Endovascular management of a coronary artery to pulmonary artery fistula with detachable balloons: a case report
Introduction
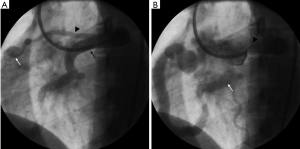
A 28-year-old woman was referred for cardiac evaluation of a continuous murmur. The murmur was detected on physical examination by a gynaecologist during work up for possible hysterectomy for severe menorrhagia. The patient had no chest pain or palpitations but reported dyspnoea on exertion. There was no history of congenital cardiac anomalies or surgical interventions on the chest. On examination, the peripheral pulses were normal and there was no pedal oedema. A continuous murmur was again noted on auscultation of the heart. An electrocardiogram was normal with no signs of myocardial ischemia or arrhythmia. An echocardiography demonstrated a large coronary artery to pulmonary artery fistula between the left anterior descending (LAD) coronary artery and the main pulmonary artery. The heart chambers were normal with normal valvular function. There was no pericardial effusion. The left ventricular ejection fraction was normal. Subsequently, a coronary angiography was performed through a trans-femoral route. This confirmed the presence of a large fistula between the LAD coronary artery and the main pulmonary artery. The fistulous connection was seen as a tortuous vessel coursing in a curvilinear fashion towards the main pulmonary artery (Figure 1). In addition, another small fistula was seen between the proximal LAD coronary artery to the main pulmonary artery. There was relative hypo-perfusion of the branches of the LAD coronary artery and the left circumflex coronary artery. Given the presence of exertional dyspnoea and coronary hypoperfusion secondary to the fistula, endovascular occlusion of the fistula was considered. Detachable balloons were considered for occlusion of the large fistula due to the size of the fistula and the tortuous course of the artery. Endovascular coil embolization was considered for the small fistula given its size and straighter course.
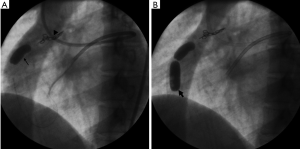
The left main coronary artery ostium was catheterized with a 7-French guiding catheter. The proximal accessory branch to the fistula was catheterized using the SL-10 microcatheter (Boston Scientific Corporation, Natick, USA) and the feeding artery was occluded with micro-coils of 3 mm diameter (Target coils, Boston Scientific Corporation, Natick, USA). The larger fistula was then catheterized with 3F coaxial microcatheter (Nycomed Ingenor, Paris, France) and the feeding artery was occluded with two detachable valved latex balloons of 10 mm diameter (Nycomed Ingenor, Paris, France). The balloons were inflated with 50% non-ionic contrast material. The second balloon was used as a safety to prevent recurrence of the fistula (Figure 2). Post embolization, angiography showed successful occlusion of the coronary artery to pulmonary artery fistula with excellent flow to the branches of the left circumflex coronary artery. The flow in the LAD coronary artery was slow with no obvious opacification of branch vessels suggesting atresia of these branches (Figure 3).
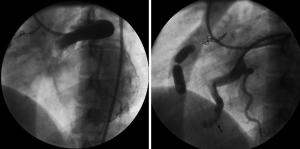
The patient was discharged on dual anti-platelet drugs and remained asymptomatic for 7 years. Later, she presented to another hospital with symptoms of acute myocardial infarction. Coronary angiography demonstrated acute thrombosis of LAD artery. The patient was treated with a coronary bypass. She is still alive and continues to be asymptomatic.
Discussion
Coronary arteriovenous (AV) fistulae are relatively rare congenital anomalies of the heart with a reported incidence of 0.6% to 1%. These were first described in 1841 (1). There is no racial or sex predilection (2). There are two types of coronary artery fistula, namely coronary-cameral fistula—that which communicates with a chamber of the heart and coronary AV fistula—that which communicates with the pulmonary circulation. Coronary AV fistulae may be congenital or acquired. Congenital lesions are often isolated, although they can be associated with anomalies with decreased pulmonary perfusion or increased systemic resistance, e.g., critical pulmonary stenosis or atresia with an intact interventricular septum and pulmonary artery branch stenosis, tetralogy of Fallot, coarctation of the aorta, hypoplastic left heart syndrome, and aortic atresia. Acquired lesions are rare and are often associated with penetrating injuries. Smaller fistulae remain asymptomatic and are almost always incidentally detected. Larger fistulae become symptomatic, enlarge over time, and result in complications related to volume overload, shunting and vascular steal (3). One hypothesis is that coronary artery fistulae are a result of persistent sinusoidal connections between the lumens of the primitive tubular heart that supply myocardial blood flow in the early embryologic period. Patients may remain asymptomatic for years or may present with symptoms of angina or myocardial infarction (2).
Coronary AV fistulae lead to complications in 11% of patients younger than 20 years and in 35% of patients older than 20 years (2). The pathophysiology of symptoms may be related to coronary steal phenomenon (myocardial infarction, angina) or secondary angioarchitectural abnormalities (congestive heart failure, endocarditis, aneurysm formation and rupture). In infancy the condition presents with signs of low output congestive heart failure owing to myocardial ischemia. In older children and adults, signs of high output cardiac failure are more evident. Physical examination, apart from features of heart failure, is marked by a characteristic continuous murmur, which is lower in position as compared to that of patent ductus arteriosus (PDA), and accentuated during the diastole, in contrast with the systolic accentuation in a PDA. Coronary-cameral fistulae are associated with only a diastolic murmur, as there is no flow through fistula in the systole.
Electrocardiography may be normal or may show signs of ischemia or left ventricular volume overload. Echocardiography will show enlarged left heart chambers, dilated coronary artery, and hyperdynamic flow patterns, along with the fistulous communication if meticulously searched for. Cross sectional imaging with multi-detector computed tomography (CT) or magnetic resonance imaging (MRI) are useful in treatment planning as they help characterise the fistula and structural cardiac anomalies in great detail. Catheter angiography remains the gold standard. It delineates the exact anatomy of the fistula and intra-cardiac and intra-arterial pressure studies provide additional physiological information.
Smaller asymptomatic fistulae can be safely monitored. A few of these close spontaneously over time. Larger fistulae invariably lead to complications and are better treated at the time of detection. Conventional treatment for these fistulae has been surgery where the communication is ligated and/or the fistula excised (4). There have been several publications on endovascular treatment of these fistulae, especially using fibered coils (3,5,6) and vascular plugs (7). The number of cases reported using detachable balloons has been relatively rare (8). Endovascular treatment is avoided in patients with multiple connections, circuitous routes, and acute angulations that make catheter positioning difficult or impossible, for whom surgery is the treatment of choice.
Embolization is less invasive and is associated with decreased morbidity compared to surgery. Small fistulae with near-normal sized feeding arteries do extremely well with endovascular treatment. However, occlusion of a large feeding artery often leads to in situ thrombosis from slow flow. These patients require oral anticoagulant therapy or dual anti-platelets to prevent coronary artery thrombosis. Coronary artery thrombosis can occur either acutely during the procedure or after years of therapy and requires urgent surgical bypass (6). Although there are quite a few case reports and a small number of case series, there is, till date, no large-scale study reporting mortality or morbidity associated with the treatment modalities.
The case report demonstrates the feasibility of endovascular treatment using detachable balloons. Detachable balloons catheters are small and allow excellent navigation of the embolic material to the desired location even in an extremely tortuous anatomy. They are easy to deploy and precise deployment can be confirmed before detaching the balloon. This ability to reposition the balloon during the procedure allows high accuracy in treating these high flow fistulae. Currently these balloons are no longer available, but newer microvascular plugs (Medtronic, Minneapolis, USA) that can be delivered though a 3F microcatheter allow similar flexibility and accuracy in deployment and occlusion.
Conclusions
In conclusion, coronary AV fistulae are uncommon, endovascular therapy can be successfully applied for symptomatic fistulae with great success. Regular follow up is required following endovascular therapy of large coronary artery fistulae to detect recurrence, coronary artery thrombosis and other complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: We have not taken consent from the patient since our institution only requires consent of clinical images of the patient is involved. In this case, neither the patient’s name or clinical patient images are included.
References
- Raju MG, Goyal SK, Punnam SR, et al. Coronary artery fistula: a case series with review of the literature. J Cardiol 2009;53:467-72. [Crossref] [PubMed]
- Qureshi SA. Coronary arterial fistulas. Orphanet J Rare Dis 2006;1:51. [Crossref] [PubMed]
- Challoumas D, Pericleous A, Dimitrakaki IA, et al. Coronary arteriovenous fistulae: a review. Int J Angiol 2014;23:1-10. [Crossref] [PubMed]
- Buxton B, Ventimeglia R, Reul GJ, et al. Congenital coronary-to-pulmonary artery fistula: diagnosis and treatment. Cardiovasc Dis 1976;3:202-9. [PubMed]
- Ibrahim MF, Sayed S, Elasfar A, et al. Coronary fistula between the left anterior descending coronary artery and the pulmonary artery: two case reports. J Saudi Heart Assoc 2012;24:253-6. [Crossref] [PubMed]
- Wejner-Mik P, Lipiec P, Peruga JZ, et al. Optimal treatment of coronary-to-pulmonary artery fistula: surgery, coil or stent graft? Postepy Kardiol Interwencyjnej 2013;9:282-5. [Crossref] [PubMed]
- Lee SN, Lee J, Ji EY, et al. Percutaneous Management of Coronary Artery-to-pulmonary Artery Fistula Using an Amplatzer Vascular Plug with the Trans-radial Approach. Intern Med 2016;55:929-33. [Crossref] [PubMed]
- Reidy JF, Sowton E, Ross DN. Transcatheter occlusion of coronary to bronchial anastomosis by detachable balloon combined with coronary angioplasty at same procedure. Br Heart J 1983;49:284-7. [Crossref] [PubMed]


