Echocardiographic and electrocardiographic abnormalities in adults living with human immunodeficiency virus: a cross-sectional study in the Yaoundé Central Hospital, Cameroon
Introduction
Human immunodeficiency virus (HIV) infection still remains a public health problem, especially in low-income settings despite a downward trend in new infections. According to UNAIDS, the number of new infections decreased from 3.4 million in 2001 to 2.3 million in 2012 (33% decrease). The number of people living with HIV (PLHIV) still remains high, with about 35.3 million cases worldwide. In Cameroon, a low-income setting in sub-Saharan Africa (SSA), the number of cases was estimated at 620,000 (prevalence of 3.9%) in 2015 (1). The severity of HIV infection is strongly correlated with the degree of immune deficiency, which exposes patients to opportunistic infections, and associated cardiovascular diseases. The cardiovascular manifestations could be due to HIV or opportunistic infections, or to the effects of anti-retroviral (ARV) medicines, especially protease inhibitors (2). Protease inhibitors can induce metabolic disorders (dyslipidemia, insulin resistance, lipodystrophy) that result in cardiovascular disorders. The latter constitutes the fourth cause of death and hospitalization in HIV-infected patients in high-income settings (3). Thus, Grunfel et al. suggested that HIV should be considered as a cardiovascular risk factor per se (4). Nzuobontane et al. showed that paucisymptomatic cardiovascular abnormalities are frequent in HIV infected adults, and there is an association between low CD4 cell counts and the occurrence of dilated cardiomyopathy (5). Menanga et al. showed that advanced HIV infection in adults is associated with symptomatic cardiovascular diseases especially dilated cardiomyopathy and pericarditis (6). The excess cardiovascular burden due to HIV infection is not well known in this setting. This work aimed at studying the profile of ECG and echocardiographic abnormalities in HIV-infected adults compared to non-infected adults in a low-income setting in SSA.
Methods
Study design and study setting
We carried out a case-control study at the Yaounde Central Hospital (YCH) over a 3-month period (March to May 2016). The YCH is the biggest teaching Hospital in Cameroon (SSA), with a catchment population of over two million inhabitants. The HIV treatment unit of this hospital is the biggest and the oldest in the country, with over fourteen thousand patients receiving regular care.
Study participants
Participants were HIV-infected adults (cases) of both sexes, aged ≥21 years, and age–sex matched controls (HIV-negative). Participants (cases) with discordant HIV serology, and a previous history of heart disease—before the diagnosis of HIV—were excluded. Participants with any symptom suggestive of heart disease—heart failure and or arrhythmia—at the time of the study were excluded.
Procedure and measurements
HIV-infected patients were recruited from the waiting room of the HIV treatment unit of the hospital, while the HIV-negative controls were recruited at the time of withdrawing their results of HIV test in the unit. We collected the following data at the initial visit after a face-to-face interview: resting blood pressure (BP) in mmHg using an automated BP machine (Omron® M6 Confort IT), resting pulse rate in beats per minute, respiratory rate in cycles per minute, weight in kilogram (kg) with a commercial bathroom scale (Camry®), and height in meter (m) using a stadiometer. The height and weight was used to calculate the body mass index (kg/m2). During the second visit, we performed a resting ECG with a MAC 1,200 machine, and a transthoracic echocardiography using a Philips echography, with the participant in the left lateral decubitus position. On the ECG, we looked for arrhythmia, abnormalities of the QRS complex, hypertrophy of the ventricles, atrial dilation, heart rate, and abnormal repolarization. On echocardiography, we looked for abnormal chamber dimensions, global and regional contractility abnormalities, trans-mitral inflow velocity pattern, ejection fraction (EF), wall thickness, and calcifications. The ECG was read by the same cardiologist blinded to the echocardiography findings. The same cardiologist (BH) performed all cardiac ultrasounds.
Definitions
Advanced HIV disease was defined as WHO stage 3 or 4 and/or CD4 cell count <200/mm3. Left ventricular hypertrophy was present when the Sokolow index (SV1 + RV5/6 ≥35 mm), and right ventricular hypertrophy was present when the R/S ratio in V1 >1. Left atrial dilation was present if the P wave duration was >120 ms, and right atrial dilation was present if the P-wave amplitude was >2.5 mm in lead II. Abnormal repolarization was present if there was T wave inversion in at least two concordant electrodes. The left ventricle was dilated if the end-diastolic diameter was >31 mm/m2 in men and >32 mm/m2 in women. The left ventricular end-diastolic wall thickness was high if it was >11 mm in men, and >10 mm in women. There was left ventricular hypertrophy if the indexed LV mass was >115 g/m2 in men, and >95 g/m2 in women. The EF was low if it was <55%. The diastolic function was assessed from the trans-mitral flow using pulse wave Doppler, and presented using the Appleton classification. Wall motion abnormality was visually assessed. All the data were recorded as absent or present.
Sample size
The minimum number of participants for each group was calculated using the Schlesselman formula. Using the prevalence of HIV infection in the city of Yaoundé to be 6.3% (Cameroon Health Demographic: EDS-MICS 2011), with a standardized power of 84% to detect a significant difference, and an accepted error of 5% or less, the estimated sample size was at least 42 participants for each group.
Data analysis
The data were analyzed with Epi-Info version 3.5.4. Discrete variables were presented as proportions with their corresponding standard deviation. Proportions were compared using Chi-squared test or Fisher exact test where appropriate. Continuous variables are presented as means with their standard deviation. Means were compared using the Student t-test. Multivariate logistic regression was used to determine factors associated with the occurrence of cardiovascular diseases in HIV infected and HIV negative adults. The level of statistical significance was set for a P value <0.05.
Ethical considerations
Ethical clearance was obtained from the Cameroon National Ethical Committee. Administrative authorization was obtained from the YCH administration. This work was carried out according to the declarations of Helsinki and reported according to the STROBE guidelines.
Results
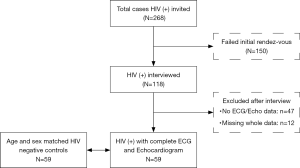
During the 3-month study period, a total of 855 people were screened for HIV, of which 268 patients (cases) tested positive. Of the cases, 150 did not come for the second visit (interview phase). Fifty-nine of them had a complete clinical, ECG, and echocardiographic assessment. These cases were age and sex matched with 59 HIV-uninfected adults (controls), giving a total of 118 participants (Figure 1). Of those living with HIV, 47 (79.7%) were on ARV, and 49 (83.1%) had CD4 cell count >200/mm3. Most patients with HIV (81.2%) were in WHO stage I or II of the disease. All patients on ARV were on a fixed drug combination associating Tenofovir-Lamivudine-Efavirenz.
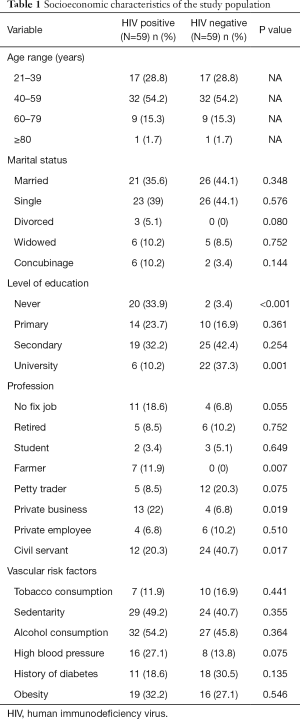
The sociodemographic characteristics and vascular risk factors of the study population are shown in Table 1. There were more females (32.2% versus 67.8%). In both cases and controls, the 40–59 years old group was the most frequent with 32 (54.2%) participants. Overall, their mean age was 47±12.7 years, and ranged from 21 to 84 years. The vascular risk factors were similarly distributed between groups.
Full table
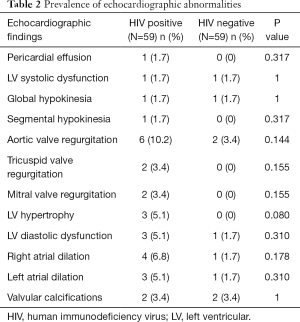
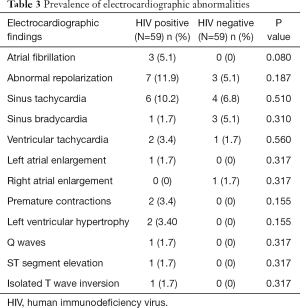
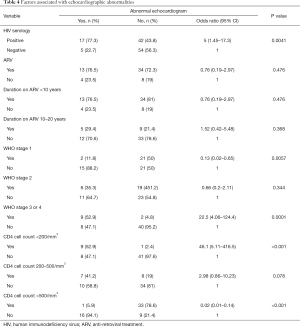
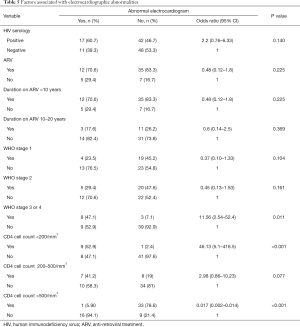
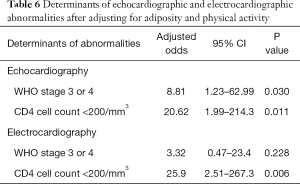
The prevalence of echocardiographic abnormalities (cases versus controls) is shown in Table 2. This was 28.8% (n=17) versus 8.5% (n=5) (P=0.005). The prevalence of ECG abnormalities (cases versus controls) is shown in Table 3. This was 28.8% (n=17) versus 18.6% (n=11) (P=0.195). Factors associated with echocardiographic abnormalities are shown in Table 4. HIV infection was significantly associated with the occurrence of an abnormal echocardiogram [OR 5, (95% CI: 1.45–17.27), P=0.004]. WHO stage 3 or 4 disease had very high risk of having an echocardiographic abnormality [OR 22.5, (95% CI: 4.06–124.39)]. A very low CD4 cell count <200/mm3 was also associated with a very high risk of having echocardiographic abnormalities [OR 46.13 (95% CI: 5.11–416.5), P<0.001]. Factors associated with electrocardiographic (ECG) abnormalities are shown in Table 5. Overall, HIV infection was not significantly associated with the occurrence of ECG abnormalities. However, WHO stage 3 or 4 disease [OR 11.56, (95% CI: 2.54–52.41), P<0.001] and CD4 cell count <200/mm3 [OR 46.13, (95% CI: 5.1–416.5), P<0.001) were associated with the occurrence of ECG abnormalities. In multivariate analysis (adjusting for adiposity and physical exercise), the factors independently associated with echocardiographic abnormalities in PLHIV were WHO stage 3 of 4 diseases [OR 8.8, (95% CI: 1.2–63), P=0.03], and a CD4 cell count <200/mm3 [OR 20.6, (95% CI: 1.98–214.3), P=0.011). A CD4 cell count <200/mm3 [OR 25.9, (95% CI: 2.5–267.3), P=0.006)] was independently associated with an abnormal ECG in PLHIV (Table 6).
Full table
Full table
Full table
Full table
Full table
In the group of HIV infected patients with no ECG and Echocardiogram (n=47), there were 15 (31.9%) males and 32 (68.1%) females. Their mean age was 46.6±11.1 years (range, 33–66 years). High BP was seen in 12 (25.5%), history of diabetes in 9 (19.1%), obesity in 17 (36.2%), tobacco use in 7 (14.9%), excessive alcohol intake in 21 (44.7%), and sedentarity in 23 (48.9%). Their age, sex, and distribution of the vascular risk factors were similar to those who had a complete ECG/Echocardiogram pair.
Discussion
We carried out a case control study with the aim of assessing the profile of echocardiographic and ECG abnormalities in the course of HIV disease in a group of patients (cases), compared to non–HIV infected participants (controls) in SSA. The vascular risk factors were similarly distributed between groups. HIV infected adults have significantly more echocardiographic and ECG abnormalities compared to non-infected adults. The ECG and echocardiographic anomalies varied, and depends on the severity of immune deficiency.
There were twice as more females than males living with HIV. This female predominance in HIV infection corroborates with that reported by UNAIDS (1). This female predominance was also reported by Kingue et al. (56.6%), and Traore et al. (65%). Their mean age was 47 years and ranged from 21 to 84 years. Those in the 40–59 age group were the most represented (54.2%), corresponding to the age group potentially exposed to cardiovascular risk factors. There was a statistically significant association between HIV infection and the presence of structural cardiac abnormalities on ultrasound. This was similarly reported in the literature, with rates that ranged from 22% to 80% (7–9). The most frequent echocardiographic abnormalities in both groups were aortic valvular regurgitation, right atrial dilation, diastolic dysfunction, and LVH. This is contrary to that frequently described in the literature, where pericardial disease was the most frequent, and followed by myocardial disorders (6,10,11). This difference can be explained by the fact that our study population consisted solely of asymptomatic participants seen at the out-patient unit, and who also had concomitant cardiovascular risk factors. This LVH could be as a result of the interplay between HIV infection and the classical cardiovascular risk factors on the heart muscle. The fact that most patients with HIV were on treatment, and had good clinical and immunological status could modify the occurrence of abnormalities. This suggests that the non-protease ARV regimen could reduce the occurrence of echocardiographic abnormalities in patients with HIV. This could be through indirect mechanisms such as improved clinical and immune status, as the prolonged exposure to ARV did not show any significant interaction with the occurrence of abnormalities.
Electrical abnormalities were slightly more frequent in those living with HIV, and these were mostly atrial fibrillation and premature contractions. Menanga et al. found more cases of repolarization disorders and sinus tachycardia (6). These abnormalities were more frequent in those with severe immune deficiency (CD4 <200/mm3) and advanced stages of the infection (WHO stage 3 or 4). It is not certain if there is a direct link between HIV disease and myocardial excitability. The sub-clinical chronic myocarditis associated with HIV could play a role in the genesis of cardiac arrhythmia in HIV. This hypothesis needs to be studied in a cohort study.
These findings should be interpreted in the light of some limitations. This was a single center study in the center region of Cameroon. More participants originated from the center region. Thus, we cannot extrapolate our findings to the general population of Cameroon, which is genetically diverse. Also, the cross-sectional design does not permit us to establish a causal relationship between HIV infection and the cardiac abnormalities observed. There was a high rate of the classical cardiovascular risk factors in both groups, which could interact with HIV disease. The study was not sufficiently powered to study these interactions. Our study population was highly selected. Those with a past history or symptoms suggestive of heart disease at the time of the study—heart failure or arrhythmia—were excluded from the main analysis. Thus our findings are essentially sub-clinical abnormalities in the study population. The control group was probably not very healthy despite a negative HIV serology as they presented for HIV screening. A high rate of a history of diabetes and other vascular risk factors were noted in this population, which could be associated with poor health. Their unfavorable health condition could contribute to the cardiovascular burden. This was however comparable to those infected with HIV. Most of the patients with HIV were on treatment, and had good clinical and immunological status. This could influence the occurrence of echocardiographic and ECG abnormalities. There was a high rate of drop-outs, especially for the interview phase. This could be explained by the fact that the pioneer treatment center of HIV cares for patients who come from near and far away. The cost of transportation for subsequent visits could explain the high dropouts—as this was incurred by the participants. Despite these limitations, this study has some merits due to its case control design. We have shown the excess burden of HIV on the heart in patients living in SSA. It is the first of its kind in the HIV treatment center, and the findings form the basis for further research in our setting.
Conclusions
HIV infected participants had significantly more echocardiographic and ECG disorders than those not infected. The ECG and echocardiographic expressions of cardiac involvement are varied, and depends on the severity of the immune deficiency. They were mainly repolarization disorders and aortic valvular regurgitation. The influence of traditional cardiovascular risk factors should be taken into account in immunocompromised patients in the development of these different abnormalities.
Acknowledgements
We thank the patients who participated in this study and the staff of the Day Hospital, YCH, for assisting with this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Cameroon National Ethical Committee (No. CEI-UD/019/05/2016T).
References
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Geneva: Joint United Nations Program on HIV/AIDS, 2013. Available online: http://files.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf
- World health organization 2006.WHO case definition of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adult and children. World Health Organization. Available online: http://www.who.int/hiv/pub/guidelines/hivstaging/en/
- Kwong GP, Ghani AC, Rode RA, et al. Comparison of the risks of atherosclerotic events versus death from others causes associated with antiretroviral use. AIDS 2006;20:1941-50. [Crossref] [PubMed]
- Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:1045-52. [PubMed]
- Nzuobontane D, Blackett KN, Kuaban C. Cardiac involvement in HIV infected people in Yaounde, Cameroon. Postgrad Med J 2002;78:678-81. [Crossref] [PubMed]
- Menanga AP, Kougang CK, Jingi AM, et al. Patterns of cardiovascular disease in a group of HIV-infected adults in Yaoundé, Cameroon. Cardiovasc Diagn Ther 2015;5:420-7. [PubMed]
- Gouëllo JP, Chennebault JM, Loison J, et al. Echocardiographic abnormalities in stage IV of HIV infection. Presse Med 1993;22:712-6. [PubMed]
- Kane A, Thiam S, Sarri M, et al. Echocardiographic aspects of HIV / AIDS infection in Senegal. Cardiol Trop 1998;24:3-9.
- Milei J, Grana D, Fernández Alonso G, et al. Cardiac involvement in acquired immunodeficiency syndrome: a review to push action. The committee for the study of Cardiac Involvement in AIDS. Clin Cardiol 1998;21:465-72. [Crossref] [PubMed]
- Bouramoue C, Ekoba J. Heart and AIDS. Med Trop (Mars) 1996;56:33-9. [PubMed]
- Longo-Mbenza B, Tonduangu K, Kintonki Vita E, et al. The effect of HIV infection on high incidence of heart diseases in Kinshasa (Zaire). Echocardiographic study. Ann Cardiol Angeiol (Paris) 1997;46:81-7. [PubMed]


