Renovascular hypertension: endovascular therapy in complicated aortic Stanford type B dissection
Case presentation
A 63 years old male (183 cm, 90 kg, BMI 26.9) with a history of arterial hypertension presented with a current onset of chest pain and discrete headaches accompanied by dizziness. His history did not reveal any pain attacks or further cardiovascular risk factors but sigmoid diverticulosis. However, blood pressures had worsened, measuring as high as 210/110 mmHg on both sides at presentation. Daily antihypertensive medication initially consisted of an AT-receptor-inhibitor (valsartan 2×20 mg) and a loop diuretic (torasemide 1×5 mg) which was then modified by switching to candesartan (1×32 mg), hydrochlorothiazide diuretic (1×25 mg) and an additional calcium channel blocker (lercanidipine 1×10 mg).
A following 24 hours measurement showed a reversed circadian rhythm with an average of 150/90 mmHg during night time (Figure 1). The patient’s EKG showed an indifference type and a ST-segment-depression in II, III and aVF compatible with unspecific echocardiographic signs of hypertensive heart disease (diastolic septal thickness 14 mm, E/A 0.85). Due to difficult to control blood pressure a bicycle ergometry was not feasible. While renal function appeared inconspicuous the added abdominal Duplex-sonography revealed an inconclusive renal resistance index (RRI) on the right side which was borderline and varied in an unexpectedly broad range; the renal artery itself could not be demonstrated. Finally, invasive angiography could rule out relevant coronary stenosis, but demonstrated a high-grade dynamic ostial stenosis on the right renal artery caused by an aortic membrane (Figure 2). After repeated careful intubation of the affected right renal artery beyond the dissected proximal segment the approximate pressure gradient (derived by catheter pull-back) varied between 5 and 35 mmHg. The following CT angiography demonstrated pronounced kinking of the thoracic aorta and confirmed a Stanford type B dissection involving the right renal artery causing reduced perfusion of the right kidney—apparently by the false lumen mainly (Figure 2).

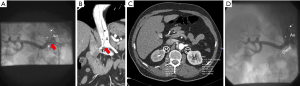
Facing the above we decided for intervention (1): in a second procedure stent placement was successfully performed. This way the right renal artery was kept open by fixing the reno-aortic dissection flap in order to prevent it from progressing into the right kidney (Figure 2).
At a follow-up examination about 1.5 years later the patient presented with an arterial blood pressure of 120/90 mmHg under his unchanged daily medication. An improved blood pressure control allowed for a persisting physiologic drop of blood pressure during night-time (Figure 1). Serum creatinine showed normal values and an in-stent-restenosis could be ruled out.
Discussion
Chronic dissections of the distal aorta (Stanford type B) is considered to be present if >14 days have elapsed since the acute event or—as in our case—the dissection is found incidentally (<10%) and typically associated with arterial hypertension (2). In patients with aortic dissection relevant renal malperfusion has been reported in 8% (3). Most importantly renal malperfusion in this setting is known to be an independent predictor of in hospital mortality (4), presumably resulting from thrombosis, ischemia-reperfusion injury, or a systemic inflammatory response syndrome (1). Clinical suspicion is usually based on worsening arterial hypertension and/or signs of renal dysfunction. Depending on the configuration of the aortic flap and its movement throughout the cardiac cycle it may cause either a high-grade static or dynamic obstruction of the affected renal artery (5). Activation of the renin-angiotensin-aldosterone system (RAAS) results in the classic reno-vascular hypertension pathway (Goldblatt phenomenon).
In the present case invasive angiography proved the formal diagnosis according to the recently introduced clinical “classification of renal artery dissection”—a so-called “combined renal artery dissection with aortic dissection” (Figure 2) presenting with “dynamic obstruction” [class IIB (5)]. We had demonstrated a pulsatile blood flow in the aorta resulting in a variable pressure gradient of between 5 and 35 mmHg (1). However, in the present case diagnostic work-up considering RRI was inconclusive. A CT-scan ordered to differentiate between aortic dissection and reno-vascular morphology (Figure 2), finally, proved prognostically relevant renal malperfusion and ruled out underlying atherosclerosis and/or fibromuscular dysplasia.
In view of (I) symptomatic secondary arterial hypertension; (II) therapy resistance; and (III) poor prognosis of aortic type B dissection with renal involvement we decided for endovascular therapy. Based on invasive and non-invasive imaging there was no need for central aortic fenestration. Thus, with exploring the true lumen isolated renal stenting was effectively performed (Figures 1 and 2).
There is still controversy with regard to the clinical value of endovascular therapy in case of renal artery stenoses possibly causing renovascular hypertension (6). Without discussing current data in detail, clinical studies support renal stenting only in selected patient populations. The present case describes another variety of renal artery pathology (7,8) requiring pathophysiologically justified endovascular therapy of this vascular region.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: We confirm that the informed consent was obtained from the patient.
References
- Barnes DM, Williams DM, Dasika NL, et al. A single-center experience treating renal malperfusion after aortic dissection with central aortic fenestration and renal artery stenting. J Vasc Surg 2008;47:903-10; discussion 910-1. [Crossref] [PubMed]
- Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J 2001;22:1642-81. [Crossref] [PubMed]
- Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg 2001;33:1185-92. [Crossref] [PubMed]
- Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation 2003;108 Suppl 1:II312-7. [Crossref] [PubMed]
- Jain A, Tracci MC, Coleman DM, et al. Renal malperfusion: spontaneous renal artery dissection and with aortic dissection. Semin Vasc Surg. 2013;26:178-88. [Crossref] [PubMed]
- Zeller T, Macharzina R, Rastan A, et al. Renal artery stenosis: Up-date on diagnosis and treatment. Vasa 2014;43:27-38. [Crossref] [PubMed]
- Demming T, Frey N, Langer C. Pickering syndrome: high-risk stenting of a renal artery stenosis in a multimorbid patient presenting with progressive congestive heart failure. Clin Res Cardiol 2013;102:615-7. [Crossref] [PubMed]
- Jularic M, Cupa J, Rosenberg M, et al. Takotsubo cardiomyopathy in complicated Pickering syndrome: endovascular therapy of an occluded renal artery. Clin Res Cardiol 2014;103:759-61. [Crossref] [PubMed]


