison to acute anterior myocardial infarction using two-dimensional (2D) strainechocardiography. Echocardiography 2011;28:715-9. [Crossref] [PubMed]
- Heggemann F, Hamm K, Brade J, et al. Right ventricular function quantification in Takotsubo cardiomyopathy using two-dimensional strain echocardiography. PLoS One 2014;9:e103717. [Crossref] [PubMed]
- Cai L, Addetia K, Medvedofsky D, et al. Myocardial strain may be useful in differentiating Takotsubo cardiomyopathy from left anterior descending coronary artery ischemia. Int J Cardiol 2017;230:359-63. [Crossref] [PubMed]
- Vicario ML, Caso P, Martiniello AR, et al. Effects of volume loading on strain rate and tissue Doppler velocity imaging in patients with idiopathic dilated cardiomyopathy. J Cardiovasc Med (Hagerstown) 2006;7:852-8. [Crossref] [PubMed]
- Kurowski V, Kaiser A, von Hof K, et al. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest 2007;132:809-16. [Crossref] [PubMed]
- Mansencal N, Abbou N, N’Guetta R, et al. Apical-sparing variant of tako-tsubo cardiomyopathy: prevalence and characteristics. Arch Cardiovasc Dis 2010;103:75-9. [Crossref] [PubMed]
- Haghi D, Papavassiliu T, Flüchter S, et al. Variant form of the acute apical ballooning syndrome (takotsubo cardiomyopathy): observations on a novel entity. Heart 2006;92:392-4. [Crossref] [PubMed]
- Madhavan M, Borlaug BA, Lerman A, et al. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart 2009;95:1436-41. [Crossref] [PubMed]
- Ahmed KA, Madhavan M, Prasad A. Brainnatriureticpeptideinapicalballooning syndrome (Takotsubo/stress cardiomyopathy): comparison with acute myocardial infarction. Coron Artery Dis 2012;23:259-64. [Crossref] [PubMed]
- Fröhlich GM, Schoch B, Schmid F, et al. Takotsubo cardiomyopathy has a unique cardiac biomarker profile: NT-proBNP/myoglobin and NT-proBNP/troponin T ratios for the differential diagnosis of acute coronary syndromes and stress induced cardiomyopathy. Int J Cardiol 2012;154:328-32. [Crossref] [PubMed]
- Nguyen TH, Neil CJ, Sverdlov AL, et al. N-terminal pro-brain natriuretic protein levels in takotsubo cardiomyopathy. Am J Cardiol 2011;108:1316-21. [Crossref] [PubMed]
- Meimoun P, Passos P, Benali T, et al. Assessment of left ventricular twist mechanics in tako-tsubo cardiomyopathy by two-dimensional speckle-tracking echocardiography. Eur J Echocardiogr 2011;12:931-9. [Crossref] [PubMed]
- Citro R, Piscione F, Parodi G, et al. Role of echocardiography in takotsubo cardiomyopathy. Heart Fail Clin 2013;9:157-66. [Crossref] [PubMed]
- Chong CR, Neil CJ, Nguyen TH, et al. Dissociation between severity of takotsubo cardiomyopathy and presentation with shock or hypotension. Clin Cardiol 2013;36:401-6. [Crossref] [PubMed]
- Redfors B, Shao Y, Ali A, et al. Rat models reveal differences in cardiocirculatory profile between Takotsubo syndrome and acute myocardial infarction. J Cardiovasc Med (Hagerstown) 2015;16:632-8. [Crossref] [PubMed]
- Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523-9. [Crossref] [PubMed]
- Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol 2002;39:1450-5. [Crossref] [PubMed]
- Citro R, Bossone E, Parodi G, et al. Clinical profile and in-hospital outcome of Caucasian patients with takotsubo syndrome and right ventricular involvement. Int J Cardiol 2016;219:455-61. [Crossref] [PubMed]
- Elesber AA, Prasad A, Bybee KA, et al. Transient cardiac apical ballooning syndrome: prevalence and clinical implications of right ventricular involvement. J Am Coll Cardiol 2006;47:1082-3. [Crossref] [PubMed]
- Fitzgibbons TP, Madias C, Seth A, et al. Prevalence and clinical characteristics of right ventricular dysfunction in transient stress cardiomyopathy. Am J Cardiol 2009;104:133-6. [Crossref] [PubMed]
- Kagiyama N, Okura H, Tamada T, et al. Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur Heart J Cardiovasc Imaging 2016;17:210-6. [Crossref] [PubMed]
- Citro R, Caso I, Provenza G, et al. Right ventricular involvement and pulmonary hypertension in an elderly woman with tako-tsubo cardiomyopathy. Chest 2010;137:973-5. [Crossref] [PubMed]
- Piran S, Veldtman G, Siu S, et al. Heart failure and ventriculardysfunction in patients with single or systemic right ventricles. Circulation 2002;105:1189-94. [Crossref] [PubMed]
- Izumo M, Shiota M, Nalawadi S, et al. Determinants of secondary pulmonary hypertension in patients with takotsubo cardiomyopathy. Echocardiography 2015;32:1608-13. [Crossref] [PubMed]
- Chockalingam A, Xie GY, Dellsperger KC. Echocardiography in stress cardiomyopathy and acute LVOT obstruction. Int J Cardiovasc Imaging 2010;26:527-35. [Crossref] [PubMed]
- El Mahmoud R, Mansencal N, Pilliére R, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in tako-tsubo syndrome. Am Heart J 2008;156:543-8. [Crossref] [PubMed]
- Citro R, Previtali M, Bovelli D, et al. Chronobiological patterns of onset of tako-tsubo cardiomyopathy: a multicenter Italian study. J Am Coll Cardiol 2009;54:180-1. [Crossref] [PubMed]
- Izumo M, Nalawadi S, Shiota M, et al. Mechanisms of acute mitral regurgitation in patients with takotsubo cardiomyopathy: an echocardiographic study. Circ Cardiovasc Imaging 2011;4:392-8. [Crossref] [PubMed]
- Parodi G, Del Pace S, Salvadori C, et al. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol 2007;50:647-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277-86. [PubMed]
- Schneider B, Athanasiadis A, Schwab J, et al. Complications in the clinical course of tako-tsubo cardiomyopathy. Int J Cardiol 2014;176:199-205. [Crossref] [PubMed]
- Yoshida S, Miwa K, Matsubara T, et al. Stress-induced takotsubo cardiomyopathy complicated with wall rupture and thrombus formation. Int J Cardiol 2012;161:e18-20. [Crossref] [PubMed]
- Kurisu S, Inoue I, Kawagoe T, et al. Incidence and treatment of left ventricular apical thrombosis in Tako-tsubo cardiomyopathy. Int J Cardiol 2011;146:e58-60. [Crossref] [PubMed]
- de Gregorio C, Grimaldi P, Lentini C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol 2008;131:18-24. [Crossref] [PubMed]
- Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-65. [Crossref] [PubMed]
- Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J 2008;155:408-17. [Crossref] [PubMed]
- Schultz T, Shao Y, Redfors B, et al. Stress-induced cardiomyopathy in Sweden: evidence for different ethnic predisposition and altered cardio-circulatory status. Cardiology 2012;122:180-6. [Crossref] [PubMed]
- Singh K, Carson K, Usmani Z, et al. Systematic review and meta-analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol 2014;174:696-701. [Crossref] [PubMed]
- Izumo M, Akashi Y, Suzuki K, et al. Recurrent takotsubo cardiomyopathy with variant forms of left ventricular dysfunction. J Cardiol Cases 2010;2:e37-40. [Crossref]
- Eitel I, Moeller C, Graf T, et al. Recurrence of takotsubo cardiomyopathy with different ballooning patterns. Int J Cardiol 2014;177:25-6. [Crossref] [PubMed]


