Echocardiographic feature of diabetic cardiomyopathy: where are we now?
Introduction
The concept of diabetic cardiomyopathy was first described in 1972 by Rubler et al. in their manuscript titled “New type of cardiomyopathy associated with diabetic glomerulosclerosis” (1). They found that 15% of postmortem cases with diabetic glomerulosclerosis had no hypertension (HTN) and no significant coronary artery disease (CAD) but left ventricular hypertrophy (LVH), cardiomegaly and congestive heart failure (HF). Their histopathologic study revealed diffuse fibrosis, myofibrillar hypertrophy, microvascular disease and deposition of acid mucopolysaccharide material. Their speculation on the pathophysiological mechanism remains valid in the current understanding: “Probably secondary to diabetic microangiopathy although the direct effects of the abnormal myocardial metabolism in diabetes mellitus (DM) could not be excluded.” Diabetic cardiomyopathy has subsequently been widely reported and used by epidemiologists and clinicians worldwide.
DM as an independent risk factor for HF
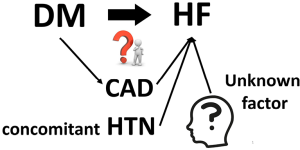
There has been a long debate whether DM causes cardiomyopathy or not (2), because some argue that CM in patients with DM could be due to confounders, such as CAD disease, concomitant HTN, and so on (Figure 1). Indeed, DM dramatically increases the risk of HF. Data from Framingham population demonstrated a 2- to 5-fold excess risk for developing new HF in individuals with DM (3). The risk is even higher (4- to 8-fold) in young patients. An almost linear increase in risk is observed: each 1% elevation in hemoglobin A1C (HbA1C) leads to an 8% increase in the frequency of HF. The independent association between DM and HTN is supported by several large sets of clinical data (Table 1).
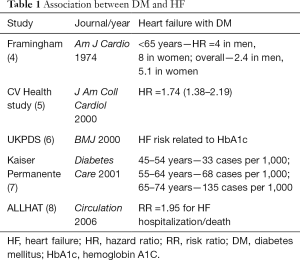
Full table
Effect of DM on HF prognosis
When a patient with DM develops HF, the prognosis generally remains dismal. Data were collected from the US Medicare which include >150,000 patients with DM and aged >65 years. Patients with DM and HF had a hazard ratio (HR) for mortality of >10 with very narrow confidence interval (CI) (HR =10.6; 95% CI: 10.4–10.9), compared with DM without HF (9). The finding is corroborated by several large clinical trials such as LIFE (HR for mortality was 5.98; 95% CI: 3.90–9.17; P<0.0001) and RENAAL (HR =3.99; 95% CI: 3.02–5.25; P<0.0001) (10).
Current understanding
Based on these data, ACCF/AHA Guidelines for HF determined diabetes as an independent risk factor for HF (11). From this revision of the Guidelines, they categorized HF into four stages (stages A to D). Stage A is at high risk for HF without structural changes or symptoms. They clearly mention that DM itself is a significant independent risk for HF. The definition of diabetic cardiomyopathy has been slightly modified from the initial definition by Rubler et al. “ventricular dysfunction that occurs independently of CAD and HTN” to “diabetes-associated structural and functional myocardial dysfunction not related to other confounding traditional factors such as CAD, HTN, congenital or valvular heart diseases” (12-14). In addition, diabetic cardiomyopathy is classified into two types: restrictive [similar to HF with preserved ejection fraction (EF) >50%] and dilated (HF with reduced EF <50%) (15).
The restrictive type is mainly characterized by coronary microvascular endothelial dysfunction and metabolic rearrangements (hyperglycemia, lipotoxicity, and obesity), whereas the dilated counterpart is characterized by cardiomyocyte cell death and autoimmune disorder [e.g., type 1 diabetes mellitus (T1DM)]. They also share common features, such as coronary microvascular rarefaction and advanced glycogen end product (AGE) deposition. Concerning further detailed mechanism, insulin resistance and hyperinsulinemia play a key role (16). They increase systemic metabolic disorders and activate the sympathetic nerve system (autonomic dysfunction); then activate renin-angiotensin-aldosterone system; prompt oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress; and impair calcium homeostasis. These effects result in cardiac fibrosis, hypertrophy, cardiomyocyte death, dysfunction of the coronary microcirculation, and eventually HF. Furthermore, these pathophysiological changes in cardiomyocytes underlie the risk factors for insulin resistance and hyperinsulinemia, which can result in a potentially vicious cycle, which prompt the following question: does HF cause DM?
Does HF cause DM?
Data from the Bezafibrate Infarction Prevention trial, which included >2,600 patients who had myocardial infarction without diabetes at baseline, showed that those with New York Heart Association (NYHA) class III symptoms had a significant increase in subsequent DM with a 1.7-fold (95% CI: 1.1–2.6) increase, but those with NYHA class II did not (HR =1.0; 95% CI: 0.8–1.3) over the 7.7 years of follow-up (17). The main underlying mechanism is insulin resistance (18). A new HF medication, sacubitril/valsartan, has greater glycemic control than angiotensin converting enzyme inhibitor (19).
Who should be screened and how?
Thus far, the brief history of diabetic cardiomyopathy and HF risk and vicious cycle has been reviewed. The next logical questions are who to be screened and how. Our meta-analysis identified four key independent risk factors: history of CAD, age (every 5 years), poor glycemic control markers (i.e., insulin use, high fasting glucose, and HbA1c of >9.0%), and HTN (20). As routine clinical practice, the screening should comprise history (above four criteria) and physical examination (including blood pressure assessment), blood tests (for glycemic control), and electrocardiography (ECG) (for CAD and LVH).
The screening should include medical encounter for medical history (age, CAD, etc.), symptoms (dyspnea, etc.), and physical examination, including blood pressure measurement, followed by blood tests for glycemic control and ECG for CAD or LVH. As screening is mostly performed in asymptomatic subjects with risks (stage A HF), cardiac imaging plays an important role. The majority of data in the literature are from echocardiography, which will be discussed in the following section.
Echocardiographic assessment of diabetic cardiomyopathy
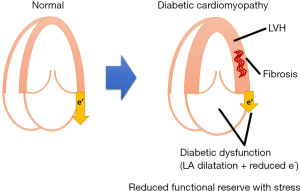
LVH and myocardial fibrosis (morphological alteration assessed by echocardiography): the main finding of diabetic cardiomyopathy was LVH in the initial autopsy study (1), where histopathologic study revealed diffuse fibrosis and myocyte hypertrophy (Figure 2). Using echocardiography, numerous studies, including population-based studies (21-28) (Table 2), confirmed LVH in a population with diabetes and in prediabetes stage, for example, impaired glucose tolerance (IGT) (21,22,25,27). In the Strong Heart Study population, Ilercil et al. identified independent associations of IGT with higher left ventricular (LV) relative wall thicknesses and LV mass/height2.7 (25). Collectively, DM, even from the prediabetes stage, is independently associated with LVH.
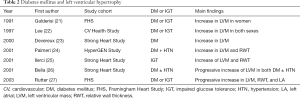
Full table
The main underlying histopathologic alteration in diabetic cardiomyopathy is fibrosis (29). Integrated backscatter (IB) is a technique used to assess echocardiographic tissue characteristic using the reflectivity of tissue to ultrasound, developed in the 1980s (30,31). From the early 1990s, the IB has been performed in patients with diabetes (32,33) (Table 3). Their primary findings are greater IB (indicating greater fibrosis) and lower cyclic variation of IB in the diabetic myocardium.
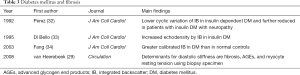
Full table
Diastolic dysfunction: the primary and early functional consequence of these morphological alterations is LV diastolic dysfunction (16) (Table 4). Early reports showed lower transmitral E/A ratios among patients with DM (35-37), followed by lower mitral annular early diastolic velocity assessment (38), greater E/E' (41,43), and larger left atrial (LA) volume (46). Subclinical LV diastolic dysfunction is associated with poor diabetic control, advancing age, HTN, metformin treatment (39), and cardiac autonomic neuropathy (44). Diastolic dysfunction in diabetes indicates worse prognosis (42,43,46). E/E'sept of >15 in patients with DM is associated with subsequent HF and increased mortality independent of HTN, CAD, or other echocardiographic parameters (43).
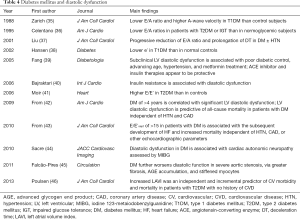
Full table
Valvular heart diseases: there have been significant increases in the prevalence of DM and valvular heart diseases (47,48). DM accelerates progression of calcific aortic stenosis (49) and worsens LV diastolic function via altering myocardial structure and cardiomyocyte stiffness (45). A small study showed the possibility that DM may accelerate aortic stenosis progression with enhanced inflammation (50). In patients with mitral regurgitation (MR), prevalence of MR is as high as 32%, mainly due to both mitral valve and myocardial abnormalities (48). As a result, patients with even mild MR have a 3.3-fold increased risk of all-cause mortality, whereas those with moderate-to-severe MR have a 5.1-fold increased risk (48).
Myocardial reserve assessed by stress echocardiography
Pharmacological and exercise stress echocardiography have provided intriguing insights into diabetic cardiomyopathy (Table 5). Patients with DM have impaired longitudinal functional reserve in both systolic (51) and diastolic functions (34). Furthermore, LV circumferential contractility and longitudinal systolic function reserves are correlated with stroke index reserve during low-dose dobutamine stress (53). Exercise stress echocardiography confirmed diminished systolic and diastolic functional reserve in this population (54,55).

Full table
Although the negative predictive value of exercise stress echocardiography for exclusion of underlying myocardial ischemia in type 2 diabetes mellitus (T2DM) is reduced compared with the nondiabetic population (57), recent data provided a favorable finding, where dual-imaging dipyridamole stress echocardiography [conventional wall motion analysis and Doppler-derived coronary flow velocity reserve (CFVR) of the left anterior descending coronary artery] has independent prognostic value in patients with DM (56). This result is in line with previous findings on reduced myocardial flow (38) and flow reserve (41) in this population. Among patients with diabetes, hypoglycemia can be fatal. As acute hypoglycemia decreases myocardial blood flow reserve in both healthy humans and patients with T1DM (58), this could be one of the underlying mechanisms.
Myocardial strain
Myocardial strain analysis brought further intriguing insights (Table 6). Others and we reported that patients with DM have impaired systolic and diastolic longitudinal strains irrespective of concomitant HTN in tissue Doppler-derived and speckle-tracking strain methods (41,52,61,62). This deteriorated longitudinal function has been demonstrated using more largely available technique, like tissue Doppler and atrioventricular plane displacement by M-mode (64). Studies using magnetic resonance imaging (MRI) tagging strain reported similar findings (59,60). Not only peak systolic circumferential and longitudinal strains and principal 3-D shortening strain were smaller in the T2DM group but also peak diastolic rate of relaxation of these strains were lower in patients with DM with normal EF (P<0.001 for each) (59). Furthermore, patients with DM have paradoxical increase in myocardial torsion (60). They had a higher resting HR (77.0±12.4 vs. 59.0±5.6 beats/min; P<0.01), higher maximal torsion by 23% (3.5±0.9 vs. 2.7±0.4 °/cm; P<0.01), and higher maximal systolic torsion rate by 25% (0.013±0.003 vs. 0.010±0.002 °/cm/s; P<0.01). Torsion did not significantly change with chronotropic stimulation by atropine injection (P=0.30).

Full table
Recently, Leung et al. reported possible reversibility in systolic and diastolic functions among patients with DM treated intensively (63). Subjects with T2DM and poor glycemic control received optimization of treatment for blood glucose, blood pressure, and cholesterol to recommended targets for 12 months. The improvement in HbA1c, from 10.3%±2.4% to 8.3%±2.0%, was associated with significant relative improvement in global longitudinal strain (GLS) of 21% and septal e' of 24%. A progressively greater improvement was observed in GLS as patients achieved a lower final HbA1c. Patients achieving an HbA1c of <7.0% had the highest improvement, whereas patients whose HbA1c worsened experienced a decline in GLS. Patients who improved their HbA1c by ≥1.0% had a significantly higher relative improvement in e' than those who did not (32% vs. 8%; P=0.003). These encouraging results lead us to the final section on treatment options.
Treatment options
Glycemic control agents: although good glycemic control is the main goal in the management of DM, a meta-analysis demonstrated that intensive glycemic control does not prevent HF (65). Metformin has been previously contraindicated, but recent data showed beneficial protective effects of the drug (66,67). A meta-analysis demonstrated that metformin was associated with reduced mortality [adjusted risk ratio 0.80 (0.74–0.87); P<0.001] (68). No increased risks were observed in those with reduced left ventricular ejection fraction (LVEF) or chronic kidney disease (CKD). Of note, no increased risk of lactic acidosis was found. Insulin has neutral effect on CVD outcome (69). Peroxisome proliferator-activated receptor (PPAR)-γ agonists increase the cardiovascular (CV) risk (70-74). Among the newer generation agents, including dipeptidyl peptidase-4 inhibitor (DPP-4i), glucagon-like peptide-1 receptor agonist (GLP-1 RA), and sodium-glucose cotransporter 2 inhibitors (SGLT2i), to date, DPP-4 inhibitor has demonstrated CV safety only without clear CV benefit (75). CV benefit has been shown in some of SGLT2i, such as empagliflozin (76) and canagliflozin (77), and GLP-1 RA, such as liraglutide (78) and semaglutide (79). Results from ongoing clinical trials are also underway: CREDENCE (ClinicalTrials.gov identifier: NCT02065791) and DECLARE-TIMI58 (ClinicalTrials.gov identifier: NCT01730534).
Of note, several cardioprotective agents have demonstrated their effectiveness among patients with DM. The effectiveness of angiotensin-converting enzyme (ACE) inhibitor in a population with DM has been confirmed in a meta-analysis (80). A discrepancy has been found among beta-blockers. Favorable effect of carvedilol has been confirmed in this population (81), but metoprolol has a neutral effect so far (82). Accordingly, ACCF/AHA Guidelines on HF recommend ACE inhibitors and beta-blockers for patients with DM, even without HF symptoms (stage A) (11).
Conclusions
Currently, diabetic cardiomyopathy is recognized as a real disease entity and is not a myth anymore. The underlying pathophysiological mechanism includes insulin resistance and cascades of metabolic disorders and autonomic disturbances, which result in clinical phenotype of LVH, diastolic dysfunction, fibrosis, and limited cardiac functional reserve. Recent reports suggest novel links between DM and valvular heart diseases, where mild degree of valvular disease may be associated with adverse prognosis. Myocardial strain analysis also demonstrated subclinical systolic dysfunction on top of diastolic dysfunction and possible reversibility. Collectively, careful cardiac imaging assessments including advanced techniques are crucial in the clinical management of patients with DM. Novel therapeutic agents, such as SGLT2i and DPP4i, are expected to improve these functions as a part of mechanisms explaining favorable prognostic effect on this population.
Acknowledgements
K Negishi is supported by an award from the Select Foundation, which had no role in the preparation of this manuscript.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Rubler S, Dlugash J, Yuceoglu YZ, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30:595-602. [Crossref] [PubMed]
- Ernande L, Derumeaux G.. Diabetic cardiomyopathy: myth or reality? Arch Cardiovasc Dis 2012;105:218-25. [Crossref] [PubMed]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035-8. [Crossref] [PubMed]
- Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29-34. [Crossref] [PubMed]
- Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol 2000;35:1628-37. [Crossref] [PubMed]
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. [Crossref] [PubMed]
- Nichols GA, Hillier TA, Erbey JR, et al. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care 2001;24:1614-9. [Crossref] [PubMed]
- Davis BR, Piller LB, Cutler JA, et al. Role of diuretics in the prevention of heart failure: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation 2006;113:2201-10. [Crossref] [PubMed]
- Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27:699-703. [Crossref] [PubMed]
- Carr AA, Kowey PR, Devereux RB, et al. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol 2005;96:1530-6. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [Crossref] [PubMed]
- Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J Diabetes Investig 2014;5:623-34. [Crossref] [PubMed]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213-23. [Crossref] [PubMed]
- Miki T, Yuda S, Kouzu H, et al. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 2013;18:149-66. [Crossref] [PubMed]
- Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 2015;36:1718-27, 1727a-c.
- Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144-53. [Crossref] [PubMed]
- Tenenbaum A, Motro M, Fisman EZ, et al. Functional class in patients with heart failure is associated with the development of diabetes. Am J Med 2003;114:271-5. [Crossref] [PubMed]
- Witteles RM, Tang WH, Jamali AH, et al. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol 2004;44:78-81. [Crossref] [PubMed]
- Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2017;5:333-40. [Crossref] [PubMed]
- Wang Y, Negishi T, Negishi K, et al. Prediction of heart failure in patients with type 2 diabetes mellitus- a systematic review and meta-analysis. Diabetes Res Clin Pract 2015;108:55-66. [Crossref] [PubMed]
- Galderisi M, Anderson KM, Wilson PW, et al. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 1991;68:85-9. [Crossref] [PubMed]
- Lee M, Gardin JM, Lynch JC, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J 1997;133:36-43. [Crossref] [PubMed]
- Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000;101:2271-6. [Crossref] [PubMed]
- Palmieri V, Bella JN, Arnett DK, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation 2001;103:102-7. [Crossref] [PubMed]
- Ilercil A, Devereux RB, Roman MJ, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am Heart J 2001;141:992-8. [Crossref] [PubMed]
- Bella JN, Devereux RB, Roman MJ, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study). Am J Cardiol 2001;87:1260-5. [Crossref] [PubMed]
- Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation 2003;107:448-54. [Crossref] [PubMed]
- Galderisi M.. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol 2006;48:1548-51. [Crossref] [PubMed]
- van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43-51. [Crossref] [PubMed]
- Mimbs JW, O'Donnell M, Bauwens D, et al. The dependence of ultrasonic attenuation and backscatter on collagen content in dog and rabbit hearts. Circ Res 1980;47:49-58. [Crossref] [PubMed]
- Shapiro LM, Moore RB, Logan-Sinclair RB, et al. Relation of regional echo amplitude to left ventricular function and the electrocardiogram in left ventricular hypertrophy. Br Heart J 1984;52:99-105. [Crossref] [PubMed]
- Pérez JE, McGill JB, Santiago JV, et al. Abnormal myocardial acoustic properties in diabetic patients and their correlation with the severity of disease. J Am Coll Cardiol 1992;19:1154-62. [Crossref] [PubMed]
- Di Bello V, Talarico L, Picano E, et al. Increased echodensity of myocardial wall in the diabetic heart: an ultrasound tissue characterization study. J Am Coll Cardiol 1995;25:1408-15. [Crossref] [PubMed]
- Fang ZY, Yuda S, Anderson V, et al. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol 2003;41:611-7. [Crossref] [PubMed]
- Zarich SW, Arbuckle BE, Cohen LR, et al. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J Am Coll Cardiol 1988;12:114-20. [Crossref] [PubMed]
- Celentano A, Vaccaro O, Tammaro P, et al. Early abnormalities of cardiac function in non-insulin-dependent diabetes mellitus and impaired glucose tolerance. Am J Cardiol 1995;76:1173-6. [Crossref] [PubMed]
- Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol 2001;37:1943-9. [Crossref] [PubMed]
- Hansen A, Johansson BL, Wahren J, et al. C-peptide exerts beneficial effects on myocardial blood flow and function in patients with type 1 diabetes. Diabetes 2002;51:3077-82. [Crossref] [PubMed]
- Fang ZY, Schull-Meade R, Downey M, et al. Determinants of subclinical diabetic heart disease. Diabetologia 2005;48:394-402. [Crossref] [PubMed]
- Bajraktari G, Koltai MS, Ademaj F, et al. Relationship between insulin resistance and left ventricular diastolic dysfunction in patients with impaired glucose tolerance and type 2 diabetes. Int J Cardiol 2006;110:206-11. [Crossref] [PubMed]
- Moir S, Hanekom L, Fang ZY, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart 2006;92:1414-9. [Crossref] [PubMed]
- From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol 2009;103:1463-6. [Crossref] [PubMed]
- From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol 2010;55:300-5. [Crossref] [PubMed]
- Sacre JW, Franjic B, Jellis CL, et al. Association of cardiac autonomic neuropathy with subclinical myocardial dysfunction in type 2 diabetes. JACC Cardiovasc Imaging 2010;3:1207-15. [Crossref] [PubMed]
- Falcão-Pires I, Hamdani N, Borbély A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 2011;124:1151-9. [Crossref] [PubMed]
- Poulsen MK, Dahl JS, Henriksen JE, et al. Left atrial volume index: relation to long-term clinical outcome in type 2 diabetes. J Am Coll Cardiol 2013;62:2416-21. [Crossref] [PubMed]
- Movahed MR, Hashemzadeh M, Jamal MM. Significant increase in the prevalence of non-rheumatic aortic valve disease in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2007;115:105-7. [Crossref] [PubMed]
- Rossi A, Zoppini G, Benfari G, et al. Mitral Regurgitation and Increased Risk of All-Cause and Cardiovascular Mortality in Patients with Type 2 Diabetes. Am J Med 2017;130:70-6.e1. [Crossref] [PubMed]
- Kamalesh M, Ng C, El Masry H, et al. Does diabetes accelerate progression of calcific aortic stenosis? Eur J Echocardiogr 2009;10:723-5. [Crossref] [PubMed]
- Natorska J, Wypasek E, Grudzien G, et al. Does diabetes accelerate the progression of aortic stenosis through enhanced inflammatory response within aortic valves? Inflammation 2012;35:834-40. [Crossref] [PubMed]
- Vinereanu D, Nicolaides E, Tweddel AC, et al. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond) 2003;105:591-9. [Crossref] [PubMed]
- Fang ZY, Najos-Valencia O, Leano R, et al. Patients with early diabetic heart disease demonstrate a normal myocardial response to dobutamine. J Am Coll Cardiol 2003;42:446-53. [Crossref] [PubMed]
- Palmieri V, Capaldo B, Russo C, et al. Left ventricular chamber and myocardial systolic function reserve in patients with type 1 diabetes mellitus: insight from traditional and Doppler tissue imaging echocardiography. J Am Soc Echocardiogr 2006;19:848-56. [Crossref] [PubMed]
- Ha JW, Lee HC, Kang ES, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart 2007;93:1571-6. [Crossref] [PubMed]
- Mizuno R, Fujimoto S, Saito Y, et al. Exercise-induced delayed onset of left ventricular early relaxation in association with coronary microcirculatory dysfunction in patients with diabetes mellitus. J Card Fail 2010;16:211-7. [Crossref] [PubMed]
- Cortigiani L, Gherardi S, Faggioni M, et al. Dual-Imaging Stress Echocardiography for Prognostic Assessment of High-Risk Asymptomatic Patients with Diabetes Mellitus. J Am Soc Echocardiogr 2017;30:149-58. [Crossref] [PubMed]
- Cortigiani L, Bigi R, Sicari R, et al. Prognostic value of pharmacological stress echocardiography in diabetic and nondiabetic patients with known or suspected coronary artery disease. J Am Coll Cardiol 2006;47:605-10. [Crossref] [PubMed]
- Rana O, Byrne CD, Kerr D, et al. Acute hypoglycemia decreases myocardial blood flow reserve in patients with type 1 diabetes mellitus and in healthy humans. Circulation 2011;124:1548-56. [Crossref] [PubMed]
- Fonseca CG, Dissanayake AM, Doughty RN, et al. Three-dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol 2004;94:1391-5. [Crossref] [PubMed]
- Chung J, Abraszewski P, Yu X, et al. Paradoxical increase in ventricular torsion and systolic torsion rate in type I diabetic patients under tight glycemic control. J Am Coll Cardiol 2006;47:384-90. [Crossref] [PubMed]
- Ng AC, Delgado V, Bertini M, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol 2009;104:1398-401. [Crossref] [PubMed]
- Yang H, Wang Y, Negishi K, et al. Pathophysiological effects of different risk factors for heart failure. Open Heart 2016;3:e000339. [Crossref] [PubMed]
- Leung M, Wong VW, Hudson M, et al. Impact of Improved Glycemic Control on Cardiac Function in Type 2 Diabetes Mellitus. Circ Cardiovasc Imaging 2016;9:e003643. [Crossref] [PubMed]
- Ballo P, Cameli M, Mondillo S, et al. Impact of diabetes and hypertension on left ventricular longitudinal systolic function. Diabetes Res Clin Pract 2010;90:209-15. [Crossref] [PubMed]
- Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011;162:938-48.e2. [Crossref] [PubMed]
- Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 2005;111:583-90. [Crossref] [PubMed]
- Aguilar D, Chan W, Bozkurt B, et al. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail 2011;4:53-8. [Crossref] [PubMed]
- Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013;6:395-402. [Crossref] [PubMed]
- ORIGIN Trial Investigators, Gerstein HC, Bosch J, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319-28. [Crossref] [PubMed]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71. [Crossref] [PubMed]
- Mahaffey KW, Hafley G, Dickerson S, et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J 2013;166:240-9.e1. [Crossref] [PubMed]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89. [Crossref] [PubMed]
- Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med 2016;374:1321-31. [Crossref] [PubMed]
- Lincoff AM, Tardif JC, Schwartz GG, et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA 2014;311:1515-25. [Crossref] [PubMed]
- Monami M, Dicembrini I, Mannucci E.. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2014;24:689-97. [Crossref] [PubMed]
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. [Crossref] [PubMed]
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017;377:644-57. [Crossref] [PubMed]
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22. [Crossref] [PubMed]
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016;375:1834-44. [Crossref] [PubMed]
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003;41:1529-38. [Crossref] [PubMed]
- Wedel H, Demets D, Deedwania P, et al. Challenges of subgroup analyses in multinational clinical trials: experiences from the MERIT-HF trial. Am Heart J 2001;142:502-11. [Crossref] [PubMed]
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-7. [Crossref] [PubMed]


