Management of vascular complications of extra-corporeal membrane oxygenation
Introduction
The first description of successful extra-corporeal membrane oxygenation (ECMO) was reported by Hill and colleagues in 1972 (1). ECMO was introduced to provide post-operative circulatory support for patients undergoing cardiac surgery. Technological advances and the H1N1 pandemic in 2009 opened the door for wider application of this lifesaving technique (2-4). Currently, it is an established treatment option for patients requiring temporary cardiopulmonary support (5-7). A circulatory bypass is established using large bore vascular cannulas to cycle the blood through an extracorporeal device which performs the physiological role of the heart and lungs. The extracorporeal device consists of a membrane oxygenator and a blood pump. The membrane oxygenator provides an efficient surface for gaseous exchange. The blood pump provides hemodynamic support. A low priming volume and low resistance to blood flow with a thrombosis-resistant coating minimize thrombotic events (8-10). The duration of support is typically 1–3 weeks (11). Patients are maintained on systemic anticoagulation while on ECMO. Vascular complications, both bleeding and thrombosis remain the leading causes of morbidity and mortality in patients treated with ECMO (12,13). This review will focus on the management of vascular complications of ECMO.
ECMO indications and configurations
ECMO circulation can have two different configurations: veno arterial (VA) and veno-venous (VV). VA ECMO is designed to remove blood from the venous system, perform the gas exchange and return the blood back to the body through the arterial system. In addition, hemodynamic support is provided using a blood pump. VA ECMO design is utilized in patients with cardiopulmonary failure. In contrast, VV ECMO is designed to perform the gas exchange and return the blood back to the venous system without hemodynamic pump support. VV ECMO design is utilized in patients with isolated pulmonary failure with preserved cardiac function (Figure 1) (14).
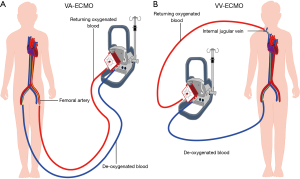
Classical indication for VA ECMO includes any potentially reversible cardiogenic shock as a bridge to recovery. Post cardiac surgery, graft failure after cardiac transplant, decompensated cardiomyopathy, myocarditis, acute coronary syndrome and drug induced cardiac decompensation are conditions where a bridge to recovery using VA ECMO is utilized. VA ECMO is also utilized as a bridge to decision making when the suitability or need for long term support is unclear (15). In other situations such as massive pulmonary embolism and pre-cardiac transplant status, VA ECMO serves the role of a bridge to destination treatment (16,17). The extracorporeal right to left shunt unloads the failing heart by reducing the preload while providing a stable blood flow to sustain end organ perfusion.
There are two main cannulation strategies for patients requiring VA ECMO: central and peripheral cannulation. Central cannulation is created in the operating room. The cannulas are larger and provide better flows than the peripheral VA ECMO. The outflow cannula is placed in the right atrium and the inflow cannula is placed in the proximal ascending aorta. Central VA ECMO provides better oxygenation for the coronary arteries and arch vessels due to the antegrade flow configuration compared to peripheral VA ECMO (18). Neurological complications due to perfusion abnormalities are lower with central VA ECMO. The vascular complications associated with central VA ECMO are related to cannula dislodgement and mediastinal hematoma which are managed surgically. Risk of infection is higher with central VA ECMO. Peripheral VA ECMO cannulation is most commonly performed in an angiography suite. In emergent situations, cannulation may need to be performed in less controlled environments such as the emergency room, intensive care unit and even at bedside. Cannulation of the vessels is performed using ultrasound guided percutaneous access or following a surgical cut-down. The most commonly used vessels are the common femoral artery (CFA) and the common femoral vein. The size of the arterial cannula varies from 16 to 21 French. The size of the venous cannula varies from 20 to 24 French. A distal limb perfusion line is required to prevent limb ischemia (19,20) (Figure 2). Perfusion abnormalities resulting from retrograde flow of oxygenated blood from the femoral artery creates a watershed phenomenon (16,21) compromising oxygenation of the aortic arch branches and coronary arteries. Monitoring the upper body perfusion using a radial access is mandatory during peripheral VA ECMO (22). Utilization of the subclavian artery and internal jugular vein for peripheral VA ECMO reduces the risk of upper body perfusion abnormality. Cannulation of the subclavian artery requires surgical cut down and has not been widely adopted (23).

Indications for VV ECMO include adult respiratory distress syndrome, pneumonia, trauma and post-lung transplantation as a bridge to recovery (24). The extracorporeal life support organization (ELSO) recommends initiation of VV ECMO in patients with Horowitz index of less than 80 (22). VV ECMO is achieved by peripheral cannulation of the femoral vein as outflow and jugular vein as inflow (Figure 1). Recently, the use of VV ECMO in non-intubated patients “Awake ECMO” has gained attention in patients awaiting lung transplants as a viable bridge to transplantation strategy (25). The introduction of a single upper body venous access using the bicaval dual lumen catheter reduces the risk of bleeding and enables mobility (26). Correct positioning of the cannula is essential for avoiding recirculation (27) (Figure 3).
Vascular complications related to ECMO
Although ECMO is a lifesaving treatment modality, patients requiring this type of support are subject to multiple complications. Major complications of ECMO include vascular, neurological, renal, hemorrhagic complications and infection. Overall survival (patient weaned off ECMO) has been reported between 20% and 65% (28). Vascular complications are the major cause of mortality in ECMO patients. A recent study showed that the survival to discharge rates for patients on ECMO was 18% and 49% in patients with and without vascular complications (29). Limb ischemia is the most common acute and late vascular complication and has been reported in 10% to 70% of patients undergoing VA ECMO (30-32). Other vascular complications include dissection, pseudoaneurysm formation, groin infection and retroperitoneal bleeding.
Limb ischemia
The combination of femoral arterial cannulation with large bore cannulas and the hemodynamic instability during peripheral VA ECMO places the limb at risk for thromboembolic complications. The risks are greater with larger cannulas (>20 Fr), in women, in young patients, and in the presence of peripheral arterial disease (19,20). The CFA is smaller in women. The CFA increases in size with age and thus younger patients are at greater risk for ischemic injury (30). The presence of peripheral vascular disease further compromises the blood flow to the lower extremity resulting in higher incidence of thromboembolic occlusion. Difficult bedside cannulation has also been identified as an additional risk factor (33). Clinical presentation varies in severity from pain, pallor, poikilothermia, motor or sensory deficit to gangrene. Doppler ultrasound and near infra-red spectroscopy (NIRS) are non-invasive tools to evaluate for vascular insufficiency. Baseline NIRS (SpO2) of greater than 40 and a distal perfusion pressure of 50 mmHg is required to prevent limb ischemia (34,35). Placement of a distal perfusion catheter (DPC) has shown to provide the required limb perfusion and reduce the incidence of limb ischemia (30,32,33) (Figure 2).
A 4 or 5 French antegrade DPC placed in the proximal superficial femoral artery with ultrasound guidance and attached to the side port of the arterial cannula provides limb perfusion. Part of the returning arterial blood from the cannula is directed to the DPC. Systemic anticoagulation also helps prevent limb ischemia. The ideal time to place a DPC is at the time of initial placement of the ECMO cannula in the CFA (19,20). Placement of a distal venous drainage catheter attached to the side port of the venous cannula has also been described to enhance the venous drainage and prevent venous thrombosis and venous ischemia (36,37). Monitoring with NIRS/Doppler ultrasound allows detection of ischemia early to institute corrective measures. Reperfusion compartment syndrome clinically presents with tense calf, pain, paresthesia, paralysis should prompt early decompression with fasciotomies. In patients requiring fasciotomies, adjustment to anticoagulation to prevent bleeding complications may be required.
Vascular injury
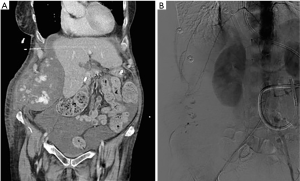
Vascular injury can lead to dissection, pseudoaneurysm and retroperitoneal bleeding. Injury usually occurs during initial placement or at time or removal of arterial cannulas. These complications occur in 7–14% of patients (38) and are compounded by coagulative abnormalities. Pseudoaneurysm presents with a painful pulsatile swelling at the access site and is confirmed with Doppler ultrasound. Pseudoaneurysms with a narrow neck can be managed with ultrasound guided thrombin injection. Large pseudoaneurysms and those with a wider neck or those associated with an arteriovenous communication require surgical intervention. Most of the arterial dissections are asymptomatic. When arterial occlusion results from dissection, differentiation from thromboembolic occlusion is imperative. Management of symptomatic dissection includes placement of a stent and relocating the arterial cannula to a different site. Due to the systemic anticoagulation, these patients are prone to develop large internal hematoma with minor vascular injuries (Figure 4). Such vascular injuries can be suspected with hemodynamic changes or decrease in hematocrit and can be confirmed on cross sectional imaging. The patients are managed with correction of anticoagulation and transfusion. Endovascular embolization is required if conservative measures do not work. Rarely open surgical management is warranted.
Infection
Infection rates at groin cannulation site have been reported from 7% to 20% (19,20,32). Malnourishment and obesity increase the risk of groin infection. Cellulitis, drainage around the cannula, induration indicate superficial infection. Systemic signs including fever, hypotension, leukocytosis and bacteremia portend development of systemic sepsis. Strict aseptic technique during placement of the cannula and subsequent handling are very critical to prevent infections. Once an infection is detected, antibiotic treatment and need for surgical intervention should be determined and initiated.
Summary
The utilization of ECMO for critically ill patients with cardiac and/or respiratory failure is increasing. Although these procedures can be lifesaving, vascular complications play a major role in successful outcomes. Understanding the different types of ECMO circulation and associated potential vascular complications as outlined in this review is vital for successful management of these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Hui DSC, Lee N, Chan PKS. A clinical approach to the threat of emerging influenza viruses in the Asia-Pacific region. Respirology 2017;22:1300-12. [Crossref] [PubMed]
- Zarychanski R, Stuart TL, Kumar A, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 2010;182:257-64. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Schuerer DJ, Kolovos NS, Boyd KV, et al. Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 2008;134:179-84. [Crossref] [PubMed]
- Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care 2000;4:156-68. [Crossref] [PubMed]
- Weber TR. Extending the uses of ECMO. Chest 2004;126:9-10. [Crossref] [PubMed]
- Muller T, Philipp A, Luchner A, et al. A new miniaturized system for extracorporeal membrane oxygenation in adult respiratory failure. Crit Care 2009;13:R205. [Crossref] [PubMed]
- Khoshbin E, Roberts N, Harvey C, et al. Poly-methyl pentene oxygenators have improved gas exchange capability and reduced transfusion requirements in adult extracorporeal membrane oxygenation. ASAIO J 2005;51:281-7. [Crossref] [PubMed]
- Reul HM, Akdis M. Blood pumps for circulatory support. Perfusion 2000;15:295-311. [Crossref] [PubMed]
- Marasco SF, Lukas G, McDonald M, et al. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008;17 Suppl 4:S41-7. [Crossref] [PubMed]
- Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care 2013;17:R73. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015;29:90-101. [Crossref] [PubMed]
- Haneya A, Philipp A, Puehler T, et al. Ventricular assist device implantation in patients on percutaneous extracorporeal life support without switching to conventional cardiopulmonary bypass system. Eur J Cardiothorac Surg 2012;41:1366-70. [Crossref] [PubMed]
- Napp LC, Kuhn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Belohlavek J, Rohn V, Jansa P, et al. Veno-arterial ECMO in severe acute right ventricular failure with pulmonary obstructive hemodynamic pattern. J Invasive Cardiol 2010;22:365-9. [PubMed]
- Jayaraman AL, Cormican D, Shah P, et al. Cannulation strategies in adult veno-arterial and veno-venous extracorporeal membrane oxygenation: Techniques, limitations, and special considerations. Ann Card Anaesth 2017;20:S11-8. [Crossref] [PubMed]
- Lamb KM, DiMuzio PJ, Johnson A, et al. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J Vasc Surg 2017;65:1074-9. [Crossref] [PubMed]
- Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg 2013;28:190-2. [Crossref] [PubMed]
- Hoeper MM, Tudorache I, Kuhn C, et al. Extracorporeal membrane oxygenation watershed. Circulation 2014;130:864-5. [Crossref] [PubMed]
- Napp LC, Brehm M, Kuhn C, et al. Heart against veno-arterial ECMO: Competition visualized. Int J Cardiol 2015;187:164-5. [Crossref] [PubMed]
- Javidfar J, Brodie D, Costa J, et al. Subclavian artery cannulation for venoarterial extracorporeal membrane oxygenation. ASAIO J 2012;58:494-8. [Crossref] [PubMed]
- Hoeper MM, Wiesner O, Hadem J, et al. Extracorporeal membrane oxygenation instead of invasive mechanical ventilation in patients with acute respiratory distress syndrome. Intensive Care Med 2013;39:2056-7. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Bermudez CA, Rocha RV, Sappington PL, et al. Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg 2010;90:991-5. [Crossref] [PubMed]
- Bonacchi M, Harmelin G, Peris A, et al. A novel strategy to improve systemic oxygenation in venovenous extracorporeal membrane oxygenation: the “chi-configuration”. J Thorac Cardiovasc Surg 2011;142:1197-204. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Tanaka D, Hirose H, Cavarocchi N, et al. The Impact of Vascular Complications on Survival of Patients on Venoarterial Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2016;101:1729-34. [Crossref] [PubMed]
- Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during femoral cannulation for cardiopulmonary support. J Vasc Surg 2010;52:850-3. [Crossref] [PubMed]
- Aziz F, Brehm CE, El-Banyosy A, et al. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg 2014;28:178-83. [Crossref] [PubMed]
- Bisdas T, Beutel G, Warnecke G, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg 2011;92:626-31. [Crossref] [PubMed]
- Zimpfer D, Heinisch B, Czerny M, et al. Late vascular complications after extracorporeal membrane oxygenation support. Ann Thorac Surg 2006;81:892-5. [Crossref] [PubMed]
- Huang SC, Yu HY, Ko WJ, et al. Pressure criterion for placement of distal perfusion catheter to prevent limb ischemia during adult extracorporeal life support. J Thorac Cardiovasc Surg 2004;128:776-7. [Crossref] [PubMed]
- Steffen RJ, Sale S, Anandamurthy B, et al. Using near-infrared spectroscopy to monitor lower extremities in patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg 2014;98:1853-4. [Crossref] [PubMed]
- Russo CF, Cannata A, Vitali E, et al. Prevention of limb ischemia and edema during peripheral venoarterial extracorporeal membrane oxygenation in adults. J Card Surg 2009;24:185-7. [Crossref] [PubMed]
- Le Guyader A, Lacroix P, Ferrat P, et al. Venous leg congestion treated with distal venous drainage during peripheral extracorporeal membrane oxygenation. Artif Organs 2006;30:633-5. [Crossref] [PubMed]
- Roussel A, Al-Attar N, Alkhoder S, et al. Outcomes of percutaneous femoral cannulation for venoarterial extracorporeal membrane oxygenation support. Eur Heart J Acute Cardiovasc Care 2012;1:111-4. [Crossref] [PubMed]


