Diabetes and antiplatelet therapy: from bench to bedside
Introduction
Diabetes mellitus (DM) is a metabolic condition currently affecting over 150 million people worldwide, largely due to type 2 DM, representing a global pandemic (1). The prevalence of DM is in continuous increase and the global prevalence of DM among adults is estimated to be 7.7% (439 million individuals) by 2030 (2,3). In the United States, the costs related to DM have been projected at $172 billion in 2007, while they are anticipated to rise to $192 billion by 2020 (4).
Dysfunction in insulin secretion and insulin action at the target tissues leads to chronic hyperglycemia and multiple comorbidities affecting the cardiovascular and many other systems (5). Cardiovascular disease, predominantly coronary artery disease (CAD) deriving from accelerated atherosclerosis, is the primary cause of morbidity and mortality in DM patients (6). A Danish population-based study on 3.3 million people showed that 5 years cardiovascular mortality in DM patients without a history of CAD was the same as non-DM patients with a history of myocardial infarction (MI) (6). In addition, DM is the main cause of accelerated atherogenesis and atherothrombosis detected in this patient cohort (7). Furthermore, the negative prognosis associated with DM status is maintained across the acute coronary syndrome (ACS) spectrum. This includes unstable angina, non-ST-segment elevation MI (NSTEMI) (8), ST-segment elevation MI (STEMI) treated medically (9), and ACS undergoing percutaneous coronary intervention (PCI) (10,11).
These findings highlight the significance of antiplatelet therapy for secondary prevention of atherothrombotic recurrences in patients with DM. This is challenged by the fact that DM patients have heightened platelet reactivity, which may warrant the need for more potent therapies to reduce their ischemic risk (12). This article reviews currently available antiplatelet agents and provides an update on the advances and drawbacks of these agents used for secondary prevention in patients with DM experiencing an ACS or undergoing PCI.
Diabetes & atherothrombosis
Multiple mechanisms contribute to the accelerated atherogenesis and atherothrombotic risk in DM patients (12). Patients with DM have endothelial dysfunction and metabolic disorders, including obesity, hyperglycemia, dyslipidemia, insulin resistance, and increased oxidative stress (12). The prothrombotic and inflammatory state in patients with DM contribute to the accelerated progression of atherosclerosis as well as to the exacerbated risk of thrombosis in response to rupture of an atherosclerotic plaque (5,13,14). Platelets of DM patients are hyperreactive with intensified adhesion, activation, and aggregation (15,16). The prothrombotic status is the result of this increased platelet reactivity. However, other factors also contribute to this condition including: high levels of procoagulant agents (e.g., fibrinogen, tissue factor, von Willebrand factor, factor VII, platelet factor 4); impaired endogenous fibrinolysis (e.g., elevated levels of plasminogen activator inhibitor-1); and decreased concentrations of endogenous anticoagulants (e.g., antithrombin III and protein C) (Figure 1) (12).
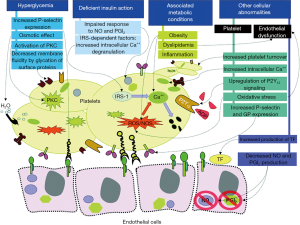
Hyperglycemia and platelet reactivity
Several mechanisms can contribute to increased platelet reactivity in patients with DM (12). Hyperglycemia is one of the most characteristic features for DM that may prompt the expression of the adhesion molecule P-selectin on the cell surface (17,18). Correlation between P-selectin expression and levels of fasting glucose has also been reported (19).
Several mechanisms by which hyperglycemia may increase platelet reactivity have been proposed. These include glycation of platelet surface proteins resulting in a decrease in membrane fluidity and an increase in platelet adhesion (20,21); osmotic effect of glucose (22), and activation of protein kinase C, a mediator of platelet activation (23). Early intensive glucose control with insulin in patients with ACS presenting with hyperglycemia was found to decrease platelet reactivity (24). The Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction (DIGAMI) trial showed intensive glucose-lowering treatment to reduce mortality in DM patients presenting with an ACS after 3.4 years of follow-up (25).
Insulin resistance and platelet reactivity
Type 2 DM is produced by the combination of resistance to insulin in the target tissues and an inadequate compensatory secretion of insulin leading to insulin deficiency (26). Reduced insulin secretion and tissue response is one of the factors contributing to platelet dysfunction in DM (27). Platelets express both insulin-like growth factor-1 receptors (IGF-1) and insulin receptors (28,29). When insulin binds to platelets, it increases the surface expression of adenylate cyclase-linked prostacyclin receptor (30). However, the expression of the insulin receptor has a tendency to be relatively low because the majority of its subunits heterodimerize with those of the IGF-1 receptor to conform an insulin/IGF-1 hybrid receptor, which avidly binds IGF-1 but not insulin (29). IGF-1 is present in the granules of platelets, and its receptor is expressed on the platelet surface, contributing to the amplification of platelet reactivity in DM patients leading to a prothrombotic status. Moreover, IGF-1 stimulates tyrosine phosphorylation of insulin receptor substrate (IRS)-1 and IRS-2 and their subsequent binding to the p85 subunit of the phosphoinositide-3 kinase, leading to phosphorylation of protein kinase B, involving several cellular responses to insulin, IGF-1, and modulation of platelet reactivity (31).
Insulin resistance induces a rise in intracellular calcium, promoting enhanced platelet degranulation and aggregation (31). However, the exact mechanism by which calcium concentration is increased is not yet well-established (32,33). It has been suggested that IRS-1 mediates inhibition of Ca2+ mobilization by insulin via the inhibitory G-protein Gi (34). Type 2 DM patients who are carriers of the C allele of the rs956115 marker of the IRS-1 gene have a hyperreactive platelet phenotype and increased risk of ischemic events (35). IRS independent pathways are also involved in platelet hyperreactivity caused by insulin resistance, including impairment in platelet sensitivity to nitric oxide (NO) and prostacyclin (36,37). These mediators are released by the endothelium and reduce platelet activation. Consequently, impaired response to NO and prostacyclin is associated with increased platelet reactivity. The thiazolidinedione rosiglitazone is an insulin sensitizer associated with improved sensitivity to NO in platelets and reduced P-selectin expression in DM and non-DM patients, respectively (38,39). Clinical trials have also shown a benefit of insulin-sensitizer therapy over insulin-providing therapy on atherosclerosis progression and cardiovascular outcomes in DM patients (40,41). However, in DM patients treated with aspirin and clopidogrel therapy, the adjunctive use of pioglitazone was not associated with enhanced inhibition of platelet P2Y12 mediated signaling (42).
Concomitant metabolic conditions and platelet reactivity
Multiple metabolic conditions like obesity, dyslipidemia, and systemic inflammation are commonly associated with type 2 DM. However, other factors present in obese individuals can contribute to platelet dysfunction such as high mean platelet volume and elevated platelet count (43), increase systolic calcium concentration (44), increase oxidative stress and high blood leptin concentrations (45) causing an enhanced effect for the platelet activation and adhesion. Abnormalities of the lipid profile are commonly found in DM subjects. Hypertriglyceridemia can lead to a higher platelet activation (46). It has been suggested that this effect is mediated by the apolipoprotein E content of the very-low-density lipoprotein particles, which are rich in triglycerides (47,48). Systemic inflammation may also contribute to increased platelet reactivity (49). Expression of platelet FcgammaRIIA receptor, which is heightened in DM patients and involved in platelet activation, has shown to be modulated by inflammation (50,51).
Other disorders associated with platelet reactivity
Dysregulation of calcium metabolism is a disorder present in DM platelets and the mechanisms involved in calcium signaling abnormalities are not yet fully explained. Some of the suggested factors that may play a role are an augmented oxidative stress (52), insulin resistance, change in the activity of calcium ATPases and a disproportionate influx of calcium through the sodium/calcium exchanger (53). Oxidative stress is a disorder commonly associated with DM, especially the overproduction of reactive oxygen and nitrogen species with a reduction of platelet antioxidant levels that lead to impaired platelet function (54,55). The surge in reactive oxygen species enhances the production of advanced glycation end products, which has been proposed to play a role in atherosclerosis through the activation of the receptor for advanced glycation end products (56). In addition, the oxidative stress present in DM patients enhances endothelial dysfunction decreasing the production of NO and prostacyclin leading an increase in platelet reactivity and enhancing the prothrombotic state through increased production of tissue factor (Figure 1) (57,58).
Patients with DM have accelerated platelet turnover, which is represented by a greater number of reticulated platelets (59). These platelets are larger and more sensitive resulting in enhanced platelet reactivity with a reduced response to antiplatelet therapies like aspirin and clopidogrel (60). In addition to this mechanism, there is upregulation of platelet ADP P2Y12 receptor signaling that suppresses cAMP leading to a reduced response to insulin, increasing the adhesion, aggregation and pro-coagulative state (61,62).
Oral antiplatelet therapies and diabetes mellitus
Aspirin and a P2Y12 receptor antagonist, frequently used in combination and known as dual antiplatelet therapy (DAPT), represents the standard of care oral antiplatelet treatment for the prevention of recurrent ischemic events in patients with CAD, particularly those presenting with an ACS and undergoing PCI (63,64). In the section below, the efficacy and safety of these drugs, particularly for secondary prevention in DM patients, are described. The role of oral antiplatelet therapy (i.e., aspirin) for primary prevention in DM patients goes beyond the scope of this manuscript and described in details elsewhere (65).
Aspirin
Aspirin inhibits cyclooxygenase 1 (COX-1) activity of platelet prostaglandin-endoperoxide synthase 1 by selectively and irreversibly acetylating the hydroxyl group of a serine residue at position 529 (Ser529). In turn, this prevents the conversion of arachidonic acid into multiple downstream bioactive prostanoids including thromboxane A2 (TXA2), prostaglandins and prostacyclin (66).
Therefore, aspirin diminishes platelet activation mediated by the G protein-coupled thromboxane and prostaglandin endoperoxide (TP) receptor pathway leading to changes in platelet shape and enhancement of recruitment and aggregation of platelets. Due to the fact that platelets are enucleated this effect is irreversible and therefore they are unable to resynthesize COX-1 (67). Aspirin blocks TXA2 induced platelet activation and prostacyclin-induced counter-regulation, which plays a central role in gastric mucosal protection. Prostacyclins derive from both COX-1 and more deeply from COX-2 especially with low-dose aspirin. This suggests that significant prostacyclin synthesis is preserved with low-dose aspirin therapy. With increasing doses of aspirin, COX-2 inhibition increases in a dose-dependent manner, which results in reduced prostacyclin generation resulting in a dose-dependent increase of bleeding, but with comparable reduction in ischemic risk (66).
The advantages of aspirin are well demonstrated in the collaborative meta-analysis (Antithrombotic Trialists’ Collaboration) which comprised 43,000 person-years from 16 secondary prevention trials. In secondary prevention, aspirin significantly reduced major CV events (rate ratio 0.80, 95% CI, 0.73–0.88), including a 31% risk reduction (rate ratio 0.69, 95% CI, 0.60–0.80) in nonfatal MI (68-70). Aspirin was also effective in reducing the risk of ischemic stroke (rate ratio 0.78, 95% CI, 0.61–0.99), although it increased the risk of hemorrhagic stroke (rate ratio 1.67, 95% CI, 0.97–2.90) (67). Aspirin also increased major gastrointestinal and other extracranial bleeds [0·10% vs. 0·07% per year; RR 1.54 (1.30–1.82), P<0.0001]. However, the ischemic recurrences remains elevated (~20%) despite aspirin use. The persistence of this high percentage can be attributed to the increased platelet reactivity, enhanced platelet turnover, more reticular platelets, increased TXA2 synthesis or early recovery of COX-1 activity characteristics present in patients with DM (12).
A number of studies have shown that twice-daily administration of aspirin enhance platelet inhibition to a greater extent than once-daily administration and a dose-dependent suppression of serum TXB2 levels in patients with DM (71,72) However, the clinical implications of a twice-daily dosing regimen are largely unknown and are being evaluated in the ongoing ANDAMAND (Aspirin Twice a Day in Patients With Diabetes and Acute Coronary Syndrome) trial (clinicaltrials.gov identifier NCT02520921). Higher aspirin doses (325 vs. 81 mg) have also been associated with greater platelet inhibition and decreased rates of aspirin resistance in DM patients (73). To a certain extent this was assessed in the CURRENT-OASIS 7 study (Clopidogrel and aspirin optimal dose usage to reduce recurrent events-7th organization to assess strategies in ischemic syndromes) which did not show significant differences in efficacy between high (300 to 325 mg daily) and low (75 to 100 mg daily) dose aspirin and observing a trend towards higher rates of gastrointestinal bleeds (74). Ultimately, studies are currently being conducted to identify aspirin formulations that are associated with more favorable pharmacodynamics effects (75,76).
P2Y12 receptor antagonists
The platelet ADP signaling pathways play a key role in platelet activation and aggregation via the P2Y1 and P2Y12 receptors (77,78). While both receptors are required for aggregation, ADP-stimulated effects on platelets are mediated primarily by Gi-coupled P2Y12 receptor activation, leading to persistent platelet aggregation and stabilization of the platelet aggregate, whereas P2Y1 is responsible for an initial weak, transient phase of platelet aggregation (79). The importance of this signaling pathway is underscored by the clinical benefit associated with the use of oral P2Y12 inhibitors. There are two main classes of oral P2Y12 inhibitors: thienopyridines (ticlopidine, clopidogrel, and prasugrel) and non-thienopyridine (ticagrelor) agents. Thienopyridines are nondirect (requiring metabolism to generate an active metabolite), orally administered, irreversible P2Y12 receptor inhibitors. Ticlopidine was a first-generation thienopyridine which is mostly no longer used due to safety concerns, in particular, bone marrow suppression (80). Clopidogrel and prasugrel are second and third generation thienopyridines, respectively (67). Ticagrelor, on the contrary, is a non-thienopyridine appertaining to a drug class called cyclopentyltriazolopyrimidine (CPTP) which is a direct (no metabolism required), orally administered agent, with reversible binding properties to the P2Y12 receptor (67). With the exception of clopidogrel, which was studies in a head-to-head comparison with aspirin in the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) trial (81), all other reported studies of P2Y12 inhibitors in CAD patients were tested on a background of aspirin therapy.
Clopidogrel
Clopidogrel is a prodrug which, following intestinal absorption, is largely (~85%) hydrolyzed by human carboxylesterase-1 into an inactive acid metabolite, while the remaining 15% is metabolized by two-step oxidation processes using several hepatic cytochrome P-450 (CYP) isoenzymes to generate an active metabolite (67). The CYP2C19 isoenzyme is involved in both metabolic steps. Therefore, genetic polymorphisms associated with reduced enzymatic activity or drugs that interfere with enzyme activity (e.g., proton pump inhibitors), may impair clopidogrel’s effects leading to potential complications (82-84).
In the CAPRIE study (n=19,185), composed of patients with recent MI, recent ischemic stroke or established peripheral artery disease (PAD), clopidogrel (75 mg daily) led to a significant 8.7% relative risk reduction of the composite endpoint (ischemic stroke, MI, or vascular death) compared with aspirin (325 mg daily) (Table 1) (81). In this study, DM patients (n=3,866) had more ischemic event rates than non-DM patients in both the clopidogrel and aspirin groups (15.6% vs. 11.8% in clopidogrel group; 17.7% vs. 12.7% in aspirin group). In this subgroup, clopidogrel was associated with a 21% risk reduction of the primary endpoint compared with aspirin (Table 1). Importantly, compared with aspirin, clopidogrel significantly reduced (37% relative risk reduction) any bleeding event, mainly driven by gastrointestinal bleeding.

Full table
The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) study was the first to assess the benefits of DAPT consisting of aspirin and clopidogrel compared with aspirin monotherapy in patients (n=12,562) with non-ST-segment elevation ACS (NSTE-ACS) (85,86). After a mean of 9-month treatment, DAPT was associated with a 20% relative risk reduction in the combined endpoint of cardiovascular death, nonfatal MI, and stroke in comparison with aspirin alone at the expense of 38% increased risk of major bleeding. However, life-threatening bleedings were not increased. DM was present in 22.6% of the study population (n=2,840) and there were consistent benefits of DAPT on the primary outcome with non-DM patients (Table 1) (85,86). DAPT with a aspirin and clopidogrel was subsequently tested in other studies of high-risk patients, including CREDO, CLARITY, and COMMIT, consistently showing superiority in terms of reducing ischemic events compared with aspirin monotherapy, albeit at the expense of bleeding complications (87-89). However, the net benefit was in favor of the use of DAPT making this a standard of care approach in high-risk patients with CAD, such as those with ACS and undergoing PCI. The outcomes were consistent according to DM status. On the contrary, in a study of lower-risk patients called the CHARISMA trial, there was no ischemic benefit of DAPT, which was also associated with harm over aspirin therapy due to the increased risk of bleeding (90).
While the role of maintaining DAPT for up to 12 months in patients undergoing PCI was supported by clinical trial evidence, there has been much debate surrounding the need to prolong DAPT beyond 12 months (91). This was largely attributed to stent thrombosis, particularly with first generation drug-eluting stents, that were still occurring after 12 months (92,93). The benefit of 12 vs. 30 months of DAPT, most consisting of aspirin and clopidogrel, was tested in the DAPT trials (94). The study included patients (n=9,961) who had not experienced ischemic and bleeding events at 1 year after PCI. Prolonging DAPT by 18 months significantly reduced the composite outcome of all-cause mortality, MI, or stroke (4.3% vs. 5.9%; HR, 0.71; 95% CI, 0.59–0.85; P<0.001) and in-stent thrombosis (0.4% vs. 1.4%; HR, 0.29; 95% CI, 0.17–0.48; P<0.001). Notably, 55% of the ischemic benefit from DAPT prolongation derived from MI not related to the stent. However, this occurred at the expense of increased moderate or severe bleeding (2.5% vs. 1.6%; HR, 1.61; 95% CI, 1.21–2.16; P=0.001) (94). In patients with DM (n=3,037), there was a trend in reduced stent thrombosis (0.5% vs. 1.1%; HR, 0.47; 95% CI, 0.21–1.05; P=0.06) with prolonged DAPT, but this strategy was not effective in reducing the composite outcome (6.6% vs. 7.0%; HR, 0.92; 95% CI, 0.71–1.20; P=0.55) (95). Nevertheless, in a post hoc analysis to develop a risk prediction model, known as the DAPT score, DM was a predictor of stent thrombosis or MI (94,96).
Although in the studies mentioned above DAPT with aspirin and clopidogrel showed consistent benefits irrespective of DM status, DM patients had more ischemic recurrences than non-DM patients. These have led to investigations to define why DM patients continued to have high atherothrombotic events despite clopidogrel therapy. Pharmacodynamic investigations have consistently shown patients with DM to persists with high levels of platelet reactivity compared with non-DM patients (97-100). There are multiple mechanisms contributing to poor clopidogrel-induced antiplatelet effects. However, this appears to be most significantly due to impaired generation of the active metabolite (97). These observations have led to studies aimed to optimize platelet inhibition in DM patients. In the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus) study, while doubling the clopidogrel maintenance dose (MD) enhanced platelet inhibition in DM patients, 60% of patients persisted as poor responders, highlighting the need for alternate therapies (101).
Prasugrel
Similarly to clopidogrel, prasugrel also needs to be converted into an active metabolite through an oxidation process by hepatic CYP isoenzymes to exert its effects of irreversible inhibition of the P2Y12 receptor (67). However, prasugrel has more effective bioactivation leading to >5-fold higher active metabolite levels compared with clopidogrel leading to greater platelet inhibition and less interindividual response variability making it an attractive agent in DM patients (102). The OPTIMUS-3 study showed that standard- dose prasugrel [60 mg of loading dose (LD) plus 10 mg of maintenance dose (MD)] achieved enhanced platelet inhibition than double-dose clopidogrel (600 mg of LD plus 150 mg of MD) in DM patients (103). Nevertheless, patients with DM have reduced generation of active metabolites and platelet inhibition in comparison with non-DM patients even with prasugrel therapy (104).
Nevertheless, its ischemic benefits are supported by the results of the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) study. This trial included ACS patients undergoing PCI (n=13,608) (105). At 15-months, prasugrel significantly reduced (19% relative risk reduction) ischemic events (cardiovascular death, non-fatal MI or non-fatal stroke). Notably, prasugrel was associated with a 52% relative risk reduction of stent thrombosis. However, this occurred at the expense of a 32% risk increase of TIMI major bleeding, including a significant increase in fatal and life-threatening bleeding, than clopidogrel (105). Subgroup analysis showed a neutral effect of prasugrel in certain patient cohorts, including those ≥75 year of age or weighing <60 kg, because the ischemic benefit was outweighed by the higher risk of bleeding complications compared with clopidogrel in these subjects (105). Importantly, in patients with a previous history of stroke or transient ischemic attack (TIA), there was harm, including more ischemic and bleeding events (including intracranial hemorrhage) with the use of prasugrel (105).
In a prespecified subgroup analysis, a greater magnitude of reduction in ischemic events was observed in DM (n=3,146) compared with non-DM patients (12.2% vs. 17.0%; HR, 0.70; P<0.001 in DM patients, and 9.2% vs. 10.6%; HR, 0.86; P=0.02 in non-DM patients, P for interaction=0.09) (Table 1) (106). There were no significant differences in TIMI major bleeding in DM patients (106). The magnitude of the net clinical benefit with prasugrel was enhanced in DM patients (14.6% vs. 19.2%; HR, 0.74; P=0.001) compared with non-DM patients (11.5% vs. 12.3%; HR, 0.92; P=0.16, P for interaction=0.05) (88). DM patients ≥75 year of age also attained a significant 36% risk reduction in the primary endpoint with prasugrel use (106).
The TRITON TIMI 38 trial specifically studied ACS patients undergoing PCI and did not include medically managed patients, which were selectively studied in the TRILOGY ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) trial (107). In this study, although the bleeding end points of non-CABG-related severe or life-threatening events occurred with similar frequency among patients under the age of 75 years in the two study groups (clopidogrel vs. prasugrel), prasugrel use was not associated with a reduction in the primary endpoint (a composite of CV death, nonfatal MI and nonfatal stroke) among NSTE-ACS patients who were medically managed (n=7,243) (107). Event rates were higher in DM patients irrespective of treatment group (17.8% in prasugrel group vs. 20.4% in clopidogrel group) than non-DM patients (11.5% in prasugrel group vs. 13.2% in clopidogrel group) and the clinical effect of prasugrel was consistent irrespective of DM status (P for interaction=0.71) (107).
In light of these observations, prasugrel (60 mg LD and 10 mg MD) is only indicated for ACS patients after PCI; it not recommended for those undergoing non-invasive treatment or being medically managed (108,109). Prasugrel is contraindicated in patients with a prior history of stroke/TIA, at high risk of bleeding or hypersensitivity. In elderly patients (≥75 years old), prasugrel at standard dosing can be cautiously used only in high-risk patients with a history of DM or prior MI according to the United States Food and Drug Administration (FDA); according to the European Medical Agency (EMEA), prasugrel should be used at a reduced dose (5 mg MD) in the elderly. In low weight patients (<60 kg), both the FDA and EMEA recommend that prasugrel be used at a reduced dose (5 mg MD) (108,109).
Ticagrelor
Ticagrelor is a CPTP that reversibly blocks ADP-induced conformational changes of the P2Y12 receptor and downstream signal transduction through the G-coupled protein (67). It is an active drug (i.e., not requiring an activation process) that undergoes a rapid absorption accompanied by degradation to its main active metabolite (AR-C124910XX) and inactive metabolite AR-C133913XX via CYP3A4 and 3A5 (67). Ticagrelor also inhibits adenosine uptake through the adenosine transporter ENT1 (type 1 equilibrated nucleoside transporter) providing adenosine-related effects (110). In addition to favoring platelet inhibition, the adenosine-related effects can contribute the anti-inflammatory effects, coronary vasodilation, inhibition of atrioventricular conduction and the sensation of dyspnea associated with ticagrelor therapy (110). Ticagrelor provides faster and more potent antiplatelet effects compared with clopidogrel, including in patients with DM (111-113). Furthermore, ticagrelor achieves comparable or higher platelet inhibition than prasugrel, a finding that was also shown in DM patients (114-116).
In the PLATO (Platelet Inhibition and Patient Outcomes) trial, compared with clopidogrel, ticagrelor significantly reduced the rate of the primary end point (composite of death from vascular causes, MI or stroke at 12 months; 10.2% vs. 12.3%; HR, 0.84; P=0.0001) in ACS patients (n=18,624) treated either medically or undergoing revascularization (117). The trial also showed a reduction in the rate of cardiovascular death (4.0% vs. 5.1%; HR, 50.79; P=0.001) and the occurrence of definitive/probable stent thrombosis (2.2% vs. 2.9%; HR, 0.75; P=0.02) in the subgroup of patients undergoing PCI. Ticagrelor was not associated with an increase in protocol-defined major bleeding (11.6% vs. 11.2%; HR =0.03), although a higher rate of major bleeding not related to CABG occurred (4.5% vs. 3.8%; HR, 1.19; P=0.030) (117). DM patients (n=4,662) had significantly higher risks of both ischemic and bleeding events than non-DM patients (n=13,951) (Table 1) (118). The rates of primary endpoint and all-cause death among DM patients were respectively 66% and 84% higher compared with non-DM patients (118). DM patients had a 41% higher risk of major bleeding than non-DM patients (118). The benefits of ticagrelor in DM patients were consistent with the overall cohort and there was no heterogeneity in relation to DM status (14.1% vs. 16.2%; HR, 0.88; 95% CI, 0.76–1.03 in DM patients; 8.4% vs. 10.2%; HR, 0.83; 95% CI, 0.74–0.93 in non-DM patients; P for interaction=0.49) (118). Notably, compared with clopidogrel, ticagrelor reduced the primary endpoint by 20%, all-cause death by 22%, and stent thrombosis by 48% in patients with poor metabolic control (HbA1c ≥6%) without increasing major bleeding (8.4% vs. 8.2%).
The PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) trial evaluated the clinical efficacy of prolonged ticagrelor use in patients >50 years old with a history of MI 1–3 years prior to enrollment. Patients also needed to have at least 1 additional risk factor, among which DM requiring pharmacological treatment was included (119). Patients were randomized to either one of 2 doses of ticagrelor (90 or 60 mg b.i.d.) or placebo in addition to aspirin. At 3 years, a similar reduction of the primary efficacy endpoint (CV death, MI, and stroke) was observed in patients treated with either dose of ticagrelor (7.85% in 90 mg b.i.d. vs. 7.77% in 60 mg b.i.d. vs. 9.04% in placebo; P=0.004 for 90 mg b.i.d. vs. placebo and P=0.008 for 60 mg b.i.d. vs. placebo) (119). However, both ticagrelor doses were associated with increased major bleeding (but not fatal or intracranial bleeding) compared with placebo, albeit a numerically lower rate was shown with the 60 mg dose than with the 90 mg dose (2.60% in 90 mg b.i.d. vs. 2.30% in 60 mg b.i.d. vs. 1.06% in placebo; P<0.001 both for 90 mg b.i.d. vs. placebo and 60 mg b.i.d. vs. placebo) (119). A dose-dependent increase in dyspnea resulted in a higher treatment discontinuation rate in ticagrelor users (6.5% in 90 mg b.i.d. vs. 4.55% in 60 mg b.i.d. vs. 0.79% in placebo; P<0.001 for each ticagrelor dose vs. placebo (119). There was a higher rate of the primary efficacy endpoint in DM patients (n=6,806, 32%) (10.1% in 90 mg b.i.d. vs. 10.0% in 60 mg b.i.d. vs. 11.60% in placebo) compared to non-DM patients (6.77% in 90 mg b.i.d. vs. 6.68% in 60 mg b.i.d. vs. 7.83% in placebo) (120). Nevertheless, the relative risk reduction in MACE with ticagrelor was consistent for the pooled doses versus placebo in DM patients (HR, 0.84; 95% CI, 0.72 to 0.99; P=0.035) and non-DM patients (HR, 0.84; 95% CI, 0.74 to 0.96; P=0.013; P interaction=0.99). Because DM patients have higher event rates, their absolute risk reduction was greater than in non-DM patients (1.5% vs. 1.1%, with corresponding 3-year number needed to treat of 67 vs. 91). Additionally, in DM patients, ticagrelor reduced cardiovascular death by 22% and coronary heart disease death by 34%. Similar to non-DM patients, there was increased TIMI major bleeding in DM patients (HR, 2.56; 95% CI, 1.52 to 4.33; P=0.0004) (119,120). A pharmacodynamic substudy of the trial showed consistently high level of platelet inhibition with ticagrelor irrespective of DM status, even in insulin-treated patients. Patients with diabetes did not have an increased incidence of high platelet reactivity in either ticagrelor group (121,122). Platelet reactivity was comparable in DM patients treated with ticagrelor 60 vs. 90 mg bid. Pharmacokinetics of ticagrelor were not affected by DM status (121,122). The ongoing THEMIS (Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study NCT01991795) study is currently testing the clinical impact of ticagrelor in patients with type 2 DM and CAD but without prior MI.
Based on these results, ticagrelor (180 mg LD and 90 mg b.i.d. MD) is recommended for the management of ACS patients irrespective of planned treatment approach (invasive or non-invasive). After 1 year from an ACS, ticagrelor is recommended for secondary prevention at a lower dosing regimen (60 mg b.i.d.). Ticagrelor is contraindicated in patients with a history of intracranial bleeding, active pathological bleeding, severe hepatic dysfunction or hypersensitivity to the drug (123,124).
New therapeutic agents
Although DAPT has significantly reduced the risk of ischemic events in high-risk CAD patients, recurrences still occur particularly in patients with DM. This has underscored the need to define alternative strategies or newer antiplatelet therapies to reduce the risk of ischemic recurrences. Cilostazol is a phosphodiesterase III inhibitor clinically approved for the reduction of claudication symptoms in patients with peripheral arterial disease (125). However, cilostazol also has antiplatelet properties and has been tested in adjunct to DAPT, also known as triple antiplatelet therapy. The benefits of adding cilostazol to aspirin and clopidogrel in reducing ischemic events has been demonstrated particularly in patients with DM (125,126). This is also supported by pharmacodynamic studies specifically conducted in DM patients (127,128). However, this approach is not largely utilized following the introduction of the newer generation P2Y12 receptor inhibitors. A number of other antiplatelet agents that inhibit other pathways of platelet activation have been under development, including thromboxane receptor inhibitors and thrombin receptor inhibitors, among others (129-131). However, of these only vorapaxar, a PAR-1 receptor inhibitor, has been approved for clinical use.
Vorapaxar
Vorapaxar is a PAR-1 inhibitor which blocks thrombin-mediated platelet activation without affecting fibrinogen cleavage in the coagulation cascade (67,132-134). A combination of thrombin and P2Y12 receptor inhibitors has a synergistic effect in the inhibition of thrombin-induced platelet activation. Vorapaxar was studied in 2 large-scale phase 3 clinical trials. In the TRACER (Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome) trial (n=12,944) conducted in NSTE-ACS patients, compared with placebo, vorapaxar (40 mg LD and 2.5 mg daily MD) utilized on top of standard DAPT, did not reduce the primary efficacy outcome (CV death, MI, stroke, recurrent ischemia with rehospitalization, or urgent coronary revascularization) at 2 years (135,136). Vorapaxar was associated with a significant increase in major bleeding and intracranial hemorrhage, and patients with previous stroke had a higher risk for intracranial hemorrhage (135).
The TRA 2P-TIMI 50 (Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis) study, including patients (n=26,449) with a history of atherosclerosis (defined as MI or ischemic stroke within the previous 2 weeks to 12 months or PAD), showed that, compared with placebo, vorapaxar (2.5 mg daily MD without LD) added on top of aspirin and/or a thienopyridine was associated with a significant 13% relative risk reduction of the primary endpoint (a composite of CV death, MI, stroke)after a median of 30 months’ follow-up (136). In a subgroup analysis of the TRA 2P-TIMI 50 study including 16,896 patients with a history of MI, 3,623 (21%) had DM (137). The occurrence of the primary endpoint was more frequent in DM patients than in non-DM patients (14.3% vs. 7.6%; HR, 1.47; P<0.001) (136). Vorapaxar reduced the primary endpoint in both DM- and non- DM patients (Table 1) but increased the incidences of moderate or severe bleeding in both DM patients (4.4% vs. 2.6%; HR, 1.60; 95% CI, 1.07–2.40; P=0.02), and non- DM patients (2.9% vs. 1.9%; HR, 1.56; 95% CI, 1.22–2.00; P<0.001) (P for interaction=0.93) (137). Despite the increased risk of bleeding, vorapaxar enhanced the net clinical benefit (combined outcome of CV death, MI, stroke, recurrent ischemia leading to revascularization, and moderate or severe bleeding) in DM patients (16.4% vs. 19.6%; HR, 0.79; 95% CI, 0.67–0.93; P=0.005), whereas the net clinical benefit of vorapaxar was not significant in non-DM patients (10.4% vs. 10.9%; HR, 0.95; 95% CI, 0.85–1.06; P=0.32) (137).
Future perspectives and conclusions
Patients with DM are at increased atherothrombotic risk and have elevated rates of ischemic recurrences. Abnormalities in platelet function profiles which characterize this patient population can contribute to these observations. While currently approved antiplatelet treatment strategies have proven successful in improving outcomes in ACS, DM patients continue to experience high rates of adverse outcomes. Even though the use of more potent antiplatelet therapies, as well as prolonging intensified therapy, reduces ischemic events, the increase in bleeding complications represents a major concern. Therefore, strategies aimed at reducing ischemic events while minimizing the risk of bleeding complications represent an area on ongoing research. Emerging strategies include dropping aspirin and maintaining therapy with a potent P2Y12 receptor inhibitor as well as the use of low-dose oral anticoagulant therapy in adjunct to aspirin (138). Ongoing trials are testing these strategies and will provide important understandings into optimizing outcomes in patients with DM.
Acknowledgements
None.
Footnote
Conflicts of Interest: DJ Angiolillo reports receiving payments as an individual for: (I) Consulting fee or honorarium from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; (II) Participation in review activities from CeloNova and St. Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions; in addition, Dr. Angiolillo is recipient of a funding from the Scott R. MacKenzie Foundation and the NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH/NHGRI U01 HG007269, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782-7. [Crossref] [PubMed]
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414-31. [Crossref] [PubMed]
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14. [Crossref] [PubMed]
- American Diabetes Association. National Diabetes Fact Sheet, Diabetes Statistics. Available online: http://www.diabetes.org/diabetes-statitics
- Lüscher TF, Creager MA, Beckman JA, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation 2003;108:1655-61. [Crossref] [PubMed]
- Schramm TK, Gislason GH, Kober L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945-54. [Crossref] [PubMed]
- Creager MA, Luscher TF, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy. Part I. Circulation 2003;108:1527-32. [Crossref] [PubMed]
- Ferreiro JL, Angiolillo DJ. Challenges and perspectives of antiplatelet therapy in patients with diabetes mellitus and coronary artery disease. Curr Pharm Des 2012;18:5273-93. [Crossref] [PubMed]
- Mak KH, Moliterno DJ, Granger CB, et al. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction: GUSTO-I Investigators: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Am Coll Cardiol 1997;30:171-9. [Crossref] [PubMed]
- Roffi M, Topol EJ. Percutaneous coronary intervention in diabetic patients with non-ST-segment elevation acute coronary syndromes. Eur Heart J 2004;25:190-8. [Crossref] [PubMed]
- Stuckey TD, Stone GW, Cox DA, et al. Impact of stenting and abciximab in patients with diabetes mellitus undergoing primary angioplasty in acute myocardial infarction (the CADILLAC trial). Am J Cardiol 2005;95:1-7. [Crossref] [PubMed]
- Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011;123:798-813. [Crossref] [PubMed]
- Biondi-Zoccai GG, Abbate A, Liuzzo G, et al. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003;41:1071-7. [Crossref] [PubMed]
- Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23:1979-89. [Crossref] [PubMed]
- Rollini F, Franchi F, Muñiz-Lozano A, et al. Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res 2013;6:329-45. [Crossref] [PubMed]
- Ferroni P, Basili S, Falco A, et al. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost 2004;2:1282-91. [Crossref] [PubMed]
- Schneider DJ. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care 2009;32:525-7. [Crossref] [PubMed]
- Vaidyula VR, Boden G, Rao AK. Platelet and monocyte activation by hyperglycemia and hyperinsulinemia in healthy subjects. Platelets 2006;17:577-85. [Crossref] [PubMed]
- Yngen M, Norhammar A, Hjemdahl P, et al. Effects of improved metabolic control on platelet reactivity in patients with type 2 diabetes mellitus following coronary angioplasty. Diab Vasc Dis Res 2006;3:52-6. [Crossref] [PubMed]
- Winocour PD, Watala C, Perry DW, et al. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost 1992;68:577-82. [Crossref] [PubMed]
- Watala C, Golan’ski J, Boncler MA, et al. Membrane lipid fluidity of blood platelets: a common denominator that underlies the opposing actions of various agents that affect platelet activation in whole blood. Platelets 1998;9:315-27. [Crossref] [PubMed]
- Keating FK, Sobel BE, Schneider DJ. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol 2003;92:1362-5. [Crossref] [PubMed]
- Assert R, Scherk G, Bumbure A, et al. Regulation of protein kinase C by short-term hyperglycaemia in human platelets in vivo and in vitro. Diabetologia 2001;44:188-95. [Crossref] [PubMed]
- Vivas D, García-Rubira JC, Bernardo E, et al. Effects of intensive glucose control on platelet reactivity in patients with acute coronary syndromes. Results of the CHIPS Study ("Control de Hiperglucemia y Actividad Plaquetaria en Pacientes con Síndrome Coronario Agudo"). Heart 2011;97:803-9. [Crossref] [PubMed]
- Malmberg K. Prospective randomized study of intensive insulin treatment on long-term survival after acute myocardial infarction in patients with diabetes mellitus: DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 1997;314:1512-5. [Crossref] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33:S62-9. [Crossref] [PubMed]
- Randriamboavonjy V, Fleming I. Insulin, insulin resistance, and platelet signaling in diabetes. Diabetes Care 2009;32:528-30. [Crossref] [PubMed]
- Hajek AS, Joist JH. Platelet insulin receptor. Methods Enzymol 1992;215:398-403. [Crossref] [PubMed]
- Hunter RW, Hers I. Insulin/IGF-1 hybrid receptor expression on human platelets: consequences for the effect of insulin on platelet function. J Thromb Haemost 2009;7:2123-30. [Crossref] [PubMed]
- Kahn NN. Insulin-induced expression of prostacyclin receptors on platelets is mediated through ADP-ribosylation of Gi alpha protein. Life Sci 1998;63:2031-8. [Crossref] [PubMed]
- Hers I. Insulin-like growth factor-1 potentiates platelet activation via the IRS/PI3Kalpha pathway. Blood 2007;110:4243-52. [Crossref] [PubMed]
- Ishida M, Ishida T, Ono N, et al. Effects of insulin on calcium metabolism and platelet aggregation. Hypertension 1996;28:209-12. [Crossref] [PubMed]
- Algenstaedt P, Antonetti DA, Yaffe MB, et al. Insulin receptor substrate proteins create a link between the tyrosine phosphorylation cascade and the Ca2-ATPases in muscle and heart. J Biol Chem 1997;272:23696-702. [Crossref] [PubMed]
- Ferreira IA, Eybrechts KL, Mocking AI, et al. IRS-1 mediates inhibition of Ca2+ mobilization by insulin via the inhibitory G-protein Gi. J Biol Chem 2004;279:3254-64. [Crossref] [PubMed]
- Angiolillo DJ, Bernardo E, Zanoni M, et al. Impact of insulin receptor substrate-1 genotypes on platelet reactivity and cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2011;58:30-9. [Crossref] [PubMed]
- Betteridge DJ, El Tahir KE, Reckless JP, et al. Platelets from diabetic subjects show diminished sensitivity to prostacyclin. Eur J Clin Invest 1982;12:395-8. [Crossref] [PubMed]
- Anfossi G, Mularoni EM, Burzacca S, et al. Platelet resistance to nitrates in obesity and obese NIDDM, and normal platelet sensitivity to both insulin and nitrates in lean NIDDM. Diabetes Care 1998;21:121-6. [Crossref] [PubMed]
- Randriamboavonjy V, Pistrosch F, Bölck B, et al. Platelet sarcoplasmic endoplasmic reticulum Ca2_-ATPase and mu-calpain activity are altered in type 2 diabetes mellitus and restored by rosiglitazone. Circulation 2008;117:52-60. [Crossref] [PubMed]
- Sidhu JS, Cowan D, Tooze JA, et al. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces circulating platelet activity in patients without diabetes mellitus who have coronary artery disease. Am Heart J 2004;147. [Crossref] [PubMed]
- Nissen SE, Nicholls SJ, Wolski K, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008;299:1561-73. [Crossref] [PubMed]
- McGuire DK, Newby LK, Bhapkar MV, et al. Association of diabetes mellitus and glycemic control strategies with clinical outcomes after acute coronary syndromes. Am Heart J 2004;147:246-52. [Crossref] [PubMed]
- Suryadevara S, Ueno M, Tello-Montoliu A, et al. Effects of pioglitazone on platelet P2Y12-mediated signaling in clopidogrel-treated patients with type 2 diabetes mellitus. Thromb Haemost 2012;108:930-6. [Crossref] [PubMed]
- Muscari A, De Pascalis S, Cenni A, et al. Determinants of mean platelet Volume (MPV) in an elderly population: relevance of body fat, blood glucose and ischaemic electrocardiographic changes. Thromb Haemost 2008;99:1079-84. [Crossref] [PubMed]
- Scherrer U, Nussberger J, Torriani S, et al. Effect of weight reduction in moderately overweight patients on recorded ambulatory blood pressure and free cytosolic platelet calcium. Circulation 1991;83:552-8. [Crossref] [PubMed]
- Sugiyama C, Ishizawa M, Kajita K, et al. Platelet aggregation in obese and diabetic subjects: association with leptin level. Platelets 2007;18:128-34. [Crossref] [PubMed]
- de Man FH, Nieuwland R, van der Laarse A, et al. Activated platelets in patients with severe hypertriglyceridemia: effects of triglyceride-lowering therapy. Atherosclerosis 2000;152:407-14. [Crossref] [PubMed]
- Pedreño J, Hurt-Camejo E, Wiklund O, et al. Platelet function in patients with familial hypertriglyceridemia: evidence that platelet reactivity is modulated by apolipoprotein E content of very-low density lipoprotein particles. Metabolism 2000;49:942-9. [Crossref] [PubMed]
- Olufadi R, Byrne CD. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemost Thromb 2006;35:281-91. [Crossref] [PubMed]
- Lim HS, Blann AD, Lip GY. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: relationships to cardiovascular disease and risk factor intervention. Circulation 2004;109:2524-8. [Crossref] [PubMed]
- Calverley DC, Hacker MR, Loda KA, et al. Increased platelet Fc receptor expression as a potential contributing cause of platelet hypersensitivity to collagen in diabetes mellitus. Br J Haematol 2003;121:139-42. [Crossref] [PubMed]
- Belostocki K, Pricop L, Redecha PB, et al. Infliximab treatment shifts the balance between stimulatory and inhibitory Fcgamma receptor type II isoforms on neutrophils in patients with rheumatoid arthritis. Arthritis Rheum 2008;58:384-8. [Crossref] [PubMed]
- Schaeffer G, Wascher TC, Kostner GM, et al. Alterations in platelet Ca2+ signaling in diabetic patients is due to increased formation of superoxide anions and reduced nitric oxide. Diabetologia 1999;42:167-76. [Crossref] [PubMed]
- Dean WL, Chen D, Brandt PC, et al. Regulation of platelet plasma membrane Ca2_-ATPase by cAMP-dependent and tyrosine phosphorylation. J Biol Chem 1997;272:15113-9. [Crossref] [PubMed]
- Freedman JE. Oxidative stress and platelets. Arterioscler Thromb Vasc Biol 2008;28:s11-6. [Crossref] [PubMed]
- Seghieri G, Di Simplicio P, Anichini R, et al. Platelet antioxidant enzymes in insulin-dependent diabetes mellitus. Clin Chim Acta 2001;309:19-23. [Crossref] [PubMed]
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787-90. [Crossref] [PubMed]
- Schäfer A, Bauersachs J. Endothelial dysfunction, impaired endogenous platelet inhibition and platelet activation in diabetes and atherosclerosis. Curr Vasc Pharmacol 2008;6:52-60. [Crossref] [PubMed]
- Kario K, Matsuo T, Kobayashi H, et al. Activation of tissue factor-induced coagulation and endothelial cell dysfunction in non-insulin-dependent diabetic patients with microalbuminuria. Arterioscler Thromb Vasc Biol 1995;15:1114-20. [Crossref] [PubMed]
- Guthikonda S, Lev EI, Patel R, et al. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J Thromb Haemost 2007;5:490-6. [Crossref] [PubMed]
- Guthikonda S, Alviar CL, Vaduganathan M, et al. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelets therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol 2008;52:743-49. [Crossref] [PubMed]
- Ferreira IA, Mocking AI, Feijge MA, et al. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2006;26:417-22. [Crossref] [PubMed]
- Ueno M, Ferreiro JL, Tomasello SD, et al. Functional profile of the platelet P2Y12 receptor signaling pathway in patients with type 2 diabetes mellitus and coronary artery disease. Thromb Haemost 2011;105:730-2. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082-115. [Crossref] [PubMed]
- Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60. [Crossref] [PubMed]
- Capodanno D, Angiolillo DJ. Aspirin for Primary Cardiovascular Risk Prevention and Beyond in Diabetes Mellitus. Circulation 2016;134:1579-94. [Crossref] [PubMed]
- Patrono C, García Rodríguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373-83. [Crossref] [PubMed]
- Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: From aspirin to the present day. Drugs 2012;72:2087-116. [Crossref] [PubMed]
- Baigent C, Blackwell L, Collins R, et al. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomized trials. Lancet 2009;373:1849-60. [Crossref] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [Crossref] [PubMed]
- Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81-106. [Crossref] [PubMed]
- Capodanno D, Patel A, Dharmashankar K, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv 2011;4:180-7. [Crossref] [PubMed]
- Spectre G, Arnetz L, Östenson CG, et al. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascularcomplications. Thromb Haemost 2011;106:491-9. [Crossref] [PubMed]
- DiChiara J, Blinden KP, Tantry US, et al. The effect of aspirin dosing on platelet function in diabetic and nondiabetic patients: an analysis from the aspirin-induced platelet effect (ASPECT) study. Diabetes 2007;56:3014-9. [Crossref] [PubMed]
- Mehta SR, Bassand JP, Chrolavicius S, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930-42. [Crossref] [PubMed]
- Bhatt DL, Grosser T, Dong JF, et al. Enteric Coating and Aspirin Nonresponsiveness in Patients With Type 2 Diabetes Mellitus. J Am Coll Cardiol 2017;69:603-12. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Chaudhary R, et al. Antiplatelet Effect Durability of a Novel, 24-Hour, Extended-Release Prescription Formulation of Acetylsalicylic Acid in Patients With Type 2 Diabetes Mellitus. Am J Cardiol 2016;118:1941-7. [Crossref] [PubMed]
- Storey RF, Newby LJ, Heptinstall S. Effects of P2Y (1) and P2Y (12) receptor antagonists on platelet aggregation induced by different agonists in human whole blood. Platelets 2001;12:443-7. [Crossref] [PubMed]
- Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost 2001;86:222-32. [Crossref] [PubMed]
- Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood 2001;98:3340-5. [Crossref] [PubMed]
- Bertrand ME, Rupprecht HJ, Urban P, et al. Double-blind study of the safety of clopidogrel with and without a loading dose in combi¬nation with aspirin compared with ticlopidine in combina¬tion with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation 2000;102:624-9. [Crossref] [PubMed]
- CAPRIE Steering Committee. A randomized, blinded, trial of Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE). Lancet 1996;348:1329-39. [Crossref] [PubMed]
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol 2007;49:1505-16. [Crossref] [PubMed]
- Marín F, González-Conejero R, Capranzano P, et al. Pharmacogenetics in cardiovascular antithrombotic therapy. J Am Coll Cardiol 2009;54:1041-57. [Crossref] [PubMed]
- Angiolillo DJ, Gibson CM, Cheng S, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 2011;89:65-74. [Crossref] [PubMed]
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary. the PCI-CURE study. Lancet 2001;358:527-33. [Crossref] [PubMed]
- Steinhubl SR, Berger PB, Mann JT III, et al. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411-20. [Crossref] [PubMed]
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocaridal Infarction with ST- Segment Elevation. N Engl J Med 2005;352:1179-89. [Crossref] [PubMed]
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo controlled trial. Lancet 2005;366:1607-21. [Crossref] [PubMed]
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706-17. [Crossref] [PubMed]
- Montalescot G, Brieger D, Dalby AJ, et al. Duration of dual antiplatelet therapy after coronary stenting: A review of the evidence. J Am Coll Cardiol 2015;66:832-47. [Crossref] [PubMed]
- Giustino G, Samantha S, Menran R, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systemic review and Meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298-310. [Crossref] [PubMed]
- Moon JY, Franchi F, Rollini F, et al. Evolution of coronary stent technology and implications for duration of dual antiplatelet therapy. Prog Cardiovasc Dis 2018;60:478-90. [Crossref] [PubMed]
- Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155-66. [Crossref] [PubMed]
- Meredith IT, Tanguay JF, Kereiakes DJ, et al. Diabetes Mellitus and Prevention of Late Myocardial Infarction After Coronary Stenting in the Randomized Dual Antiplatelet Therapy Study. Circulation 2016;133:1772-82. [Crossref] [PubMed]
- Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA 2016;315:1735-49. [Crossref] [PubMed]
- Angiolillo DJ, Jakubowski JA, Ferreiro JL, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 2014;64:1005-14. [Crossref] [PubMed]
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes 2005;54:2430-5. [Crossref] [PubMed]
- Angiolillo DJ, Bernardo E, Ramírez C, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol 2006;48:298-304. [Crossref] [PubMed]
- Angiolillo DJ, Bernardo E, Sabaté M, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 2007;50:1541-7. [Crossref] [PubMed]
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007;115:708-16. [Crossref] [PubMed]
- Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol 2010;50:126-42. [Crossref] [PubMed]
- Angiolillo DJ, Badimon JJ, Saucedo JF, et al. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: Results of the Optimizing anti- Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 Trial. Eur Heart J 2011;32:838-46. [Crossref] [PubMed]
- Erlinge D, Varenhorst C, Braun OO, et al. Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol 2008;52:1968-77. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis In Myocardial Infarction 38. Circulation 2008;118:1626-36. [Crossref] [PubMed]
- Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297-309. [Crossref] [PubMed]
- FDA. Prasugrel full prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022307s003lbl.pdf
- EMA. Assessment Report for Prasugrel. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/ human/000984/WC500021975.pdf
- Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: Evidence and potential clinical relevance. J Am Coll Cardiol 2014;63:2503-9. [Crossref] [PubMed]
- Sweeny JM, Angiolillo DJ, Franchi F, et al. Impact of Diabetes Mellitus on the Pharmacodynamic Effects of Ticagrelor Versus Clopidogrel in Troponin-Negative Acute Coronary Syndrome Patients Undergoing Ad Hoc Percutaneous Coronary Intervention. J Am Heart Assoc 2017.6. [PubMed]
- Angiolillo DJ, Franchi F, Waksman R, et al. Effects of Ticagrelor Versus Clopidogrel in Troponin-Negative Patients With Low-Risk ACS Undergoing Ad Hoc PCI. J Am Coll Cardiol 2016;67:603-13. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation 2010;121:1188-99. [Crossref] [PubMed]
- Laine M, Frère C, Toesca R, et al. Ticagrelor versus prasugrel in diabetic patients with an acute coronary syndrome. Thromb Haemost 2014;111:273-8. [Crossref] [PubMed]
- Alexopoulos D, Xanthopoulou I, Mavronasiou E, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with diabetes. Diabetes Care 2013;36:2211-6. [Crossref] [PubMed]
- Franchi F, Rollini F, Aggarwal N, et al. Pharmacodynamic Comparison of Prasugrel Versus Ticagrelor in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 Study. Circulation 2016;134:780-92. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2010;31:3006-16. [Crossref] [PubMed]
- Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791-800. [Crossref] [PubMed]
- Bhatt DL, Bonaca MP, Bansilal S, et al. Reduction in Ischemic Events With Ticagrelor in Diabetic Patients With Prior Myocardial Infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 2016;67:2732-40. [Crossref] [PubMed]
- Storey RF, Angiolillo DJ, Bonaca MP, et al. Platelet Inhibition With Ticagrelor 60 mg Versus 90 mg Twice Daily in the PEGASUS-TIMI 54 Trial. J Am Coll Cardiol 2016;67:1145-54. [Crossref] [PubMed]
- Thomas MR, Angiolillo DJ, Bonaca MP, et al. Consistent platelet inhibition with ticagrelor 60 mg twice-daily following myocardial infarction regardless of diabetes status. Thromb Haemost 2017;117:940-7. [Crossref] [PubMed]
- FDA. Ticagrelor full prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022433s017lbl.pdf
- EMA.Ticagrelor assessment report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/ human/001241/WC500100492.pdf
- Goto S. Cilostazol: Potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl 2005;6:3-11. [Crossref] [PubMed]
- Bundhun PK, Qin T, Chen MH. Comparing the effectiveness and safety between triple antiplatelet therapy and dual antiplatelet therapy in type 2 diabetes mellitus patients after coronary stents implantation: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2015;15:118. [Crossref] [PubMed]
- Angiolillo DJ, Capranzano P, Goto S, et al. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: Results of the OPTIMUS-2 study. Eur Heart J 2008;29:2202-11. [Crossref] [PubMed]
- Angiolillo DJ, Capranzano P, Ferreiro JL, et al. Impact of adjunctive cilostazol therapy on platelet function profiles in patients with and without diabetes mellitus on aspirin and clopidogrel therapy. Thromb Haemost 2011;106:253-62. [Crossref] [PubMed]
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12:30-47. [Crossref] [PubMed]
- Capra V, Bäck M, Angiolillo DJ, et al. Impact of vascular thromboxane prostanoid receptor activation on hemostasis, thrombosis, oxidative stress, and inflammation. J Thromb Haemost 2014;12:126-37. [Crossref] [PubMed]
- Moon JY, Franchi F, Rollini F, et al. Role for Thrombin Receptor Antagonism With Vorapaxar in Secondary Prevention of Atherothrombotic Events: From Bench to Bedside. J Cardiovasc Pharmacol Ther 2018;23:23-37. [Crossref] [PubMed]
- FDA. Vorapaxar full prescribing information. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204886s000lbl.pdf
- EMA. Vorapaxar assessment report. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/ human/002814/WC500183331.pdf
- Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J 2010;31:17-28. [Crossref] [PubMed]
- Tricoci P, Huang Z, Held C, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20-33. [Crossref] [PubMed]
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-13. [Crossref] [PubMed]
- Cavender MA, Scirica BM, Bonaca MP, et al. Vorapaxar in patients with diabetes mellitus and previous myocardial infarction: Findings from the thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events-TIMI 50 trial. Circulation 2015;131:1047-53. [Crossref] [PubMed]
- Moon JY, Franchi F, Rollini F, et al. The quest for safer antithrombotic treatment regimens in patients with coronary artery disease: new strategies and paradigm shifts. Expert Rev Hematol 2018;11:5-12. [Crossref] [PubMed]


