Acute pulmonary embolism: endovascular therapy
Introduction
Pulmonary embolism (PE) is a leading cause of morbidity and mortality worldwide. The incidence of PE is approximately 600,000 cases annually with approximately 100,000–180,000 deaths related to PE (1). PE is the most preventable cause of death among hospitalized patients (2).
Multidisciplinary management of PE
PE is a complex disease with a highly variable presentation. The available treatment options for PE are expanding rapidly. Traditionally, the initial evaluating physician consulted a specialist at his or her discretion. However, the expansion of therapeutic options and strategies, spanning multiple specialties, means that no one specialty can claim an exclusive or comprehensive domain over the management of PE.
Recognizing this, many institutions have created multidisciplinary pulmonary embolism response teams (PERTs) (3). The PERT model has been described as a combination of rapid response teams that respond to critically ill patients immediately and heart teams that leverage input from a multidisciplinary team to make clinical decisions on complex patients (4). The functions of a PERT are to respond rapidly to a request for assistance, evaluate the patient, form a consensus treatment plan, and execute it (5). The composition of a PERT can vary, but PERTs often include physicians from cardiology, pulmonary/critical care, interventional radiology, cardiac surgery, hematology, and vascular medicine (6-8).
When a PERT receives a consult, a designated member of the team will evaluate the patient and review imaging and lab results. This person will risk stratify the patient and assess the risk of bleeding. The rest of the team will convene either in person, via telephone, or via Health Insurance Portability and Accountability Act (HIPAA) compliant virtual meeting software. The evaluating team member will present the case to the group, and the team members will discuss the case and formulate a treatment plan. The plan will be shared with the referring physician as resources are mobilized (i.e., cath lab or OR for intervention or emergency medical services for inter-facility transport) (5,8,9).
The PERT model is relatively new, but several existing PERTs have begun to publish on their initial experiences (6,7,9). Though data is scant at present, the PERT model has the potential to effectively streamline care and provide an evidence-based approach towards management of severe PE.
Risk stratification
Risk stratification guidelines have been developed based on short term mortality and clinical deterioration. The 2011 American Heart Association’s (AHA) Scientific Statement for venous thromboembolism management separates patients into low-risk PE, submassive PE and massive PE (Table 1) (12). Low-risk PE patients are normotensive [systolic blood pressure (BP) ≥90 mmHg], have no right ventricular (RV) dysfunction, and no evidence of myocardial necrosis. Low-risk PE patients represent 55% of the PE population and have an excellent prognosis. Massive PE patients have sustained hypotension (systolic BP <90 mmHg for ≥15 minutes), require inotropic support, and/or have persistent profound bradycardia (HR <40 beats per minute with signs or symptoms of shock). Massive PE patients represent 5% of the PE population and have a 58% mortality risk (13).

Full table
Defining the submassive/intermediate risk PE population is more difficult given the broad range of clinical presentations and the wide mortality range reported in the literature. Submassive PE patients represent 25–40% of the PE population with a mortality risk ranging from 2–3% to 21% at 3 months (13,14). According to the AHA risk stratification statement, submassive PE patients are systemically normotensive (systolic BP ≥90 mmHg) but have evidence of RV dysfunction and/or myocardial necrosis. RV dysfunction is defined as an RV/left ventricular (LV) ratio of >0.9 on CT scan or echocardiogram (Figure 1). Myocardial necrosis is defined by a B-type natriuretic peptide (BNP) or troponin elevation. In addition, patients may have electrocardiography (ECG) changes such as new right bundle branch block (RBBB), anteroseptal systemic thrombolysis (ST) elevation or anteroseptal T-wave inversion.
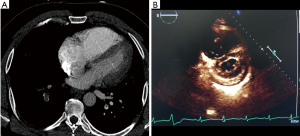
Given the broad range of clinical presentations of submassive PE patients, the 2014 European Society of Cardiology (ESC) Guidelines further delineate submassive/intermediate patients into low and high risk (15,16). The stratification is dependent on a simplified pulmonary embolism severity index (sPESI) score. Intermediate-low risk patients have an sPESI of one or more with either RV dilation on imaging (echo or CT) or biomarker elevation, or neither of these. Intermediate-high risk patients have a sPESI of one or more with RV dilatation on echo/CT and biomarker elevation.
Systemic and catheter directed lysis
Rationale for thrombolysis
Anticoagulation (AC) alone does not dissolve thrombus. Rapid restoration of pulmonary perfusion using thrombolytics can benefit select patients. However, determining which patients benefit from thrombolysis is challenging, as outcomes vary substantially depending on patient characteristics (13). According to the AHA guidelines, aggressive reperfusion therapy [i.e., ST, catheter-directed thrombolysis (CDT), and/or surgical embolectomy] is recommended for massive PE. Such measures are unnecessary for low-risk PE. However, clinical judgement is required for submassive PE, as the decision is not as straightforward (10,12). Furthermore, the bleeding risk of thrombolytics, discussed below, must also be considered.
Like the AHA guidelines, the ESC recommends thrombolytics for high risk PE and AC only for low-risk PE. Clinical judgement is recommended for intermediate risk patients. Thrombolytics may be considered for those in the intermediate-high risk category who are at high risk for clinical deterioration, whereas AC with close monitoring is recommended for those with intermediate-low risk PE.
A separate concern is that PE patients are at risk for long-term functional impairment, shortness of breath, and decreased quality of life. The elevated pulmonary vascular resistance and RV pressure due to occlusive thrombus can damage the RV and subsequently result in decreased ability to adapt to exercise. A prospective study examined 109 previously healthy patients with first-time submassive PE treated with AC only (17). At follow-up 6 months after PE, 41% of patients had cardiopulmonary abnormalities: 17% had only RV dilatation or hypokinesis on echocardiography, 17% had functional limitation as defined by a New York Heart Association (NYHA) heart failure score > II or a 6 minutes walk distance <330 meters, and 8% had both. Additionally, 20% of patients indicated that their quality of life was worsened by at least one of the following three indices: diminished health status, inability to shop, or perceived need for home oxygen. The most severe form of this condition is chronic thromboembolic pulmonary hypertension (CTEPH), a condition found in about 4% of patients at 2-year follow-up, which frequently requires major surgery (18). However, a higher proportion of patients suffer chronic disability without resting pulmonary hypertension, referred to by some as the post-PE syndrome and by others as chronic thromboembolic disease (CTED) (19).
ST
ST is the administration of a thrombolytic agent through a peripheral IV. A large systemic dose is required for adequate delivery of medication to the pulmonary arteries. In the United States, the Food and Drug Administration (FDA) approved regimen is the continuous infusion of 100 mg of alteplase over 2 hours.
ST appears to reduce recurrence or death compared with AC alone in massive PE (20). As such, thrombolysis is indicated for massive or high risk PE. In the absence of hemodynamic instability, such as submassive or intermediate-risk PE, its use is controversial. There have been a few randomized clinical trials specifically investigating the effect of ST and AC vs. AC alone in submassive PE patients. The 2002 trial by Konstantinides et al. randomized 256 patients with submassive PE to 100 mg alteplase infusion or heparin alone and found no difference in mortality, but patients receiving ST less frequently required treatment escalation (10.2% vs. 24.6%, P=0.004) (21). The larger and more recent 2014 PEITHO trial randomized 1,005 patients with intermediate risk PE to bolus tenecteplase or AC alone and found a lower rate of death or hemodynamic decompensation in the tenecteplase group (2.6% vs. 5.6%, P=0.02) (14). However, overall mortality was not different between the two groups. Additionally, three meta-analyses examining ST were completed in 2014. All three showed that, in the setting of normotensive acute PE, ST reduced the incidence of clinical deterioration when compared to AC alone (11,22,23). One of the three studies showed a reduction in overall mortality that extended to patients with intermediate-risk PE (11).
These short-term clinical improvements are tempered by the increased risk of major and intracranial bleeding with ST. The frequency of intracranial hemorrhage associated with ST is approximately 2% (24). As such, many patients have contraindications to ST. In the 2014 PEITHO trial, 6.3% of patients in the thrombolysis arm vs. 1.2% of patients who received placebo experienced major extracranial bleeding complications (P<0.001) (14). Additionally, 2.0% of patients receiving thrombolysis had hemorrhagic stroke vs. 0.2% of those who received placebo. All three of the 2014 meta-analyses also showed an increased rate of bleeding complications with ST (11,22,23).
Long-term outcomes after PE may be improved by initial treatment with ST. In one prospective, observational study of 162 patients with acute submassive PE who either received ST or AC alone (25), 27% of the patients who received AC alone had an RV systolic pressure that was higher at follow up than baseline, compared with 0% of the patients who received thrombolysis. Additionally, a greater proportion of the patients who did not receive thrombolysis had a NYHA score >2 or 6-minute walk distance under 330 minutes at 6 months follow-up. The 2013 MOPETT randomized trial of ST vs. AC alone also showed that patients who received ST had a lower incidence of pulmonary hypertension, defined as a pulmonary artery (PA) systolic pressure ≥40 mmHg, at long-term follow-up (28±5 months) (26).
CDT
Percutaneous catheter-directed methods to remove pulmonary thrombi include mechanical fragmentation, thrombus aspiration, and targeted thrombolytic drug delivery. CDT is the targeted delivery of a thrombolytic drug using a multi-sidehole infusion catheter. The side holes of the catheter are embedded within the thrombus, maximizing the surface area of thrombus in contact with the thrombolytic agent. As a result of the targeted intrathrombus delivery, a much lower dose is required to achieve clot lysis with CDT vs. ST. A standard regimen is an infusion rate of 1 mg/hour of alteplase, with 15–30 mg delivered in total during the course of the treatment. If two infusion catheters are used, an infusion rate of 0.5 mg/hour/catheter is typical, with the total dose not to exceed 30 mg. The EkoSonic catheter (BTG, West Conshohocken, PA, USA) is an alternative infusion catheter (Figure 2) for directed thrombolysis that contains an ultrasonic transducer at its core, which is intended to use sound waves to improve drug penetration into the thrombus and increase access to fibrin.
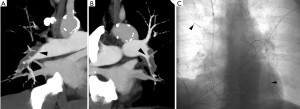
Compared to ST, there is a dearth of data regarding the clinical performance of CDT. There have been three prospective studies demonstrating that CDT effectively lyses pulmonary arterial thrombi, improves pulmonary blood flow at 48 hours, and rapidly restores RV function (27-29). of the studies had an intracranial hemorrhage. However, one found that 10% (15/150) of patients who received CDT experienced major extracranial bleeding complications requiring transfusion (27). One patient with a groin hematoma became transiently hypotensive, requiring vasopressor support. The remainder was normotensive.
There are many unanswered questions regarding CDT, as the aforementioned prospective studies were efficacy studies with methodological limitations (10). It is unclear whether short- and long-term clinical outcomes are improved and whether it is truly safer than ST. Large randomized prospective trials are necessary to define CDT’s optimal use.
Mechanical thrombectomy (MT)
Mechanical fragmentation or MT involves the use of catheters, suction devices, or other tools to remove or decrease the clot burden in the pulmonary arteries to reduce RV afterload and reverse RV failure and shock. MT may resolve hemodynamic instability in massive PE patients faster than thrombolytics alone, or may be used as an adjunct to targeted thrombolytic drug delivery or as a stand-alone therapy in patients with contraindications to lytic therapy.
Indications and patient selection
MT alone is suggested as a treatment for patients with massive PE (12) and contraindications to ST. Contraindications to thrombolysis include active internal bleeding, recent intracranial or intraspinal surgery or serious head trauma, intracranial pathology that increases bleeding risk, bleeding diathesis, and current severe hypertension (30). Even when screened, the rate of major hemorrhage has been reported to be as high as 20% in patients receiving ST (31). MT may also be used when there is insufficient time for ST to take effect in a hemodynamically unstable patient. MT may theoretically decrease the amount of thrombolytic agent required by increasing the surface area exposed to thrombolytics (32).
A meta-analysis published in 2009 (n=594; 6 prospective studies, 29 retrospective studies) showed a high success rate (86.5%) and a low major complication rate (2.4%) in patients who received MT, with some patients additionally receiving local thrombolytics, though the studies comprising the meta-analysis had methodologic limitations. Several studies have shown that MT combined with targeted thrombolytic drug delivery may improve the clinical success rate for massive and sub-massive PE (28,32,33).
Thrombectomy devices
The only percutaneous catheter device that was designed for and approved specifically for PE intervention was the Greenfield catheter, a device developed in the 1960s that is rarely used. Thrombectomy devices that are commonly used in patients with PE were originally designed for removing clots from dialysis fistulas or peripheral arteries (34). Since these devices were designed for use in smaller caliber vessels than the pulmonary arterial system, the clearance of thrombus in the pulmonary arteries is not always achieved (34). There have been multiple retrospective and prospective trials that have shown preliminary success; however, these trials have been hampered by small sample sizes (32). Different strategies for MT include rheolytic devices (Angiojet, Minneapolis, MN, USA), suction devices, and fragmentation devices.
Pigtail catheter
A pigtail catheter can function as a rudimentary MT device. The catheter is placed over a wire into the PE. The clot can then be aspirated and agitated to decrease the clot burden in the central pulmonary arteries. Advantages include wide availability and low cost (32). In some cases, rotating pigtail catheter fragmentation has resulted in distal embolization requiring adjunctive aspiration to correct elevated PA pressure (35). Despite this risk, fragmentation of the clot into smaller fragments is typically more hemodynamically favorable than a large, more proximal occlusion because of the larger volume of peripheral branches (34,36). A small meta-analysis (n=121) published in 2007 found that rotation pigtail catheter thrombectomy in conjunction with local thrombolytic therapy resulted in a reduction of PA pressure (33 to 22 mmHg) and a clinical success rate of 95%, with only 2 instances of major bleeding (33).
Angiojet
The Angiojet (a rheolytic thrombectomy device) injects pressurized saline or thrombolytic from a catheter into the thrombus and subsequently aspirates clot via a separate port. Despite the ease of use and high clinical success rate, there is a high rate of major complications associated with the Angiojet system (heart block, hemoglobinuria, temporary renal insufficiency, major hemoptysis, major hemorrhage, and procedure related death) (32). In their meta-analysis, Kuo et al. found a 28% major complication rate in 68 patients who received Angiojet. Additionally, the release of adenosine from affected platelets can result in bradycardia and vasospasm. The bradyarrhythmias caused by adenosine release can be prevented by administration of glycopyrrolate (37) or treated by a temporary pacemaker wire. However, given the high complication rate, Angiojet has a black box warning from the FDA.
Rotational thrombectomy
Several rotational thrombectomy devices have potential uses in the treatment of PE. Most of these devices have limited descriptions in the literature. First, the Arrow-Trerotola percutaneous thrombolytic device (Teleflex Inc., Morrisville, NC, USA) uses a rotating basket to break up the clot; as the basket is pulled back, clot can be withdrawn from the PA. The device comes in an over-the-wire version, which uses an 0.025-inch wire, and 6 and 7 Fr versions which are not over the wire and require a sheath for delivery to the PA (38). Figure 3 demonstrates a case of submassive PE treated with the Trerotola device in the right interlobar PA.
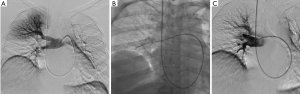
Another rotating thrombectomy device that may be potentially useful in PE is the Cleaner (Argon Medical Devices, Plano, TX, USA). The Cleaner uses a sinusoidal rotating wire to disrupt thrombus. It requires delivery via a 6-Fr sheath (39).
Dumantepe et al. describe the use of the Aspirex Catheter (Straub Medical, Wangs, Switzerland) in 36 patients with massive and submassive PE (40). They were able to significantly decrease the PA pressures from 53±5.8 to 25.6±6.3 mmHg in patients with massive PE (P<0.01) and 46±7.7 to 22±3.6 mmHg in patients with submassive PE (P<0.01) using the Aspirex catheter for rotational thrombectomy. The devices travels over a 0.018-inch wire and uses a high-speed rotating spiral located in the body of the catheter that creates negative pressure through a system that macerates the thrombus and removes it by aspiration.
AngioVac
The AngioVac system (AngioDynamics, Latham, NY, USA) utilizes suction for MT. The system uses a large 26 French catheter that communicates with the clot and aspirates the material using suction generated from a system resembling a cardiopulmonary-bypass circuit. Aspirated clot and blood is run through a filter, and blood is returned to the circulation. Pasha et al. published a case report in 2014 detailing the successful usage of an AngioVac in a woman with an acute massive PE (41). Generally, however, the AngioVac has difficulty navigating through the right side of the heart to the PA due to its bulk and inflexibility (36).
Indigo thrombectomy device
The Indigo system (Penumbra Inc., Alameda, CA, USA) utilizes suction for MT (Figure 4). The device catheter is inserted into the clot and is directly aspirated; a thin “separator” at the distal tip of the catheter continuously breaks up the clot, in order to maintain lumen patency during aspiration. A small retrospective study (n=6) published in 2017 found a significant reduction in systolic PA pressure (58.2 vs. 43.0 mmHg) and RV/LV ratio (1.7 vs. 1.1) without any complications (42).
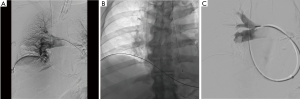
Conclusions
Considerable work and research have improved PE risk stratification and defined techniques and tools for treating severe PE. However, data is still insufficient to determine patient selection for therapeutic escalation, as short term and long-term efficacy and safety outcomes, especially following catheter-directed therapies, have not been defined (43). Additionally, the optimal thrombolytic doses for both ST and CDT require further evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: AK Sista received research grant from Penumbra, Inc., administered through NYU Department of Radiology, and he is an unpaid scientific advisory board member of Thrombolex. The other authors have no conflicts of interest to declare.
References
- Rahimtoola A, Bergin JD. Acute pulmonary embolism: an update on diagnosis and management. Curr Probl Cardiol 2005;30:61-114. [Crossref] [PubMed]
- Rathbun S. Cardiology patient pages. The Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation 2009;119:e480-2. [Crossref] [PubMed]
- Dudzinski DM, Piazza G. Multidisciplinary Pulmonary Embolism Response Teams. Circulation 2016;133:98-103. [Crossref] [PubMed]
- Dudzinski DM, Horowitz JM. Start-up, Organization and Performance of a Multidisciplinary Pulmonary Embolism Response Team for the Diagnosis and Treatment of Acute Pulmonary Embolism. Rev Esp Cardiol (Engl Ed) 2017;70:9-13. [Crossref] [PubMed]
- Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract (1995) 2014;42:31-7. [Crossref] [PubMed]
- Kabrhel C, Jaff MR, Channick RN, et al. A multidisciplinary pulmonary embolism response team. Chest 2013;144:1738-9. [Crossref] [PubMed]
- Sista AK, Friedman OA, Dou E, et al. A Pulmonary Embolism Response Team's initial 20 month experience treating 87 patients with submassive and massive pulmonary embolism. Vasc Med 2018;23:65-71. [Crossref] [PubMed]
- Reza N, Dudzinski DM. Pulmonary embolism response teams. Curr Treat Options Cardiovasc Med 2015;17:387. [Crossref] [PubMed]
- Bloomer TL, Thomassee EJ, Fong PP. Acute Pulmonary Embolism Network and Multidisciplinary Response Team Approach to Treatment. Crit Pathw Cardiol 2015;14:90-6. [Crossref] [PubMed]
- Sista AK, Kuo WT, Schiebler M, et al. Stratification, Imaging, and Management of Acute Massive and Submassive Pulmonary Embolism. Radiology 2017;284:5-24. [Crossref] [PubMed]
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014;311:2414-21. [Crossref] [PubMed]
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011;123:1788-830. [Crossref] [PubMed]
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-9. [Crossref] [PubMed]
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-11. [Crossref] [PubMed]
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315. [Crossref] [PubMed]
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-69, 3069a-k.
- Stevinson BG, Hernandez-Nino J, Rose G, et al. Echocardiographic and functional cardiopulmonary problems 6 months after first-time pulmonary embolism in previously healthy patients. Eur Heart J 2007;28:2517-24. [Crossref] [PubMed]
- Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. [Crossref] [PubMed]
- Sista AK, Klok FA. Late outcomes of pulmonary embolism: The post-PE syndrome. Thromb Res 2018;164:157-62. [Crossref] [PubMed]
- Wan S, Quinlan DJ, Agnelli G, et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004;110:744-9. [Crossref] [PubMed]
- Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 2002;347:1143-50. [Crossref] [PubMed]
- Nakamura S, Takano H, Kubota Y, et al. Impact of the efficacy of thrombolytic therapy on the mortality of patients with acute submassive pulmonary embolism: a meta-analysis. J Thromb Haemost 2014;12:1086-95. [Crossref] [PubMed]
- Marti C, John G, Konstantinides S, et al. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 2015;36:605-14. [Crossref] [PubMed]
- Kanter DS, Mikkola KM, Patel SR, et al. Thrombolytic therapy for pulmonary embolism. Frequency of intracranial hemorrhage and associated risk factors. Chest 1997;111:1241-5. [Crossref] [PubMed]
- Kline JA, Steuerwald MT, Marchick MR, et al. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009;136:1202-10. [Crossref] [PubMed]
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the "MOPETT" Trial). Am J Cardiol 2013;111:273-7. [Crossref] [PubMed]
- Piazza G, Hohlfelder B, Jaff MR, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-92. [Crossref] [PubMed]
- Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest 2015;148:667-73. [Crossref] [PubMed]
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-86. [Crossref] [PubMed]
- Tapson VF, Friedman O. Systemic Thrombolysis for Pulmonary Embolism: Who and How. Tech Vasc Interv Radiol 2017;20:162-74. [Crossref] [PubMed]
- Fiumara K, Kucher N, Fanikos J, et al. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol 2006;97:127-9. [Crossref] [PubMed]
- Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol 2009;20:1431-40. [Crossref] [PubMed]
- Skaf E, Beemath A, Siddiqui T, et al. Catheter-tip embolectomy in the management of acute massive pulmonary embolism. Am J Cardiol 2007;99:415-20. [Crossref] [PubMed]
- Todoran TM, Sobieszczyk P. Catheter-based therapies for massive pulmonary embolism. Prog Cardiovasc Dis 2010;52:429-37. [Crossref] [PubMed]
- Nakazawa K, Tajima H, Murata S, et al. Catheter fragmentation of acute massive pulmonary thromboembolism: distal embolisation and pulmonary arterial pressure elevation. The Br J Radiol 2008;81:848-54. [Crossref] [PubMed]
- Lumsden AB, Suarez E. Interventional Therapy for Pulmonary Embolism. Methodist Debakey Cardiovasc J 2016;12:219-24. [Crossref] [PubMed]
- Syed T, Tamis-Holland J, Coven D, et al. Can glycopyrrolate replace temporary pacemaker and atropine in patients at high risk for symptomatic bradycardia undergoing AngioJet mechanical thrombectomy? J Invasive Cardiol 2008;20:19A-21A. [PubMed]
- Rocek M, Peregrin J, Velimsky T. Mechanical thrombectomy of massive pulmonary embolism using an Arrow-Trerotola percutaneous thrombolytic device. Eur Radiol 1998;8:1683-5. [Crossref] [PubMed]
- Barjaktarevic I, Friedman O, Ishak C, et al. Catheter-directed clot fragmentation using the Cleaner™ device in a patient presenting with massive pulmonary embolism. J Radiol Case Rep 2014;8:30-6. [Crossref] [PubMed]
- Dumantepe M, Teymen B, Akturk U, et al. Efficacy of rotational thrombectomy on the mortality of patients with massive and submassive pulmonary embolism. J Card Surg 2015;30:324-32. [Crossref] [PubMed]
- Pasha AK, Elder MD, Khurram D, et al. Successful management of acute massive pulmonary embolism using Angiovac suction catheter technique in a hemodynamically unstable patient. Cardiovasc Revasc Med 2014;15:240-3. [Crossref] [PubMed]
- Al-Hakim R, Bhatt A, Benenati JF. Continuous Aspiration Mechanical Thrombectomy for the Management of Submassive Pulmonary Embolism: A Single-Center Experience. J Vasc Interv Radiol 2017;28:1348-52. [Crossref] [PubMed]
- Sista AK. Pulmonary Embolism: The Astute Interventional Radiology Clinician. Semin Intervent Radiol 2017;34:11-5. [Crossref] [PubMed]


