Abdominal aortic aneurysm screening: concepts and controversies
Introduction
Abdominal aortic aneurysms (AAAs) are defined by dilation of the abdominal aorta to a maximum diameter of at least 3 cm or 1.5 times that of the normal intervening segment (usually 2 cm in an adult) (1,2). They are highly prevalent, particularly among elderly males; arising in up to 8% of men over 65 years of age (2-4). Due to the risk of rupture, AAAs are also potentially lethal and comprise the 14th leading cause of mortality in the United States (U.S.), accounting for 4,500 deaths each year (5). Yet, AAAs pose a vexing problem: by the time symptoms arise, the aneurysms have usually already ruptured. At this point, treatment is frequently futile and fatality inevitable (6).
This clinical scenario provides an ideal backdrop for the introduction of a screening test that would allow early diagnosis of asymptomatic AAAs and timely intervention to prevent rupture and death. In this regard, ultrasound, which is both highly sensitive and specific in detecting AAAs but poses essentially no risk, comes to the forefront as the screening modality of choice (6). However, as with any screening program, the potential benefits of early detection must be weighed not only against immediate costs (e.g., technical sonography fees) but also long-term downsides such as periprocedural risk; for example, of the 45,000 AAA repairs performed annually in the U.S. to prevent rupture, 1,400 result in death (5).
Weighing the balance of evidence, the U.S. Preventive Services Task Force (USPSTF) in February 2005 for the first time recommended one-time sonographic screening for AAA in men ages 65–75 who had ever smoked as well as selected screening in other demographic groups (7). The agency reaffirmed and updated these guidelines in June 2014 (8,9). Recently, it has also begun inquiry into whether further guideline revisions may be appropriate, although no new recommendations would be available until at least November 2019 (10,11). Herein, the past and present USPSTF AAA ultrasound screening guidelines and their supporting data are reviewed as well techniques for optimal AAA sonography. Alternative guidelines are also discussed. Finally, evolving concepts and controversies in AAA screening are highlighted, including inconsistent data on screening benefits and appropriate follow-up, screening underutilization, and the possibility of clinically significant incidental findings, alternative screening methods, and redundant imaging.
USPSTF AAA screening recommendations: past and present
The 2005 USPSTF guidelines for AAA screening recommended one-time sonography in males between the ages of 65 and 75 who had ever smoked, defined as the use of ≥100 cigarettes in their lifetimes. This was the most definitive affirmative recommendation and attributed a level “B” grade, indicating that there was at least fair evidence that screening improved health outcomes and outweighed harms, with a moderate net benefit. For male never-smokers ages 65–75, the agency made no general recommendation for or against screening (grade “C”). Finally, for all women, the USPSTF advised against screening, a level “D” recommendation, indicating at least fair evidence that screening was ineffective or harm outweighed the risk (7).
The updated 2014 USPSTF AAA screening guidelines were similar but more nuanced. The agency again recommended one-time sonography in elderly male ever-smokers, with grade “B” evidence. Yet, it is noteworthy that the letter grade definitions changed after July 2012; grade “B” now indicated high certainty of moderate net benefit or moderate certainty of moderate to substantial benefit, with ultimate recommendation to provide the service. Similarly, for elderly male never-smokers, the agency again issued a letter “C” evidence grade. However, under the new definitions, this statement now meant that screening should be “selectively” offered depending on professional judgment and patient preferences, weighing factors that would increase AAA risk (such as cerebrovascular and coronary artery disease) or decrease risk (such as diabetes and African American race). Overall, the agency indicated a moderate certainty of small net benefit. For ever-smoker women ages 65–75, the USPSTF, now issued a class “I” recommendation, indicating that there was insufficient evidence to make a recommendation for or against screening. Finally, for never-smoker women of any age, the agency still recommended discouraging screening, a class “D” statement now indicating moderate or high certainty of no net benefit or on balance harms that outweigh benefits (8,9).
The impact on screening practices associated with the availability of the revised guidelines is not immediately apparent. However, in one recent retrospective study of AAA screening utilization a large tertiary academic medical center with an integrated health network, the 15-month period after the publication of the revised guidelines compared to the period before was associated with an increase in the proportion of exams performed in the elderly male ever-smoker population (most appropriate screening group). On the other hand, screening rates in other demographic groups did not significantly change (12).
Recently, the USPSTF has begun drafting a research plan to re-evaluate the evidence for AAA screening (10). No new evidence synthesis is currently available. Moreover, any new guidelines, if proposed, would not be available until at least November 2019 (11). Nevertheless, this ongoing analysis reflects the timeliness of the topic and the need for referring providers and clinical imagers to be cognizant of future potential guideline revisions and associated practice implications.
AAA screening: sonographic technique and reporting guidelines
Acknowledging interoperator technique variability, it is recommended that screening AAA ultrasounds be performed by a registered diagnostic medical sonographer with vascular expertise (or other similarly qualified personnel). Ultrasound equipment and transducers may vary but should allow for adequate penetration and resolution based on patient body habitus and other technical factors. The American Institute of Ultrasound in Medicine (AIUM) offers detailed guidelines on the proper performance and reporting of AAA screening ultrasound exams, summarized herein (13).
According to the AIUM, the abdominal aorta should be scanned in longitudinal and transverse planes along and perpendicular to the long axis of the vessel, respectively. The artery is imaged in its proximal, mid, and distal segments defined by locations below the diaphragm and near the celiac artery, near the level of the renal arteries, and above the iliac bifurcation, respectively. For each of these segments, the anteroposterior (AP) of the abdominal aorta is measured in the longitudinal plane, while the width is measured in the transverse plane. All measurements are performed outer edge to outer edge at the largest visible diameter of the abdominal aorta in each segment (Figure 1). If an aneurysm is detected, its location relative to the renal arteries and aortic bifurcation is documented as well as its maximal dimensions (Figure 2). In addition, longitudinal and transverse images of the bilateral common iliac arteries are captured just below the aortic bifurcation, documenting maximal AP and transverse dimensions from outer edge to outer edge. Finally, color and spectral Doppler with waveform analysis of the aorta and iliac arteries are performed to confirm patency of the vessels and assess for intraluminal thrombus (13).
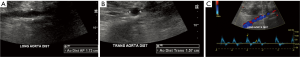
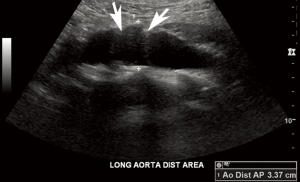
For reporting AAA screening ultrasounds, the AIUM recommends that exams be classified as “positive” (infrarenal AAA present), “negative” (infrarenal AAA absent), or indeterminate (partial or inadequate abdominal aortic visualization). If an aneurysm is detected, the maximum dimension should be indicated. Otherwise, the largest diameter of the abdominal aorta should be noted. Of note, the AIUM makes a clear demarcation between the suprarenal (above the celiac axis) and infrarenal abdominal aorta. For the suprarenal abdominal aorta, the AIUM considers an aneurysm >3.9 cm in a male or >3.1 cm in a female. In contrast, for the infrarenal abdominal aorta, the more common definition (≥3 cm or 1.5× the normal diameter) is used (13).
USPSTF recommendations: review of the evidence
The original USPSTF AAA screening guidelines were based on a meta-analysis of studies published between January 1994 and May 2004, prepared by the Agency for Healthcare Research and Quality (AHRQ) (14). The analysis primarily derived from four large randomized controlled trials, which included a combined cohort of 126,000 male subjects. One study was judged to be of “good” quality evidence according to USPSTF definitions (well-designed, well-conducted study) and the other three of “fair” quality (sufficient but limited evidence), due to lack of information on subject baseline characteristics and whether outcome raters were blinded (14,15). All of the trials included only patients over age 65 and found a reduction in AAA-related deaths associated with the invitation to attend to screening, though only statistically significant in two of the studies. The overall AAA-related mortality pooled odds ratio (OR) was 0.57 [95% confidence interval (CI), 0.45–0.74], favoring screening. However, of note, there was no significant difference in all-cause mortality. Based on statistical modeling, it was estimated that screening only ever-smokers in the 65–74 year-old male population would detect approximately 89% of all AAAs among men in this age group, thus lending credence to the ultimate USPSTF recommendations. Only one study included women (9,342 in number), in whom there was no statistically significant in AAA rupture or AAA-related mortality at 5–10 years follow-up compared to unscreened controls. There were no significant psychological harms associated with screening (14).
The 2005 AHRQ report also derived several additional important conclusions. Because no new aneurysms over 4 cm in diameter were diagnosed at 10-year follow-up after an initial screen, rescreening patients after an initial negative result did not appear beneficial. Moreover, there was no significant difference in AAA-related death or all-cause mortality in patients with aneurysms 4–5.4 cm who were managed with immediate repair rather than serial imaging. Subjects in the surveillance arm were more prone to myocardial infarction, while those in the repair group had more AAA-related hospitalizations. Data on untreated aneurysms measuring ≥5.5 cm was limited, as they are usually not observed. Still, while recognizing significant perioperative morbidity and mortality risks of AAA repair, the agency ultimately concluded that AAAs ≥5.5 cm, known to have rupture rates of more than 9%, should be repaired. Of note, endovascular aortic repair (EVAR) techniques had just been introduced in 1991 and had not been systemically studied at the time of the 2005 evidence synthesis (14).
The AHRQ prepared an updated evidence synthesis in January 2014. The analysis included the studies in the original report as well as a literature search for studies published between January 2004 and June 2012. Ultimately, 24 “fair” to “good” quality studies were examined, including 13 randomized controlled trials, 8 cohort studies, and 3 case-control studies. The overall conclusion that one-time AAA screening reduced AAA-related but not all-cause mortality was again verified; this was primarily based on the 4 trials included in the original 2005 report, with longer-follow-up available. The report also raised the possibility of risk prediction analysis to better identify the optimal screening population, noting that such factors as male sex, older age, and smoking history are associated with increased AAA prevalence, while greater years since quitting smoking, nonwhite race/ethnicity, diet, exercise, and diabetes are associated with decreased AAA prevalence. While firm conclusions could not be drawn, these concepts support the USPSTF’s ultimate recommendation to offer “selective” screening in elderly male never-smokers (16,17).
Interestingly, data on women were still limited primarily to the small cohort described in the 2005 AHRQ report; yet, the USPSTF did ultimately provide different screening recommendations for elderly female ever-smokers compared to other women. The 2014 AHRQ report did acknowledge the limitations of the small female cohort and also cited a more recent study that found the prevalence of AAA in female ever-smokers was 2.1%, compared to 0.8% in female never-smokers. In addition, the report noted across studies consistently higher rates of AAA rupture in women compared to men; however, the overall lower prevalence of AAA in females compared to males lowered the net screening benefit (16,17).
As before, the balance of evidence did not favor early medical or invasive (open repair or EVAR) therapy for small aneurysms. The report did acknowledge controversy surrounding rescreening after an initial negative exam but again noted that newly detected AAAs were usually small and unlikely to affect clinical outcomes. It is also noteworthy that the 2014 AHRQ report acknowledged the not uncommon scenario of an AAA detected incidentally on computed tomography (CT) performed for other purposes. However, the agency ultimately concluded that such CTs could not be presumed to substitute for sonographic screening due to limited data and potentially incomplete anatomic evaluation or reporting vigilance compared to a structured program (16,17).
The USPSTF recently drafted a research proposal to again systematically review the evidence for AAA screening in anticipation of possible further guideline revisions, not to appear before November 2019. The proposed questions for further study are largely the same as those appearing in previous evidence syntheses but would incorporate more recent data and longitudinal follow-up. The major issues to be studied include: the effects of one-time screening on health outcomes; variations in outcomes according to risk factors and demographic characteristics; the effects of rescreening after a negative scan; the harms of screening once or more times; the effects of medical or surgery therapy on outcomes for small AAAs <5.5 cm; and the harms associated with treating small AAAs (10,11). While the USPSTF has not substantially changed its evidence conclusions or recommendations on AAA screening since 2005, continued vigilance is needed to be cognizant of the most current data and their validity.
AAA screening: review of other guidelines
While the USPSTF recommendations are in general the most widely recognized among practitioners in the U.S., a variety of other guidelines are available. These are not substantially from the USPSTF guidelines but somewhat more inclusive. In the U.S., the American College of Cardiology (ACC) also recommends screening male ever-smokers ages 65–75 but also men ≥60 years old who are siblings or children of individuals diagnosed with an AAA. The Society for Vascular Surgery (SVS), in its most recent 2009 publication, recommended screening for all men ages 65 years or above, men ages 55 years or above with a family history of AAA, and women ages 65 years or above with a family history of AAA or past or present smoking use. Abroad, the United Kingdom National Screening Committee recommends screening all men ages 65 or above. In Canada, the Cardiovascular Society recommends screening all men ages 65–74, women ≥65 years with cardiovascular disease and a history of AAA, and mean ≥50 years with a family history of AAA. Finally, the Canadian Society for Vascular Surgery recommends screening all men ages 65–75 if they are eligible for surgery and amenable to it and consideration to screening in women above age 65 or men above age 75 with multiple risk factors; it recommends against screening other women above age 65 and any adult below age 65 (18).
Aside from guidelines, the availability of insurance coverage may ultimately drive provider and patient screening decisions. Since January 1, 2007, the U.S. Medicare program has covered the cost of ultrasound screening to ever-smoker men ages 65–75, as per USPSTF recommendations. Interestingly, adults with a family history of AAA are also covered, in a somewhat more inclusive stance compared to that of the USPSTF. AAA screening was initially only covered if referred as part of the “Welcome to Medicare” initial preventive visit. However, effective January 27, 2014, Medicare now only requires a referral from any healthcare professional with requisite ordering privileges (physician, physician assistant, nurse practitioner, or clinical nurse specialist) (19).
Emerging concepts and controversies
AAA screening has now been deemed beneficial for over 10 years. However, as new studies amass additional data with longer follow-up, and AAA diagnosis and treatment methods continue to evolve, controversies continue to arise. Indeed, the USPSTF’s recent proposal to revisit yet again the evidence for AAA screening highlights the timeliness of this topic. Some of the major emerging concepts are summarized herein.
Screening: true net benefit?
In recent years, some have questioned the validity and applicability of current AAA screening practices, supported by several arguments. First, the randomized trials on which screening guidelines were primarily based did not account for overdiagnosis of aneurysms that would never have ruptured or required surgery at follow-up. Second, the prevalence of AAA has declined in the past several decades, in part related to a decline in smoking use, reducing the effectiveness of screening. Third, the psychological stress associated with a new diagnosis of AAA can never be truly exactly quantified but may tip the balance toward relative harm from screening. Fourth, the detection of small aneurysms may inadvertently lead to overtreatment; indeed, >50% of EVARs in one series were performed on AAAs under 5.5 cm (20,21). Fifth, estimates of the cost-effectiveness of screening vary. Finally, the prevalence of AAA is known to be lower in those who undergo screening compared to those who do not undergo screening; thus, offering screening may also accentuate health care inequities without reaching the target population (20).
While screening criteria are now based primarily on demographic characteristics and high-level risk factors, further insights into the genomics of AAA formation will undoubtedly help to better inform who should be screened. Although several candidate genes have been identified, the science is still in very early stages (22). Furthermore, size criteria are predictive but crude indicators of AAA rupture. More precise noninvasive modeling of aortic hemodynamic parameters such as wall shear stress, flow displacement, and helicity is now possible with new imaging methods such as four-dimensional (4D) ultrasound and 4D flow magnetic resonance imaging (MRI) (23,24). While computationally intensive, such techniques could be the standard of care in future years.
Screening: evidence of underutilization
Assuming AAA screening should be performed as indicated in USPSTF guidelines, current research suggests a pervasive underutilization of the recommended sonography. In fact, utilization is estimated only in the range of <1% to 20% based on Medicare beneficiary data and primary care physician surveys (25-27). The elderly poor are disproportionately underscreened and prone to late AAA detection and rupture (28-30). In the study by Zucker et al., on average just under one AAA screening exam was performed per day, likely out of proportion to the size of large healthcare network that included many screening-eligible Medicare patients (12). It has been estimated that on average 1.31 years of life are gained per 10 patients screened for AAA, which is similar to estimates for breast cancer screening; thus, greater screening utilization could have a large positive impact on population health (27). While there is no “silver bullet” for ensuring recommended screening is performed, a multifaceted effort, ranging from provider and patient education to electronic health record reminders and point-of-care tools, may be optimal (12,31).
Aneurysm follow-up: a gray area
AAA screening facilitates detection of mostly small aneurysms for which early repair would cause more harm than benefit. While most agree that such aneurysms should have regular imaging follow-up to monitor their size and morphology, recommendations on appropriate follow-up are heterogeneous with limited supporting evidence (16,17). Usually, the larger the aneurysm size, the closer the screening interval is suggested; however, the optimal time to wait between exams is not known. For example, follow-up intervals ranging from 1–3 years have been suggested for aneurysms <4 cm, when considering guidelines across multiple countries. A recent meta-analysis by the RESCAN collaborators found that surveillance intervals could be lengthened to 3 years for AAAs 3.0–3.9 cm, 2 years for AAAs 4.0–4.4 cm, and annually for those 4.5–5.4 cm, while maintaining a rupture rate of <1%. At the same, the number of surveillance scans could on average by reduced by more than 50% (32).
It is also not uncommon for aneurysms to evade follow-up. For example, in one retrospective series, nearly 35% of patients did not obtain follow-up according to the minimum RESCAN standards. Most commonly, the lack of follow-up was due to provider failure to order a repeat scan. Such behavior could be due to a lack of education or robust electronic systems, although the confusion surrounding what merits appropriate follow-up could also contribute to heterogeneity in practice (33).
Alternative screening modalities and redundant imaging
The 2014 AHRQ evidence review concluded that a CT in which an AAA was incidentally detected could not be presumed to substitute for AAA screening sonography (16,17). This may be true on a purist review of the limited available data. However, clinical imagers would likely agree that the aorta is often well-imaged by other modalities such as CT or MRI with fewer technical limitations and less interoperator variability compared to ultrasound. If the interpreting imager could consistently and accurately assess the quality of the scan (i.e., adequate visualization of entire abdominal aorta) and maintain vigilance in reporting aortic sizes and aneurysms, this could produce several unique opportunities. First, those with a detected aneurysm could reasonably forego screening sonography but be referred for periodic sonographic surveillance. Second, a CT or MRI performed for other purposes might suffice in place of recommended sonographic follow-up after a diagnosis of AAA. Third, some patients without traditional risk factors such as elderly age and smoking use might be serendipitously discovered to have an AAA. Finally, if the AHRQ’s conclusion that patients in the traditional screening demographic group do not benefit from rescreening after negative sonography, a normal-caliber aorta on CT or MRI might analogously obviate the need for any additional dedicated screening.
Of course, the caveat remains that measurement technique is likely different and less accurate on sonography compared to other modalities (when a knowledgeable imager is performing the measurements). Thus, it is unclear whether traditional size cutoffs applied to other modalities can predict the same outcomes. Indeed, up to 5-mm intra- and interobserver measurement variability is considered a minimum standard for an acceptable AAA ultrasound screening program, and many centers exceed this threshold (34). While screening improves outcomes on a population level according to randomized controlled trials when this variation is effectively averaged, the effects of variation on an individual level are not known.
Nevertheless, there are likely opportunities to customize screening based on the availability of CT or MRI performed for other purposes. Several studies indicate not infrequent detection of AAAs on abdomen CT or lumbar spine MRI when the abdominal aorta is thoroughly examined (35-37). In one single-center retrospective study of over 500 male patients who underwent screening sonography, 20.7% of subjects were found to have had at least one prior radiologic test that adequately imaged the abdominal aorta when the patient was at least 65 years of age. Most commonly, an abdominopelvic CT was available, followed by lumbar spine MRI (38). While data are not robust, one study found that incomplete AAA imaging surveillance after incidental detection was associated with a decreased likelihood of elective AAA repair and an increased mortality risk (39).
Incidental findings
Just as other modalities may incidentally reveal an aneurysm, so too may AAA screening sonography incidentally detect unexpected findings that are likely or potentially clinically significant. In the series by Zucker et al., incidental findings were found in more than 15% of screening exams, most commonly iliac artery aneurysms (Figure 3) or renal masses (Figure 4) (12). These “incidentalomas” may merit clinical evaluation, additional imaging, or periodic surveillance, in turn incurring additional cost and anxiety to the patient. Of course, because patients should not be screened unless asymptomatic, the clinical significance of many incidental findings is not immediately obvious (40). Still, some findings such as an early renal neoplasm could easily evade clinical presentation for years.
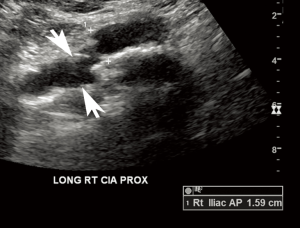
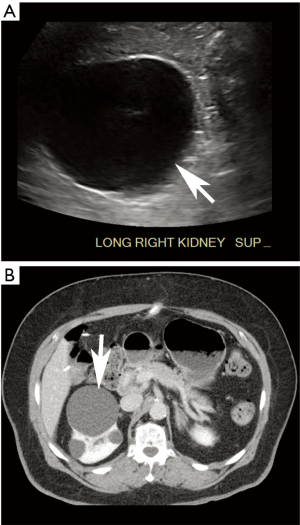
Further compounding the issue, a variety of non-radiology personnel may perform and interpret screening AAA sonography. For the sole intention of screening, such practices are not necessarily discouraged and may provide greater availability of services in areas where specialized radiologists are not available. Indeed, studies have shown that only limited sonographic training is required to perform accurate abdominal aortic measurements (41-43). Moreover, non-radiologists appear to perform similarly to radiologists in the detection and measurement of AAA (44). Nevertheless, this heterogeneous group of imagers may not be uniformly attuned to detecting and interpreting the significance of incidental findings outside the aorta. A dual radiologist/non-radiologist interpretive approach as often implemented for cardiac MRI is a possible solution, but it is not clear whether the net benefit would justify the incremental time and cost (45-47).
Conclusions
Screening AAA sonography particularly for the elderly male ever-smoker population has now been recommended for more than 10 years. Time will tell whether new USPSTF efforts to revisit the evidence for AAA screening will result in substantial guideline revisions. However, with continued affirmation of a reduction in AAA-related mortality attributed to one-time sonography, current core screening practices are likely to endure as the standard of care in the near future. Still, regardless of future UPSTF actions, there remain many areas for further study and practice improvement, including optimizing screening based on risk factors, increasing screening utilization, clarifying and ensuring appropriate follow-up intervals, managing incidental findings, and exploring the utility of alternative screening modalities. At the same time, the steady accumulation of knowledge about the genetic, physiologic, and biomechanical underpinnings of aneurysm formation, growth, and rupture, coupled with continued advanced in minimally invasive diagnostics and sophisticated imaging technologies, has the potential to transform the field and facilitate much more precise and patient-specific screening practices in coming years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kumar Y, Hooda K, Li S, et al. Abdominal aortic aneurysm: pictorial review of common appearances and complications. Ann Transl Med 2017;5:256. [Crossref] [PubMed]
- Meyermann K, Caputo FJ. Treatment of Abdominal Aortic Pathology. Cardiol Clin 2017;35:431-9. [Crossref] [PubMed]
- Moxon JV, Parr A, Emeto TI, et al. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol 2010;35:512-48. [Crossref] [PubMed]
- Robinson D, Mees B, Verhagen H, et al. Aortic aneurysms - screening, surveillance and referral. Aust Fam Physician 2013;42:364-9. [PubMed]
- Aggarwal S, Qamar A, Sharma V, et al. Abdominal aortic aneurysm: A comprehensive review. Exp Clin Cardiol 2011;16:11-5. [PubMed]
- Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 2014;371:2101-8. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med 2005;142:198-202. [Crossref] [PubMed]
- LeFevre ML. U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161:281-90. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Final update summary: abdominal aortic aneurysm: screening. Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening
- U.S. Preventive Services Task Force. Draft research plan: abdominal aortic aneurysm: primary care screening. Availabe online: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryDraft/abdominal-aortic-aneurysm-primary-care-screening
- U.S. Preventive Services Task Force. Leave comment for draft research plan. Draft research plan for abdominal aortic aneurysm: primary care screening. Available online: https://www.uspreventiveservicestaskforce.org/Comment/Collect/Index/draft-research-plan/abdominal-aortic-aneurysm-primary-care-screening
- Zucker EJ, Misono AS, Prabhakar AM. Abdominal Aortic Aneurysm Screening Practices: Impact of the 2014 U.S. Preventive Services Task Force Recommendations. J Am Coll Radiol 2017;14:868-74. [Crossref] [PubMed]
- American Institute of Ultrasound in Medicine (AIUM). AIUM practice parameter for the performance of diagnostic and screening ultrasound examinations of the abdominal aorta in adults. Available online: http://www.aium.org/resources/guidelines/abdominalAorta.pdf
- Fleming C, Whitlock E, Beil T, et al. Primary care screening for abdominal aortic aneurysm: evidence syntheses no. 35. Rockville (MD): Agency for Healthcare Research and Quality, 2005.
- U.S. Preventive Services Task Force. Grade Definitions. Available online: https://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions
- Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US), 2014.
- Guirguis-Blake JM, Beil TL, Senger CA, et al. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160:321-9. [Crossref] [PubMed]
- Ferket BS, Grootenboer N, Colkesen EB, et al. Systematic review of guidelines on abdominal aortic aneurysm screening. J Vasc Surg 2012;55:1296-304. [Crossref] [PubMed]
- Centers for Medicare & Medicaid Services (CMS). Implementation of a one-time only ultrasound screening for abdominal aortic aneurysms (AAA), resulting from a referral from an initial preventive physical examination. Available online: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5235.pdf
- Johansson M, Hansson A, Brodersen J. Estimating overdiagnosis in screening for abdominal aortic aneurysm: could a change in smoking habits and lowered aortic diameter tip the balance of screening towards harm? BMJ 2015;350:h825. [Crossref] [PubMed]
- Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848-55. [Crossref] [PubMed]
- Saratzis A, Bown MJ. The genetic basis for aortic aneurysmal disease. Heart 2014;100:916-22. [Crossref] [PubMed]
- Derwich W, Wittek A, Pfister K, et al. High Resolution Strain Analysis Comparing Aorta and Abdominal Aortic Aneurysm with Real Time Three Dimensional Speckle Tracking Ultrasound. Eur J Vasc Endovasc Surg 2016;51:187-93. [Crossref] [PubMed]
- Youssefi P, Sharma R, Figueroa CA, et al. Functional assessment of thoracic aortic aneurysms - the future of risk prediction? Br Med Bull 2017;121:61-71. [Crossref] [PubMed]
- Chun KC, Samadzadeh KM, Nguyen AT, et al. Abdominal aortic aneurysm screening in the United States. Gefässchirurgie 2014;19:534-9. [Crossref]
- Wooster DL, Dueck AD, Wooster EM. Abdominal aortic aneurysm screening is underutilized: barriers to screening and lessons learned from a survey of primary care providers. Available online: https://www.researchgate.net/publication/252938619_Abdominal_Aortic_Aneurysm_Screening_is_Underutilized_Barriers_to_Screening_and_Lessons_Learned_from_a_Survey_of_Primary_Care_Providers
- Olchanski N, Winn A, Cohen JT, et al. Abdominal aortic aneurysm screening: how many life years lost from underuse of the medicare screening benefit? J Gen Intern Med 2014;29:1155-61. [Crossref] [PubMed]
- Mell MW, Baker LC. Payer status, preoperative surveillance, and rupture of abdominal aortic aneurysms in the US Medicare population. Ann Vasc Surg 2014;28:1378-83. [Crossref] [PubMed]
- Mell MW, Baker LC, Dalman RL, et al. Gaps in preoperative surveillance and rupture of abdominal aortic aneurysms among Medicare beneficiaries. J Vasc Surg 2014;59:583-8. [Crossref] [PubMed]
- Mell MW, Hlatky MA, Shreibati JB, et al. Late diagnosis of abdominal aortic aneurysms substantiates underutilization of abdominal aortic aneurysm screening for Medicare beneficiaries. J Vasc Surg 2013;57:1519-23, 1523.e1.
- Hye RJ, Smith AE, Wong GH, et al. Leveraging the electronic medical record to implement an abdominal aortic aneurysm screening program. J Vasc Surg 2014;59:1535-42. [Crossref] [PubMed]
- Collaborators RESCAN, Bown MJ, Sweeting MJ, et al. Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 2013;309:806-13. [Crossref] [PubMed]
- Chun KC, Schmidt AS, Bains S, et al. Surveillance outcomes of small abdominal aortic aneurysms identified from a large screening program. J Vasc Surg 2016;63:55-61. [Crossref] [PubMed]
- Beales L, Wolstenhulme S, Evans JA, et al. Reproducibility of ultrasound measurement of the abdominal aorta. Br J Surg 2011;98:1517-25. [Crossref] [PubMed]
- Claridge R, Arnold S, Morrison N, et al. Measuring abdominal aortic diameters in routine abdominal computed tomography scans and implications for abdominal aortic aneurysm screening. J Vasc Surg 2017;65:1637-42. [Crossref] [PubMed]
- Kamath S, Jain N, Goyal N, et al. Incidental findings on MRI of the spine. Clin Radiol 2009;64:353-61. [Crossref] [PubMed]
- Trompeter AJ, Paremain GP. Incidental abdominal aortic aneurysm on lumbosacral magnetic resonance imaging - a case series. Magn Reson Imaging 2010;28:455-7. [Crossref] [PubMed]
- Gao G, Arora A, Scoutt L, et al. Imaging Redundancy in Screening for Abdominal Aortic Aneurysm. J Am Coll Radiol 2017;14:625-8. [Crossref] [PubMed]
- van Walraven C, Wong J, Morant K, et al. The influence of incidental abdominal aortic aneurysm monitoring on patient outcomes. J Vasc Surg 2011;54:1290-7.e2. [Crossref] [PubMed]
- Lam DL, Pandharipande PV, Lee JM, et al. Imaging-based screening: understanding the controversies. AJR Am J Roentgenol 2014;203:952-6. [Crossref] [PubMed]
- Nguyen AT, Hill GB, Versteeg MP, et al. Novices may be trained to screen for abdominal aortic aneurysms using ultrasound. Cardiovasc Ultrasound 2013;11:42. [Crossref] [PubMed]
- Bailey RP, Ault M, Greengold NL, et al. Ultrasonography performed by primary care residents for abdominal aortic aneurysm screening. J Gen Intern Med 2001;16:845-9. [Crossref] [PubMed]
- Salen P, Melanson S, Buro D. ED screening to identify abdominal aortic aneurysms in asymptomatic geriatric patients. Am J Emerg Med 2003;21:133-5. [Crossref] [PubMed]
- Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract 2014;68:1122-9. [Crossref] [PubMed]
- Greulich S, Backes M, Schumm J, et al. Extra cardiac findings in cardiovascular MR: why cardiologists and radiologists should read together. Int J Cardiovasc Imaging 2014;30:609-17. [Crossref] [PubMed]
- Chan PG, Smith MP, Hauser TH, et al. Noncardiac pathology on clinical cardiac magnetic resonance imaging. JACC Cardiovasc Imaging 2009;2:980-6. [Crossref] [PubMed]
- Wyttenbach R, Médioni N, Santini P, et al. Extracardiac findings detected by cardiac magnetic resonance imaging. Eur Radiol 2012;22:1295-302. [Crossref] [PubMed]


