Transplant artery thrombosis and outcomes
Introduction
As of June 26, 2017, the total number of kidney and liver transplants reported by the United Network for Organ Sharing (UNOS) since the beginning of 1988 was 413,277 and 151,193, respectively (www.unos.org/data). The candidate waiting lists on this same date for these two organs were 93,393 for kidney transplant and 14,394 for liver transplant. The manner in which solid organs high in demand are distributed to potential recipients on the waiting lists is in part based on detailed scoring systems and criteria that depend on standardized imaging reporting guidelines. One such set of guidelines for the selection of appropriate liver transplant recipients has been established by the Organ Procurement and Transplantation Network (OPTN)/UNOS policy for liver transplant allocation (1). Imaging thus plays an important role in stratifying potential organ recipients and has additional implications for pre-operative planning, post-transplantation organ surveillance and, when necessary, endovascular intervention.
Transplant arterial thrombosis is a major complication of solid organ transplantation and one of the leading causes of graft failure. Diagnostic evaluation of suspected thrombosis has been validated with the use of computed tomography angiogram (CTA) (2), although more contemporary postoperative imaging protocols rely heavily on Doppler ultrasound imaging, either in the operating room or in the post-anesthesia care unit. CTA remains useful as a diagnostic tool in the assessment of transplant arterial anatomy; particularly when there is significant tortuosity of the donor or recipient artery or if patient factors such as body habitus limit satisfactory sonographic evaluation.
CTA also serves as a first-line, non-invasive imaging modality for the pre-operative evaluation of hepatic arterial variations in transplant organ donors (3). These anatomic variations in hepatic arterial anatomy were first classified by Michel in 1955 on the basis of cadaveric dissection (4). Since then, numerous studies have been published on the variations described by Michel as well as many others that do not fall under the original classification scheme. The advent of CTA and digital subtraction angiography (DSA) has shed light on numerous novel anatomic variations. In a study of 100 patients using CTA with maximum intensity projection (MIP) reconstructions, Duran et al. found that 41% of potential living donors had variant arterial anatomy, with 11% of the cohort demonstrating variant anatomy outside the Michel classification. As an aside, it is worth noting that transplant artery thrombosis is a risk (albeit extremely low) not only for the organ transplant recipient, but also for the living donor in cases of segmental liver transplantation, as was demonstrated in the Duran et al. study, in which a single donor patient (1% of the cohort) developed postoperative hepatic arterial thrombosis. In a separate study of 600 patients utilizing DSA in the analysis of variant hepatic arterial anatomy (4), 39.7% had variant anatomy, in keeping with the results of the previously discussed CTA study and underlining the value of pre-transplantation imaging in living donors.
With respect to arterial considerations in transplantation, the most pertinent information regarding donor anatomy is the number of arteries, vessel diameter and stump length available for harvesting (3). These and other factors that modulate the risk of transplant artery thrombosis will be discussed individually by organ in this review, as the vascular considerations differ by type of organ. Briefly, conventional liver transplantation involves an end-to-end anastomosis between the donor hepatic artery and the recipient hepatic artery, with many variations depending on length and caliber of the vessels and patient-specific circumstances (5). The length of the resulting vessel and its susceptibility to kink may influence the risk of arterial thrombosis (5).
A standard adult living donor renal transplant utilizes a single end-to-end anastomosis between donor renal artery and recipient external iliac artery, although many variations exist including anastomoses to the external iliac artery, common iliac artery or inferior epigastric artery, particularly in the setting of multiple donor renal arteries (6). Pancreas transplantation carries unique anatomic considerations, as the donor splenic and superior mesenteric arteries are most often used in conjunction with a donor “Y-graft” comprising the donor common and external/internal iliac arteries for anastomosis with the recipient common iliac artery or external iliac artery (7).
Liver transplant
Hepatic artery thrombosis (HAT) following liver transplantation is reported to occur in up to 9% of cases in the literature (8,9), although large retrospective studies have shown rates as low as 4.8%, including early and late arterial complications (10). HAT is one of the leading cause of graft loss (>50% of cases) and mortality in liver transplantation (11). While the causes of hepatic artery thrombosis are often unknown, they can be divided into two broad categories: technical problems related to the surgery or arterial anastomosis, and donor/recipient factors that affect either the artery itself or influence secondary clinical factors that predispose to thrombosis, such as hypercoagulability, infection and liver rejection (11,12). Intraoperative factors that influence the risk for thrombosis include the use of heparin (or lack thereof), transfusion requirements, and cold ischemia time. In a study by Abou El-Ella et al. of 86 liver transplant patients, HAT occurred in 7% of transplant recipients, with the most common risk factors for thrombosis being liver rejection, the lack of heparin use intraoperatively and the need for transfusion greater than 5 units (12). Biliary complications may be the presenting sign of early arterial compromise, as the hepatic artery is the sole source of vascular supply to the bile ducts in the transplant liver (13).
The natural history of HAT varies depending on its chronicity and on the formation of collateral blood supply to the transplant liver. In the acute setting, HAT can lead to acute graft failure that may be amenable to endovascular or surgical intervention. Long-term survival of adult as well as pediatric liver transplant recipients despite sonographic evidence of hepatic artery thrombosis is well-documented in the literature, with various explanations as to the mechanisms for continued graft function. The presence of collateral flow, which is thought to occur as a result of spontaneous neovascularization of the liver via angiogenesis (14), has been documented in such cases using magnetic resonance angiography (MRA), CTA and conventional angiography as early as 3 weeks postoperative, with mean survival in one study of 4.1 years despite irreversible HAT (10). Other factors for persistent graft function despite HAT include early and more tailored interventions for biliary or infectious complications related to diminish arterial supply to the transplant liver.
Given the importance of early identification of HAT, rigorous postoperative imaging surveillance protocols have been established with immediate postoperative and short-term follow-up ultrasound and Doppler examinations of the transplant liver. A finding on Doppler ultrasound evaluation that would merit close follow-up is the presence of significantly elevated peak systolic velocities in the main hepatic artery, which may be suggestive of hepatic artery stenosis, an independent risk factor for HAT (15). A study of 148 patients by Saad et al. of primary treatment of hepatic artery stenosis with percutaneous transluminal angioplasty (PTA) found a 65% rate of thrombosis in untreated cases of stenosis within 6 months (16). Elevated velocities in the hepatic artery in and of itself; however, does not predict short term graft function (17). On the other hand, diminished intrahepatic arterial resistive indices, when less than 0.5 in the immediate postoperative period, have been associated with significant vascular complications (17). The implications of HAT may differ depending on its temporal course following transplantation, and thus it is often divided into “early” and “late” phases, with some variation on this definition across studies, but generally with “early” encompassing the immediate postoperative period and the month following surgery. A Brazilian study of 263 liver transplants showed an incidence of HAT of 7.9%, with approximately three-quarters of cases occurring early and the remainder late (9).
Interpretation of sonographic evaluation of the transplant should encompass not only intrahepatic arterial resistive indices but also changes to the spectral Doppler waveforms, as diminished hepatic arterial flow may carry different levels of gravity depending on the extent to which collateral arterial flow has been established, as discussed previously. The presence of biliary and infectious complications (Figure 1) detected clinically should prompt further vascular evaluation, as these may be manifestations of early arterial compromise (10).
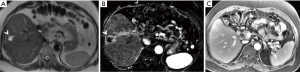
Irrespective of the theorized cause of arterial thrombosis, urgent intervention for HAT has been shown to lead to rates of survival comparable to those in patients without vascular complications. While differences may exist between pediatric and adult transplantation, urgent intervention may benefit both populations of patients. Pediatric patients have been theorized to be at greater risk for HAT due to smaller arterial vessel caliber, differences in operative technique (namely, the lack of use of an intraoperative microscope), and physiologic differences influencing hypercoagulability, however the impact of these factors has been shown to be less significant than previously thought in a relatively large series of 150 pediatric liver transplants (18). A more recent analysis of the incidence of HAT in the pediatric population following step-wise changes to microvascular operative techniques and anticoagulation protocols highlighted the importance of specific anticoagulation measures, which reduced the rate of HAT nearly 10-fold (9.5% to 1.8%) in predominantly cadaveric split liver transplantation (19).
The benefits of early intervention have been well-demonstrated in pediatric liver transplantation. Ackermann et al. showed 20-year patient survival rates of 80% despite early HAT, though urgent interventions were broad in scope and included not only surgical revascularization but intervention for secondary biliary complications and re-transplantation (20). In the same study of 590 orthotopic liver transplants, emergent revascularization was performed in 5.3% of cases, all within a 24-hour window following transplantation, with a success rate of 61%. Surgical techniques included thrombectomy with local thrombolysis as well as revision of the arterial anastomosis with an interposition graft. Such interposition grafts, which are commonly harvested from the donor iliac artery, vary widely in application according to unique anatomical considerations of both the donor and recipient anatomy (Figure 2). Recent surgical literature suggests that the use of a long graft artery may be an independent risk factor for early HAT, irrespective of donor arterial anatomic variations and the complexity of the arterial reconstruction (21).
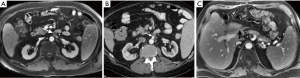
These anatomic variations come into careful consideration in the postoperative evaluation of the transplant liver. Horrow et al. in 2007 published their results using sonography for evaluation of liver transplant outcomes in cases of HAT occurring at different times following transplantation, divided into primary and secondary, with further subdivision of primary complications occurring early or late (10). Secondary complications were defined as instances of thrombosis occurring during therapeutic intervention for other hepatic artery-associated complications, such as pseudoaneurysm or stenosis. In their study of 522 transplants, the rate of HAT was 3.5% for primary, with most occurring late, in this case defined as occurring within 1 week or later post-transplantation, and 1.3% for secondary.
In cases when HAT has been identified in the early phase following transplantation, a decision must be made as to which interventional or surgical option to pursue. Given a limited organ supply and the evolution of endovascular techniques, revascularization is an attractive first-line modality in the treatment of HAT, although re-transplantation is still commonly practiced depending on the etiology of the thrombosis and other clinical factors unique to the patient. Angioplasty and stent placement for HAT has been described as early as 1994 by Vorwerk et al. in a 29-year-old female patient with HAT following re-transplantation (22) and remains a viable option today. Drug-eluting stents may also be used for primary treatment of early HAT, either alone or in conjunction with catheter-directed thrombolysis (23). In a study of 102 patients with HAT, Mourad et al. looked at outcomes following various management strategies, ranging from re-transplantation or early re-vascularization to strictly conservative management (8). Using a multivariate analysis, this group found that low donor weight, number of arterial anastomoses and prior history of HAT were independent risk factors for early thrombosis, defined in this case as occurring within 21 days post-transplantation. Independent risk factors for late HAT included previous abdominal surgery, low donor weight and recipient age less than 50 years (8).
Interventional techniques for thrombolysis utilizing tissue plasminogen activator (TPA) or urokinase have shown mixed results. The choice of thrombolytic agent, despite a stronger safety profile for TPA, may be influenced by non-clinical factors such as availability or cost (24). In a study by Kogut et al. of 26 patients with HAT, 13 of which occurred in the first 30 days post-transplantation, recanalization was achieved following intra-arterial thrombolysis in 46% of patients, however nearly half (42%) of these patients subsequently required surgical intervention for revascularization or re-transplantation (25). Zhang et al. more recently published results of urokinase thrombolytic treatment of HAT in a cohort of 34 patients, with the need for additional stent placement in a subgroup of 21 of these patients, that showed short- and long-term efficacy of these interventional techniques (24). Success rates in their study, defined technically as recanalization and clinically as improvement in liver enzymes and symptoms, reached 93%. Important considerations in the Zhang et al. study included management of splenic artery steal syndrome with splenic artery embolization, as well as the use of stents in selective hepatic arteries. The latter is particularly relevant when donor or recipient hepatic arterial anatomy is exceptionally tortuous or in cases of intrinsically narrow caliber vessels (Table 1) (23-25).

Full table
Complications of catheter-directed thrombolysis include hepatic artery dissection or perforation and bleeding complications, either locally at the access site or in the form of intra-abdominal hemorrhage. A recent retrospective review in the vascular surgery literature by Goldsmith et al. of 1,129 liver transplantations with hepatic artery stenosis showed a major complication rate of endovascular treatment of 7.5%, which encompassed target vessel dissection or rupture (26), although these complications and their rates may differ from those related to endovascular treatment of HAT. In the event of a failed attempt at re-vascularization, graft viability may persist for some time in the setting of chronic arterial comprise and in the presence of arterial collateralization, as discussed previously (27). The time course of HAT following transplantation, patient risk factors, availability of endovascular operators, thrombolytic agents or an alternative liver for re-transplantation may all influence the treatment approach.
Kidney transplant
Renal transplant arterial anatomy varies depending on whether the transplant kidney comes from a cadaveric source or a living donor. In deceased donor transplantation, the donor renal artery is often taken in conjunction with a segment of donor aorta that can be used for end-to-side anastomosis to the recipient external iliac artery (28). Living donor kidneys, in contrast, are often performed with end-to-side anastomosis from the donor renal artery to the recipient external iliac artery, or with an end-to-end anastomosis to the recipient internal iliac artery (28). Variations occur in the setting of en bloc pediatric transplantation and kidneys with greater than one renal artery. As with other solid organ transplants, vascular complications of renal transplantation include arterial thrombosis, arterial stenosis, venous thrombosis, arteriovenous fistula and pseudoaneurysm formation. In the absence of renal artery stenosis, thrombosis is exceedingly uncommon beyond the early postoperative period unless it occurs in the setting of rejection (29). This differs from HAT, which has been well described as a possible late complication (greater than one month following transplantation). The incidence of transplant renal artery thrombosis is fairly low, reported in 0.1% to 0.2% of cadaveric transplants (30).
Causes of transplant renal artery thrombosis are often multifactorial and, as with HAT in the transplant liver, broad in scope. The unique placement of the transplant kidney, most commonly in the right or left iliac fossa, predisposes it to risks to which the native kidney is not typically subject. In a case report by McCarthy et al., renal artery thrombosis was described as a direct consequence of extrinsic compression related to positioning during total hip arthroplasty (30). In a more common scenario, acute allograft dysfunction of the pelvic renal transplant may be the result of renal allograft compartment syndrome (31) and associated arterial compromise. Thrombosis has also been reported in the setting of infection, with severe cases leading to spontaneous arterial rupture secondary to invasive mucormycosis (32).
Transplant renal artery stenosis is another recognized complication of renal transplantation that often precedes thrombosis and is clinically detected based on uncontrolled-hypertension or graft dysfunction. Its incidence is generally higher than that of thrombosis, reported between 1.5% and 12.5% in the literature (33). PTA with or without stent placement is one potential treatment option for renal artery stenosis, with in-stent re-stenosis rates reported in up to 13% of cases (34). Several small case series have been published based on single center, institutional experiences in the treatment of renal artery stenosis or in-stent re-stenosis. In a small series of 3 patients, Douis et al. found the use of paclitaxel-eluting stents safe in the treatment of recurrent stenosis, with long-term patency observed in 2 of the 3 patients, while the third required balloon angioplasty (34).
In a single center study by Voiculescu et al. of 53 patients with transplant renal artery stenosis, 52 patients went on to receive invasive therapy, which included PTA, PTA with stenting or surgical intervention (33). Surgery was generally reserved for cases in which stenosis occurred at the anastomosis or bifurcation of arteries, in cases of primary dissection or in other situations in which the complexity of the arterial anatomy was not amenable to percutaneous angioplasty. Restenosis rates in the Voiculescu et al. study reached 52%, with the majority occurring in PTA alone (62%) and in PTA with stenting (30%), and only 14% occurring in the surgical group (33).
Rouvière et al. published results of a small series of four patients receiving intra-arterial fibrinolysis (using either TPA or urokinase) as a primary treatment for transplant renal artery thrombosis occurring within the first 24 hours following transplantation (29). While the study was small, revascularization was achieved in three of the four patients, although only two of these three went on to successfully discontinue dialysis in the short term. The treatment of venous complications of renal transplantation is better established in the literature. Catheter-directed thrombectomy and thrombolysis, for instance, has been used in the treatment of renal vein thrombosis in the post-transplant setting with rapid improvement in graft function and relatively low morbidity (35).
Close clinical and radiologic follow-up are essential for the early detection of renal artery thrombosis or other vascular abnormalities (Figure 3). While Doppler ultrasound evaluation is the mainstay for routine post-transplantation renal allograft surveillance, new innovative techniques for the diagnostic evaluation of vascular abnormalities have emerged, including the use of contrast-enhanced ultrasound and unenhanced MRA (36).
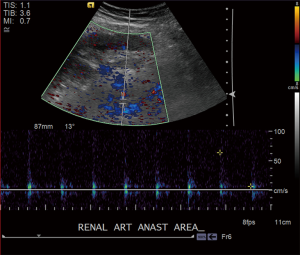
Pancreas transplant
Pancreas transplant is performed either in isolation or in conjunction with kidney transplant for patients with advanced diabetic glomerulopathy. Pancreas transplant carries uniquely challenging considerations because of its dual-blood supply. The native pancreas receives blood from branches of both the celiac and superior mesenteric arteries, specifically via the splenic artery and pancreaticoduodenal arteries (37). Transplant typically involves a “Y”-graft in which the donor splenic artery and superior mesenteric artery are brought together via a donor common iliac bifurcation graft, which is connected to the recipient external or common iliac artery (7,38). Vascular complications of pancreas transplant often result in complete graft failure, with venous thrombosis detected sonographically by the presence of pan-diastolic reversal of flow, as is seen in the setting of renal vein thrombosis (39). Most of the treatment and outcomes literature for pancreas transplantation focuses on venous thrombosis, with small studies suggesting positive results in preventing early graft loss (38).
Conclusions
Transplant artery thrombosis is a leading cause of graft failure, particularly in the early postoperative period. In liver transplantation, hepatic arterial thrombosis is typically subdivided into early and late stages, with different implications for treatment depending on the extent of arterial collateralization and on the presence of associated clinical complications such as biliary ischemia or abscess formation. Protocol-based sonographic Doppler evaluation is commonly practiced in the postoperative period and in short intervals thereafter to assess for arterial patency as well as secondary signs of arterial compromise such as increased hepatic artery velocities or changes to the arterial spectral waveforms that may suggest the presence of early stenosis or developing thrombosis. Endovascular techniques continue to evolve and include PTA, with or without stent placement, thrombectomy and intra-arterial thrombolysis with TPA or urokinase. Drug-eluting stents provide an additional, second-line endovascular therapeutic option, specifically in cases of prior treated hepatic artery stenosis with in-stent re-stenosis. Innovations in thrombolytic agents as well catheter design have the potential to expand and improve outcomes in the endovascular treatment of transplant artery thrombosis (40). Surgical intervention is required in cases of significant arterial tortuosity, arterial anatomy not amenable to an endovascular approach or in cases in which re-transplantation may be the more appropriate route for the patient in consideration of other clinical factors. Early intervention with successful re-canalization of the affected artery has the potential to prolong graft survival at rates comparable to those in patients that did not experience transplant-related arterial complications and highlights the importance of well-established postoperative imaging surveillance protocols.
Acknowledgements
R Oklu gratefully acknowledges funding from the National Institutes of Health (EB021148, CA172738, EB024403, HL137193) and the Mayo Clinic.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 2013;266:376-82. [Crossref] [PubMed]
- Legmann P, Costes V, Tudoret L, et al. Hepatic artery thrombosis after liver transplantation: diagnosis with spiral CT. AJR Am J Roentgenol 1995;164:97-101. [Crossref] [PubMed]
- Duran C, Uraz S, Kantarci M, et al. Hepatic arterial mapping by multidetector computed tomographic angiography in living donor liver transplantation. J Comput Assist Tomogr 2009;33:618-25. [Crossref] [PubMed]
- Covey AM, Brody LA, Maluccio MA, et al. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology 2002;224:542-7. [Crossref] [PubMed]
- Makowka L, Stieber AC, Sher L, et al. Surgical technique of orthotopic liver transplantation. Gastroenterol Clin North Am 1988;17:33-51. [PubMed]
- Ali-El-Dein B, Osman Y, Shokeir AA, et al. Multiple arteries in live donor renal transplantation: surgical aspects and outcomes. J Urol 2003;169:2013-7. [Crossref] [PubMed]
- Tolat PP, Foley WD, Johnson C, et al. Pancreas transplant imaging: how I do it. Radiology 2015;275:14-27. [Crossref] [PubMed]
- Mourad MM, Liossis C, Gunson BK, et al. Etiology and management of hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2014;20:713-23. [Crossref] [PubMed]
- Puliti Reigada CH, de Ataide EC, de Almeida Prado Mattosinho T, et al. Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas. Transplant Proc 2017;49:867-70. [Crossref] [PubMed]
- Horrow MM, Blumenthal BM, Reich DJ, et al. Sonographic diagnosis and outcome of hepatic artery thrombosis after orthotopic liver transplantation in adults. AJR Am J Roentgenol 2007;189:346-51. [Crossref] [PubMed]
- Pareja E, Cortes M, Navarro R, et al. Vascular complications after orthotopic liver transplantation: hepatic artery thrombosis. Transplant Proc 2010;42:2970-2. [Crossref] [PubMed]
- Abou El-Ella K, Al Sebayel M, Ramirez C, et al. Outcome and risk factors of hepatic artery thrombosis after orthotopic liver transplantation in adults. Transplant Proc 2001;33:2712-3. [Crossref] [PubMed]
- Singh AK, Nachiappan AC, Verma HA, et al. Postoperative imaging in liver transplantation: what radiologists should know. Radiographics 2010;30:339-51. [Crossref] [PubMed]
- Yedlicka JW Jr, Halloran J, Payne WD, et al. Angiogenesis after hepatic arterial occlusion in liver transplant patients. J Vasc Interv Radiol 1991;2:235-40. [Crossref] [PubMed]
- Hamby BA, Ramirez DE, Loss GE, et al. Endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg 2013;57:1067-72. [Crossref] [PubMed]
- Saad WE, Davies MG, Sahler L, et al. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol 2005;16:795-805. [Crossref] [PubMed]
- Ahmad T, Chavhan GB, Avitzur Y, et al. Doppler Parameters of the Hepatic Artery as Predictors of Graft Status in Pediatric Liver Transplantation. AJR Am J Roentgenol 2017;209:671-5. [Crossref] [PubMed]
- Heffron TG, Welch D, Pillen T, et al. Low incidence of hepatic artery thrombosis after pediatric liver transplantation without the use of intraoperative microscope or parenteral anticoagulation. Pediatr Transplant 2005;9:486-90. [Crossref] [PubMed]
- Ziaziaris WA, Darani A, Holland AJ, et al. Reducing the incidence of hepatic artery thrombosis in pediatric liver transplantation: Effect of microvascular techniques and a customized anticoagulation protocol. Pediatr Transplant 2017;21. [Crossref] [PubMed]
- Ackermann O, Branchereau S, Franchi-Abella S, et al. The long-term outcome of hepatic artery thrombosis after liver transplantation in children: role of urgent revascularization. Am J Transplant 2012;12:1496-503. [Crossref] [PubMed]
- Herrero A, Souche R, Joly E, et al. Early Hepatic Artery Thrombosis After Liver Transplantation: What is the Impact of the Arterial Reconstruction Type? World J Surg 2017;41:2101-10. [Crossref] [PubMed]
- Vorwerk D, Gunther RW, Klever P, et al. Angioplasty and stent placement for treatment of hepatic artery thrombosis following liver transplantation. J Vasc Interv Radiol 1994;5:309-11; discussion 312-4. [Crossref] [PubMed]
- Lee IJ, Kim SH, Lee SD, et al. Feasibility and Midterm Results of Endovascular Treatment of Hepatic Artery Occlusion within 24 Hours after Living-Donor Liver Transplantation. J Vasc Interv Radiol 2017;28:269-75. [Crossref] [PubMed]
- Zhang H, Qian S, Liu R, et al. Interventional Treatment for Hepatic Artery Thrombosis after Liver Transplantation. J Vasc Interv Radiol 2017;28:1116-22. [Crossref] [PubMed]
- Kogut MJ, Shin DS, Padia SA, et al. Intra-Arterial Thrombolysis for Hepatic Artery Thrombosis following Liver Transplantation. J Vasc Interv Radiol 2015;26:1317-22. [Crossref] [PubMed]
- Goldsmith LE, Wiebke K, Seal J, et al. Complications after endovascular treatment of hepatic artery stenosis after liver transplantation. J Vasc Surg 2017;66:1488-96. [Crossref] [PubMed]
- Hsiao CY, Ho CM, Wu YM, et al. Management of early hepatic artery occlusion after liver transplantation with failed rescue. World J Gastroenterol 2015;21:12729-34. [Crossref] [PubMed]
- Akbar SA, Jafri SZ, Amendola MA, et al. Complications of renal transplantation. Radiographics 2005;25:1335-56. [Crossref] [PubMed]
- Rouvière O, Berger P, Beziat C, et al. Acute thrombosis of renal transplant artery: graft salvage by means of intra-arterial fibrinolysis. Transplantation 2002;73:403-9. [Crossref] [PubMed]
- McCarthy JM, Yeung CK, Keown PA. Late renal-artery thrombosis after transplantation associated with intraoperative abdominopelvic compression. N Engl J Med 1990;323:1845. [Crossref] [PubMed]
- Ball CG, Kirkpatrick AW, Yilmaz S, et al. Renal allograft compartment syndrome: an underappreciated postoperative complication. Am J Surg 2006;191:619-24. [Crossref] [PubMed]
- Zhu X, Liu H, Wang W, et al. Two cases of transplant renal artery thrombosis and spontaneous rupture caused by mucormycosis. Transpl Infect Dis 2015;17:442-8. [Crossref] [PubMed]
- Voiculescu A, Schmitz M, Hollenbeck M, et al. Management of arterial stenosis affecting kidney graft perfusion: a single-centre study in 53 patients. Am J Transplant 2005;5:1731-8. [Crossref] [PubMed]
- Douis H, Shabir S, Lipkin G, et al. Drug-eluting stent insertion in the treatment of in-stent renal artery restenosis in three renal transplant recipients. J Vasc Interv Radiol 2008;19:1757-60. [Crossref] [PubMed]
- Kim HS, Fine DM, Atta MG. Catheter-directed thrombectomy and thrombolysis for acute renal vein thrombosis. J Vasc Interv Radiol 2006;17:815-22. [Crossref] [PubMed]
- Tang H, Wang Z, Wang L, et al. Depiction of transplant renal vascular anatomy and complications: unenhanced MR angiography by using spatial labeling with multiple inversion pulses. Radiology 2014;271:879-87. [Crossref] [PubMed]
- Vandermeer FQ, Manning MA, Frazier AA, et al. Imaging of whole-organ pancreas transplants. Radiographics 2012;32:411-35. [Crossref] [PubMed]
- Han K, Ko HK, Tsauo J, et al. Endovascular Management for the Treatment of Pancreas Transplant Venous Thrombosis: A Single-Center Experience. J Vasc Interv Radiol 2016;27:882-8. [Crossref] [PubMed]
- Foshager MC, Hedlund LJ, Troppmann C, et al. Venous thrombosis of pancreatic transplants: diagnosis by duplex sonography. AJR Am J Roentgenol 1997;169:1269-73. [Crossref] [PubMed]
- Wicky S, Pinto EG, Oklu R. Catheter-directed thrombolysis of arterial thrombosis. Semin Thromb Hemost 2013;39:441-5. [Crossref] [PubMed]


