Pulmonary arteriovenous malformations: endovascular therapy
Introduction
The natural course of pulmonary arteriovenous malformations (PAVM) is not well studied but most of the lesions remain either stable (75%) or demonstrate slow growth (25%) (1,2). However, untreated PAVM are associated with a high risk of morbidity and mortality (0–55%) compared to 3% in the treated patients (1,3). Prompt identification and treatment are indicated given the high risk of complications such as brain abscess (9%), cerebrovascular accident (2.6–25%), hemoptysis/hemothorax (8%), and hypoxemic respiratory failure (1,4-6). The risk of neurological complications has been shown to be higher with diffuse type of PAVM, a large shunt, and a feeding arterial diameter (FAD) of greater than 3 mm (7,8). Additionally, there is decreased risk of neurological complications in treated PAVM. When feasible, endovascular embolotherapy is the mainstay of treatment with less morbidity and mortality (9). Surgical resection is rarely needed and is indicated for lesions that remain refractory to endovascular therapy or when endovascular treatment is not feasible. Lung transplant is indicated only for severe bilateral diffuse disease.
Pre-procedure work up and evaluation
Typically, patients are referred from hereditary hemorrhagic telangiectasia (HHT) clinic (approximately 15–35% HHT patients have PAVM) with a positive echo bubble study (grade 4 has PPV of 1 for benefit from subsequent CT chest study) or findings of PAVM on a screening CT chest scan (4). A multidisciplinary clinic visit with a comprehensive evaluation is recommended to determine if patient status and lesion characteristics are amenable for endovascular treatment. Conversely, a patient initially presenting with PAVM diagnosis should be referred to HHT clinic for a comprehensive evaluation (80% to 90% of patients with PAVM have HHT) (1).
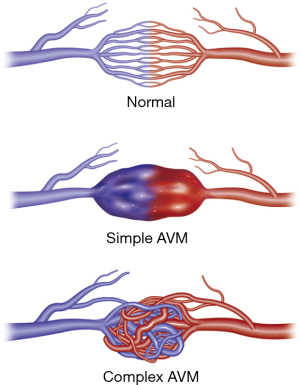
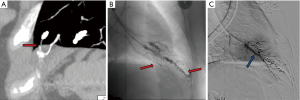
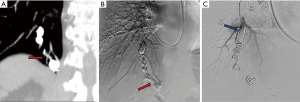
Appropriate pre-procedure work-up is outlined in Table 1. During the clinic visit, it is important to evaluate whether patient can tolerate moderate sedation and stay supine for the duration of the procedure. Patients with underlying pulmonary hypertension might require general anesthesia support during the procedure. Also, a preserved renal function is required as potentially nephrotoxic iodinated contrast materials are used during pulmonary angiography and embolization. An EKG is performed to exclude the presence of a left bundle branch block (LBBB). Catheter manipulation in right heart can result in a right bundle branch block and if the patient is known to have a LBBB, this can result in a complete heart block. Patient with a LBBB benefit from temporary pacing during pulmonary angiography. Computed tomographic angiography (CTA) is the gold standard for diagnosis, and should be reviewed to identify all possible PAVM and the feeder vessels. Specifically, it is important to identify the location, type, and the feeding vessel diameter. PAVM is classified as simple (only one feeding artery, 80–90%), complex (multiple segmental feeding arteries, 10–20%), and an uncommon diffuse subtype (<5%) which are illustrated in Figures 1,2. Conventional 3 mm FAD rule for treatment is not accurate as smaller lesions were shown to be associated with paradoxical embolization and current knowledge suggests treatment of all technically feasible lesions as low as 2 mm (2,9). Attempt should be made to treat a symptomatic lesion (neurological symptoms, embolic disease, hypoxia) irrespective of the size or FAD (10). The advent of microcatheters and technical advancements in coil and plug technology have made treatment of PAVM with smaller feeding vessels possible. Special subpopulations including pregnant patients and children need to be evaluated on a case to case basis, and should be undertaken in dedicated centers with extensive experience. Indications and contraindications for endovascular embolization of PAVM are detailed in Table 2. Risks, benefits and alternatives of the procedure should be discussed in detail, preferably during a dedicated clinical visit.
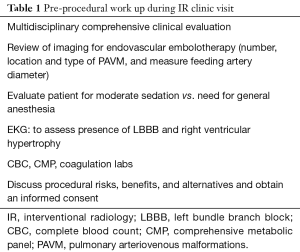
Full table

Full table
Pulmonary arteriography and embolotherapy technique
Embolization of PAVM is performed as an outpatient procedure under moderate sedation and the patient is discharged the same day after the procedure. Patients remain fasting for 6–8 hours prior to the procedure and withhold any anticoagulant medications. Use of bubble filters (0.2–0.4 micron) for IV access is mandatory to prevent paradoxical air embolism. Prophylactic antibiotic administration (1 g of cefazolin or equivalent) is optional.
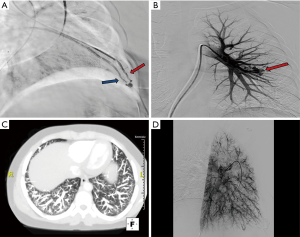
Standard aseptic sterile precautions are maintained throughout the procedure. A common femoral venous access is obtained with US guidance, preferably on the right. Alternative accesses include right internal jugular and left common femoral veins. A 7 Fr short sheath is placed within the common femoral vein and connected to air free flush system. It is important to administer heparin 3,000–5,000 units IV (and subsequently 1,000 units every hour) prior to any wire/catheter manipulations in the pulmonary system to prevent formation of thrombus within the PAVM which can embolize to the left sided circulation. It is important to monitor closely for epistaxis during the procedure. Initially, a 6 Fr APC/Grollman catheter (Cook, Bloomington, Indiana, USA) or a 5 Fr pig tail catheter is advanced into the right atrium, and the desired right or left main pulmonary artery is selected (Figure 3). Pulmonary arterial pressure is measured via a catheter based transducer system (normal—25/8 mmHg, mean—15 mmHg). Selective pulmonary arteriography (imaging at 6 frames/s, and half strength contrast material injected at a rate of 20 cc/s with a total volume of 40 cc) is performed in anterior-posterior (AP) and ipsilateral 40-degree oblique views to identify the PAVM and determine the feeding vessels. It is important for the patient to hold breath in deep inspiration and the operator to include the lung bases within the field of view during arteriography. This serves two purposes—prevents overlapping of tissues and vessels, and permits a comprehensive evaluation as PAVM occur most commonly (83%) at the lung bases (11). Once the feeder vessels are identified and mapped, a coaxial system (7 Fr guiding catheter with an inner longer 5 Fr tipped catheter combination such a Lumax system—Cook, Bloomington, Indiana, USA) is advanced over an exchange length Rosen wire (12). Meticulous care should be taken during catheter-wire exchanges to maintain water seal and also utilize closed flush system or double flush technique to prevent air suction and embolism. Maintaining a wet to wet connection during injector connection is also very important. Selective catheterization of the feeder vessel is followed by embolization. Occasionally, a guide wire or a microcatheter may be required to select small feeder vessels. After the feeder vessel is catheterized, a digital subtraction angiography (DSA) provides a road map and subsequently confirms appropriate distal selection. Additionally, measurement of the vessel diameter is important to select an appropriate embolic device size for successful occlusion. The goal of PAVM embolotherapy is to occlude the feeding artery as distally as possible and close to the sac (sac itself is not embolized) while sparing the normal lung parenchyma. Though not routinely performed, Hayashi et al. reported significantly less reperfusion with coil occlusion of the sac compared to the arterial feeders (13). It is not uncommon to see additional feeders after occlusion of the primary feeder vessel, which would then require selective catheterization and embolization of these additional feeders. A more proximal selective DSA confirms successful embolization of the PAVM. A main pulmonary arteriogram is performed to exclude unsuspected new or remaining PAVM. Any number of PAVM can be treated in a single session but each session is often limited by the complexity of the procedure, radiation dose to the patient and amount of contrast material utilized. At the end of the procedure, the venous access sheath is removed, and manual pressure is applied at the access site for hemostasis. The patient is discharged home after few hours of monitoring and recovery. Acetaminophen can be prescribed (avoid NSAID use due to risk of bleeding/epistaxis) for immediate post procedure pain.
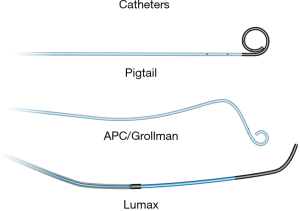
Embolic materials
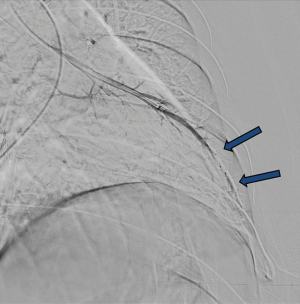
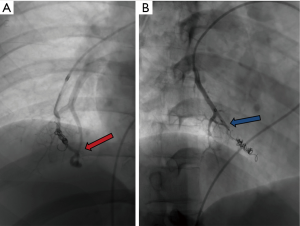
Various embolic materials are available, however, coils and plugs are the most commonly used embolics for occlusion of PAVM (see Figure 4 for illustration of embolic devices). Stainless steel or preferably platinum coils are utilized to occlude the feeding vessel (Figure 5). Detachable coils offer the advantage of repositioning before successful deployment of the coils. An anchor or scaffold technique can be utilized for safe deployment of a non-detachable coil or to prevent prolapse of the coil in to the aneurysmal sac. Alternatively, a double microcatheter technique was described for safe occlusion (14). Amplatzer vascular plugs (St. Jude Medical, St. Paul, MN, USA) offer the ability to address variable sized feeding arteries with precise deployment of the plug and less unintended migration as well as better occlusion rates with decreased procedural times and radiation dose (15). Amplatzer vascular plug 4 is usually preferred because of low profile design (5 Fr catheter) and is available in 4–8 mm diameters allowing occlusion of target vessel diameter from 2.6 to 6.2 mm (Figure 6). Amplatzer II is occasionally used when multiple contact points are required such as a high flow or large AVM and Amplatzer I is helpful when there is a short landing zone. Conventional coil embolization provides successful outcomes, however, plug embolization followed by coil embolization (Figure 7) provides a more permanent occlusion and is the preferred method at our institute (16,17). Newly available microvascular plugs (nitinol mesh and an outer PTFE membrane, Medtronic) are detachable and provide the advantage of rapid occlusion, deployment through a microcatheter (.021” and .027” microcatheter systems for MVP-3 and MVP-5 respectively) and fewer artifacts on follow up imaging (Figure 8). MVP-7 and -9 require a 0.035” lumen catheter for delivery and can be used to occlude target vessels with diameters 5–7 and 7–9 mm respectively. Early results on use of MVP for occlusion of PAVM appear promising (18). Other combinations of MVP plugs with coils are illustrated in Figures 9 and 10. It is important to oversize the coils (20–30%) and plugs (30–50%) to prevent migration as well as pack densely to prevent residual flow through the coil pack. Detachable balloons are no longer available within the US. Particles, gel foam, and liquid embolics have no role in endovascular treatment of PAVM. A stent-graft would be difficult to deploy in the tortuous small vessels, but may be the only available option in rare cases, and is a helpful alternative (Figure 11). Choice of embolic material (coil vs. plug vs. both) is largely an institutional and individual preference until further comparison data are available.
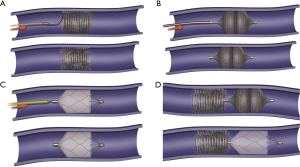
Procedural complications
The most common complication is pleuritic chest pain occurring in 15%, and the incidence increases to 31% in patients with a FAD >8 mm. Pleuritic chest pain typically occurs within the first 2 days, and is self-resolving and well controlled with NSAID medications (19). There is a small risk of thrombus dislodging during the procedure and causing stroke (0.5%) or embolus within the left sided circulation. Other complications are rare and include vessel injury or dissection, air embolism (meticulous technique and filter use can avoid this complication), non-target embolization, and lung infarct/infection. Access site complications include bleeding which can be easily managed with manual compression. Procedure related mortality is extremely rare.
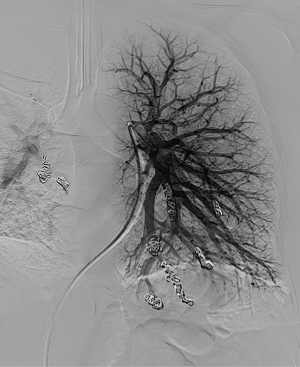
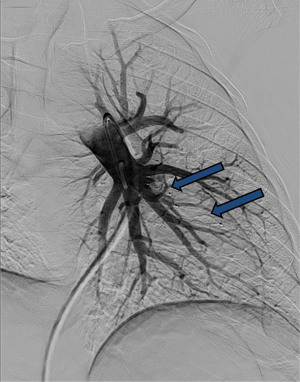
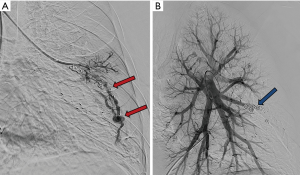
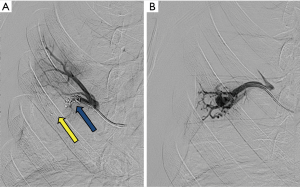
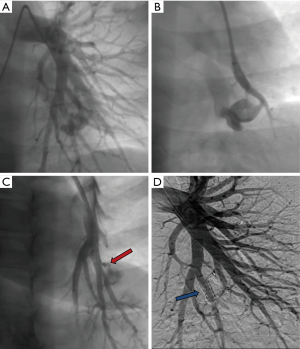
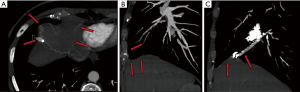
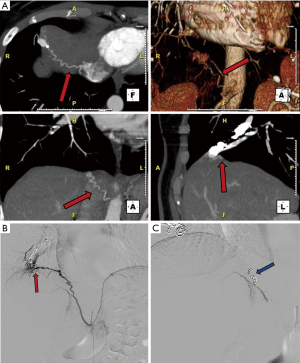
Reperfusion or new PAVM can be seen in up to 20% of treated patients, and are generally amenable for repeat embolization (20) (Figures 12 and 13). Failure of treatment or reperfusion is possible through the below mechanisms—(I) recanalization through or around the deployed embolic devices (predominant mechanism); (II) supply from previously dormant/unrecognized feeder; (III) development of new collaterals from pulmonary arteries; and (IV) development of systemic collaterals (21). Figures 14 and 15 demonstrate reperfusion from systemic collaterals. Factors associated with a higher risk of reperfusion or failure after coil embolotherapy are enumerated in Table 3 (20). New systemic (bronchial artery) collaterals can lead to massive hemoptysis. Development of new or worsening pulmonary hypertension is a rare complication but needs close monitoring and treatment, especially in high risk patients with large and multiple PAVM, pre-existing pulmonary hypertension, and >20% right to left shunt.

Full table
Outcomes
Embolotherapy of PAVM has a very high technical success (≥99%) (2,19). Outpatient single session successful occlusion was achieved in 85% of patients in one study (22). Symptomatic relief can be immediate (23). The risk of neurological complications in treated PAVM is significantly decreased compared to untreated PAVM (0–2% vs. 2.6–25%). Treated PAVM remain occluded in most of the cases (83%), however there is reperfusion or new PAVM noted in approximately 17–20% (2,11,24). Careful attention to technique of dense packing of the distal feeding artery with an appropriately sized coil is important to prevent residual flow and reperfusion. There is good response to repeat embolization of residual or recurrent lesions. There are no randomized studies comparing different treatments or embolic devices which largely remain an individual and institutional preference. Surgical resection and transplant are reserved for refractory and diffuse cases respectively.
Post procedure care and follow up
There are no evidence based guidelines on optimal post procedure follow up and monitoring. Occlusion of the PAVM while immediately apparent on catheter angiography, changes of resolution or decrease in size on CT scan are more delayed. Contrast echocardiography remains positive for an indeterminate period in spite of a successful occlusion necessitating continued pre-surgical antibiotic prophylaxis and use of filter for IV lines (25). In patients with multiple lesions requiring additional sessions, embolotherapy can be repeated in a few weeks. If all the PAVM were treated successfully, patient is evaluated in clinic at one and six months. A follow up CTA in 6 months to 1 year is performed to confirm successful occlusion and evaluate for any residual or new PAVM (9). Subsequent follow up can be with CT scan in 3–5 years or contrast echocardiography performed annually if the initial follow up CT is negative. A close follow up is necessary in patients with severe or diffuse lesions. Recurrence of symptoms at any time should prompt a contrast enhanced CT scan of the chest.
Conclusions
PAVM are associated with high morbidity and mortality if untreated, and therefore, endovascular embolotherapy is recommended as first line therapy for all lesions with feeding artery diameter greater than 3 mm. There is a growing trend to treat all PAVM that are technically accessible, especially if symptomatic, irrespective of the size of the feeding artery. Endovascular embolization has very high technical success with minimal morbidity and no mortality. The commonly used embolic materials include coils and vascular plugs or a combination and are chosen based on individual and institutional preferences. There is a high risk of recanalization or development of new lesions, and therefore, close monitoring is required.
Acknowledgements
The authors would like to thank Erin Moore, MA, Medical Illustrator, Sr. Graphic Designer, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shovlin CL, Jackson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008;63:259-66. [Crossref] [PubMed]
- Pollak JS, Saluja S, Thabet A, et al. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 2006;17:35-44. [Crossref] [PubMed]
- Pierucci P, Murphy J, Henderson KJ, et al. New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest 2008;133:653-61. [Crossref] [PubMed]
- Zukotynski K, Chan RP, Chow CM, et al. Contrast echocardiography grading predicts pulmonary arteriovenous malformations on CT. Chest 2007;132:18-23. [Crossref] [PubMed]
- Ference BA, Shannon TM, White RI Jr, et al. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest 1994;106:1387-90. [Crossref] [PubMed]
- Faughnan ME, Lui YW, Wirth JA, et al. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest 2000;117:31-8. [Crossref] [PubMed]
- Rosenblatt M. Pulmonary arteriovenous malformations: What size should be treated to prevent embolic stroke? Radiology 1992;185:134.
- Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology 2000;55:959-64. [Crossref] [PubMed]
- Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. [Crossref] [PubMed]
- Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol 2010;195:837-45. [Crossref] [PubMed]
- Remy-Jardin M, Dumont P, Brillet PY, et al. Pulmonary arteriovenous malformations treated with embolotherapy: helical CT evaluation of long-term effectiveness after 2-21-year follow-up. Radiology 2006;239:576-85. [Crossref] [PubMed]
- White RI Jr, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology 1988;169:663-9. [Crossref] [PubMed]
- Hayashi S, Baba Y, Senokuchi T, et al. Efficacy of venous sac embolization for pulmonary arteriovenous malformations: comparison with feeding artery embolization. J Vasc Interv Radiol 2012;23:1566-77; quiz p. 1581.
- Greben CR, Setton A, Putterman D, et al. Pulmonary arteriovenous malformation embolization: how we do it. Tech Vasc Interv Radiol 2013;16:39-44. [Crossref] [PubMed]
- Tau N, Atar E, Mei-Zahav M, et al. Amplatzer Vascular Plugs Versus Coils for Embolization of Pulmonary Arteriovenous Malformations in Patients with Hereditary Hemorrhagic Telangiectasia. Cardiovasc Intervent Radiol 2016;39:1110-4. [Crossref] [PubMed]
- Trerotola SO, Pyeritz RE. Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol 2010;195:766-71. [Crossref] [PubMed]
- Fidelman N, Gordon RL, Bloom AI, et al. Reperfusion of pulmonary arteriovenous malformations after successful embolotherapy with vascular plugs. J Vasc Interv Radiol 2008;19:1246-50. [Crossref] [PubMed]
- Conrad MB, Ishaque BM, Surman AM, et al. Intraprocedural Safety and Technical Success of the MVP Micro Vascular Plug for Embolization of Pulmonary Arteriovenous Malformations. J Vasc Interv Radiol 2015;26:1735-9. [Crossref] [PubMed]
- Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med J 2002;78:191-7. [Crossref] [PubMed]
- Milic A, Chan RP, Cohen JH, et al. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol 2005;16:1675-83. [Crossref] [PubMed]
- Woodward CS, Pyeritz RE, Chittams JL, et al. Treated pulmonary arteriovenous malformations: patterns of persistence and associated retreatment success. Radiology 2013;269:919-26. [Crossref] [PubMed]
- Trerotola SO, Pyeritz RE, Bernhardt BA. Outpatient single-session pulmonary arteriovenous malformation embolization. J Vasc Interv Radiol 2009;20:1287-91. [Crossref] [PubMed]
- Gupta P, Mordin C, Curtis J, et al. Pulmonary arteriovenous malformations: effect of embolization on right-to-left shunt, hypoxemia, and exercise tolerance in 66 patients. AJR Am J Roentgenol 2002;179:347-55. [Crossref] [PubMed]
- Mager JJ, Overtoom TT, Blauw H, et al. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol 2004;15:451-6. [Crossref] [PubMed]
- Lee WL, Graham AF, Pugash RA, et al. Contrast echocardiography remains positive after treatment of pulmonary arteriovenous malformations. Chest 2003;123:351-8. [Crossref] [PubMed]


