
Role of imaging in the diagnosis of vascular malformations vascular malformations
Introduction
Vascular malformations (VM) are vascular spaces lined by flat epithelium with an estimated prevalence of 4.5% in the population (1). It is usually present at birth, with enlargement proportional to child’s growth, but sudden expansion may be seen in infection, hormonal changes or trauma (2). They are the most common child hood soft tissue masses (3). VM occurs due to errors in morphogenesis however they exhibit normal cell turnover unlike vascular tumors (4-6). These lesions can be diffuse or focal, simple or combined based on subtype of vessels involved (7). Appropriate distinction between different VM lead to improved management of lesions, therefore VM can be classified by the type of vessel component (capillary, venous, lymphatic, arterial and hybrid subtype) and according to blood flow dynamics (high and slow flow lesions) (8). Moreover, this classification system combined with detailed physical exam and imaging can have up to 90% accuracy of diagnosis (9). Doppler ultrasonography (US) has been the first diagnostic modality utilized in the management of patients with VM, due to low-cost, non-ionizing technology and the ability to provide lesions’ flow characteristics (10), however magnetic resonance imaging (MRI) has proven advantageous to define extent of the lesions and guide appropriate treatment (11).
In this article, we review the classification of VM, their clinical presentation and role of imaging in their diagnosis.
Current classifications and terminology review
In the last decade, there has been increased knowledge to different aspect of vascular anomalies including histopathology, etiology, and their treatment. Due to its low incidence and heterogenous presentation of clinical and imaging findings, misdiagnosis is common and therefore, correct classification and terminology is paramount for proper clinical management.
The first classification system was proposed by Malan and Puglionisi in 1964 (12), categorizing vascular anomalies based on involvement of main trunks or peripheral vessels and then each group into three entities of arterial, venous, and other associated malformations.
In 1988, the Hamburg classification introduced embryological aspects, further subdividing them into either an extra truncular or truncular based on time of developmental arrest during embryonic life (13,14).
A binary approach initially proposed by Mulliken and Glowacki (15) with further revisions in 1996 based on pathologic features by the International Society for the Study of Vascular Anomalies (ISSVA), is now widely accepted by various subspecialists. Correlation of this system with clinical history and treatment options make this approach clinically useful.
The ISSVA divides vascular anomalies into two categories: (I) vascular neoplasm and (II) VM (Table 1) (16). While vascular neoplasms have increased rate of endothelial cell turnover, VM are structural abnormalities without cell turnover of capillary, venous, lymphatic and arterial vessels; which may have these elements alone or in combination (7). This classification also reflects hemodynamic aspect of VM and categorizes them into high flow and low flow lesions (17,18). High-flow lesions should have an arterial component and including arteriovenous malformations (AVM) and fistulas, while low-flow lesions are divided into venous malformation, lymphatic malformation and combined form. The combined form depending on the dominant component is divided further into venous dominant and lymphatic dominant.

Full table
Due to the vascular endothelial origin of these lesions, every organ in the body can be affected therefore a multi-specialty approach is required for the management of these conditions. Given the rarity of these malformations correct use of terminology will improve communication among different specialists of multidisciplinary teams.
Diagnostic imaging in VM
A detailed history and clinical examination are paramount for initial evaluation of VM, however, when imaging is required, it is important to choose the appropriate modality for the specific type of malformation. Ultrasound (US) is widely used for initial screening, and can help planning for further imaging assessment (19,20). US should be able to evaluate vascularity if it includes grayscale, color Doppler, and spectral Doppler tracings (19). Given the lack of ionizing radiation, no need for sedation for children and easy accessibility, makes it an appropriate modality for primary classification of these lesions. Another advantage of US is that it can evaluate response to treatment by detecting changes in size and flow characteristic (21,22). However, the limited field of view for large lesions and operator-dependent examination are major limitations as a primary diagnostic modality (23). Multidetector computed tomography (CT) or CT angiography is helpful because of its fast speed and wide availability, especially useful when urgent imaging is required, however, its radiation exposure limits its usage, particularly in the pediatric population (11,22).
MRI features can provide further characterization of sonographic findings and help determine the appropriate management of VM (24). Moreover, it allows to define extent of the vascular lesion and anatomic relationship to adjacent structures (10). High soft tissue resolution and fat suppression characteristics allows to discern the location of vascular anomalies in relation to surrounding soft tissues (23). MRI is also able to differentiate high flow from low flow lesions using dynamic post-contrast sequences (25). The distinction between these two types of lesions depends on rapid filling on high flow lesions and late filling on low flow lesions (26).
Different MR imaging protocol depends on type of vascularity and location of the lesion (11). To be able to classify and characterize VM, we should initially acquire non-contrast multiplanar T2-weighted fast spin echo and T1-weighted spin echo. Next, contrast in administered and dynamic sequencing images are obtained from arterial and venous phases. After that, post contrast T1 weighted spin echo images are acquired to complete the imaging acquisition (26). On contrast-enhanced MRI, the parenchymal component of VM seems so bright in T2-weighted imaged, and extent of adjacent tissue involvement is well depicted on T1, T2 or short-tau inversion recovery type images (27). Low flow malformations generally homogenous intraluminal signal on T2-weighted imaging, whereas high flow lesions contain voids (10).
Calcification and phleboliths may appear as flow-void and lead to misdiagnosis of low flow and high flow lesion (28) since these lesions are better visualized on CT, obtaining a non-contrast CT would help to better identify the lesion (29).
Since proper classification of VM is paramount for management, below we present imaging characteristic of various VM per different features.
Slow flow malformations
Slow flow VM depending on being local or diffuse have different clinical presentation. Their incidence is 1 in 10,000 in the United States (30). Typically, a detailed physical examination can separate high flow from low flow malformations. In slow flow category, typically one can differentiate them to lymphatic (LM) and venous (VM) subtype by physical exam. To define the extent of the lesions and plan for further management, US and MRI are useful modalities.
US is best primary modality for initial evaluation of patients. Slow flow vascular lesions appear heterogenous defined lesion which can be unilocular or multilocular. Cystic areas are compressible in lymphatic lesions whereas venous malformations are not compressible (31). On the other hand, MRI also can give prognostic information and includes description of extent and tissue involvement. With use of T1 weighted imaging, we can define the anatomy and with fat suppression techniques, we can increase lesion detection by suppressing g the bright fat. T1 imaging is useful in differentiating LMs from VMs, LMs enhance peripherally while VMs enhance homogenously and the chambers within a VM appear heterogenous hyperintense, whereas, LM remain hypointense and the septa appear hyperintense. In other words, the contrast medium penetrates a VM but not LM. Below is an overview of LMs and VMs in term of description of the lesions.
Venous malformation
Venous malformation is the most prevalent VM with a prevalence of 1% in general population (32). Venous malformation can be found anywhere in the body however, they are more prevalent in head and neck (40%), extremities (40%), and trunk (20%) (33). The Birmingham peripheral limb classification (34) tried to separate venous malformation as to whether they are localized or diffuses, bone joint involvement, fascia or muscle involvement whole limb involvement with or without skin involvement (35). The severity of symptoms depends on size of the lesions and adjacent structure. Lesions may progress during adolescence (75%) and childhood (25%) (36).
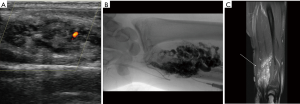
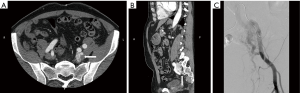
US is used as the first line modality to diagnose venous malformations and they appear as slow flow with multiple chambers (Figure 1A). Doppler sonography helps the physicians to distinguish high flow from slow flow VM and to differentiate lymphatic from venous malformations (37). VMs typically composed of small chambers on US and phleboliths are detected as a mass with acoustic shadowing and are so typical of venous malformations. CT venography is recommended for to identify underlying pathology, obstructed veins and anatomical variation and extent of venous thrombosis (38). In MRI, venous malformations look hypo- to isointense on T1 weighted images and hyperintense on T2 weighted images (Figure 1B). These features allow differentiation of VMs from AVM. On angiographic evaluation, these masses can present with a fistulous component to an adjacent artery (Figure 1C). Invasive tests may be required for when non/less invasive tests were not diagnostic. Obstructive truncular VM along iliac vein needs phlebography combined with intravenous ultrasound for proper management (39).
Lymphatic malformation
LMs account for 2.8/100,000 of hospital admissions (37,40). LMs are composed of vessels or large chambers lined by a single layer of endothelial cells. Most commonly, LMs affect head and neck (78%), and an additional 20% occurring in pelvis, axilla, and mesentery (41). Fifty percent of cases are present at birth and more than 90% are diagnosed before age 2 (42). Evaluation of LMs is highly dependent on imaging to define the size and characteristic of the lesions. They are categorized at either macrocytic lesion and microcytic lesions:
- Macrocytic: these lesions have cyst spaces of 2 cm or more and appear as soft masses with trans-illumination and don’t exhibit dependency. On US macrocytic lymphatic lesions are characterizes by fewer major anechoic chambers compared to VMs (43);
- Microcytic: these lesions can infiltrate tissues. The affected area has an overlying layer of small vesicles and present with swollen limb and can be complicated by lymphatic fluid leakage and episode of infection. Staging system based on site and extent of LM has been proposed and correlate well with surgical outcome (44).
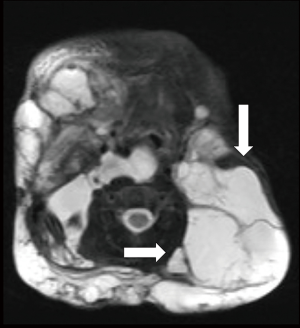
Like other VM US is the first line examination and LM appear as small (microcytic) or large (macrocytic) chambers with no detectable flow. Whether chylous or not, the lesions are usually anechoic by US. US may demonstrate hypoechoic compressible channels with absent blood flow on Doppler (45). Macrocytic LMs can be misdiagnosed with VMs but they can be distinguished from VMs based on the size of the chambers and lack of phlebolith, whereas, microcytic lesions are more easily differentiated from VMs as venous malformations show slow flow in 85% of cases (19). MRI is the modality of choice for determine the extent of LMs. LM cavities appear iso to hypointense in T1 imaging and hyperintense in T2 weighted images (Figure 2) and as they lack the flow void effect they can be differentiated from high flow lesions. Use of gadolinium facilitate differentiate venous malformation and lymphatic malformations as gadolinium only accumulate in venous malformations. As in VM, CT is never indicated for evaluation of LMs.
Fast flow malformations
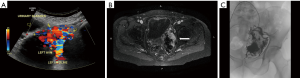
Symptoms of high flow lesions depends on the degree of arteriovenous shunting and involved area. They are pulsatile lesions without capillary transition between artery and vein, they may present during childhood and grow with child’s growth, with periods of rapid growth at puberty at times of trauma, surgery and pregnancy, they may also be due to an iatrogenic cause (11). Venous congestions can lead to pain, hemorrhage, ulceration and high output cardiac failure (19). Like other vascular lesions, US is the first diagnostic modality to both differentiate these lesions from slow flow lesions and plan for further imaging. Cross sectional can show the key feature of the high flow lesions including a mass like cluster of an arterial and venous structures (Figure 3).
Arteriovenous malformation
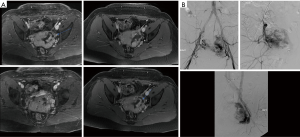
They are congenital lesion which are compose of dysplastic arteries connected to veins without capillary bed in between. They contain a nidus between venous and arterial bed. They are mainly found in the muscles, subcutaneous fat, bone and cranium (11). These lesions are most commonly found in central nervous system and less frequently on limbs (Figure 4) and trunk (Figures 3 and 4) (46). Major complications include: varicose vein bleeding, cardiac failure and aneurysm formation (45). Venous congestions can lead to pain, hemorrhage, ulceration and high output cardiac failure (47).
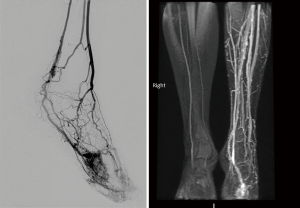
On US and Doppler sonography, AVM appear as vessels with high systolic and diastolic flow, prominent arteriovenous shunting and pulsatile venous structure (19) on contrast to other VM, CT can also provide useful information regarding adjacent organ involvement and extent of the mass (Figure 4). Contrast enhanced CT allow structural assessment of these lesions (48). On MRI they present as network of arteries and veins connected by a shunt. They demonstrate flow void in T1 an T2 weighted spin echo imaging and are hyperintense on T2 weighted gradient echo imaging indicating rapid flow (Figure 4B) (49). Unlike other VM AVM don’t present enhancement of adjacent structures on T2 weighted imaging (49). In conventional MRI findings are vascular flow voids without associated T2 hyperintensity, enhancement or mass on surrounding tissue, however, Patel et al. (50) showed 50% of lesions presented with these characteristics and these are more common in lesions that consisting of multiple tissue compartment. Awareness of this atypical features help radiologist not to misdiagnose these lesions. AVMs also require diagnostic angiography (Figures 3C) (51) and they present as dilated arteries with early opacification of dilated veins and in cases that embolization is performed during the angiography we see that additional arteries become apparent after original vessels are embolized (22,52) (Figure 5).
Arteriovenous fistula
They are usually acquired lesions and most commonly found in the brain (53) and lack the nidus present in VMs in imaging (11). Long standing fistulas may result in limb edema, high output cardiac failure and aneurysmal degeneration of the artery (54,55). Most commonly masses with AV fistula component are iatrogenic lesions from prior surgery and/or trauma and usually there is communication between large vessels. Risk factors for iatrogenic fistula include: female gender, hypertension, left femoral puncture and anticoagulation therapy (56,57).
Like other vascular anomalies, first diagnostic test of choice for diagnosis of arteriovenous fistulas is ultrasound. Arteriovenous fistulas appear as high frequency, low resistance continuous flow with elevated diastolic velocities through pulse cycle.
MRI shows arterial and venous component as high signal intensity without well-defined mass (3). T1W and T2W sequences show serpiginous signal voids without focal mass (3) which are diagnostic for fistula. Intrauterine AVFs are usually secondary to trauma and may present with mild discomfort to feeling of pressure and vaginal and rectal bleeding (58). Most asymptomatic women are diagnosed with US which present as high velocity, low resistance flow and on MRI imaging they appear as high-flow serpentine and large arteries and draining veins Figure 6A. Areas of high signal on T1 weighted images may be due to hemorrhage or flow related enhancement (58). On angiography, there are enlarged vessels with early venous drainage (Figure 6B).
Conclusions
The evaluation of vascular masses is complex and treatment is highly dependent on the imaging work-up and its characteristics. While US evaluation is the first modality for diagnosis of VM, MRI can provide significant information for treatment planning and improvement of symptoms.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Greene AK, Kim S, Rogers GF, et al. Risk of vascular anomalies with Down syndrome. Pediatrics 2008;121:e135-40. [Crossref] [PubMed]
- Fishman SJ, Mulliken JB. Hemangiomas and vascular malformations of infancy and childhood. Pediatr. Clin North Am 1993;40:1177-1200. [Crossref] [PubMed]
- Navarro OM, Laffan EE, Ngan BY. Pediatric soft tissue tumors and pseudotumors: MR imaging features with pathologic correlation. Imaging approach, pseudotumors, vascular lesions, and adipocytic tumors. Radiographics 2009;29:887-906. [Crossref] [PubMed]
- Brouillard P, Vikkula M. Vascular malformations: localized defects in vascular morphogenesis. Clin Genet 2003;63:340-51. [Crossref] [PubMed]
- Woollard HH. The development of the principal arterial stems in the forelimb of the pig. In: Contributions to Embryology. Washington, DC: Carnegie Institution of Washington, 1922;14:139-54.
- Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interv Neuroradiol 1997;3:275-81. [Crossref] [PubMed]
- Available online: http://www.issva.org/classification
- Lee BB, Bergan JJ. Advanced management of congenital vascular malformations:a multidisciplinary approach. Cardiovasc Surg 2002;10:523-33. [Crossref] [PubMed]
- Foley LS, Ann M. Vascular Anomalies in Pediatrics. Adv Pediatr 2015;62:227-55. [Crossref] [PubMed]
- Dubois J, Kulungowski AM. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 2010;40:895-905. [Crossref] [PubMed]
- Kollipara R, Odhav A, Rentas KE, et al. Vascular Anomalies in Pediatric Patients Updated Classification, Imaging, and Therapy. Radiol Clin N Am 2013;51:659-72. [Crossref] [PubMed]
- Malan E, Puglionisi A. Congenital angiodysplasia of the extremities: generalities and classification; venous dysplasias. J Cardiovasc Surg (Torino) 1964;5:87-130. [PubMed]
- Belov S. Classification of congenital vascular defects. Int Angiol 1990;9:141-6. [PubMed]
- Belov S. Anatomopathological classification of congenital vascular defects. Semin Vasc Surg 1993;6:219-24. [PubMed]
- Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 1982;69:412-22. [Crossref] [PubMed]
- Enjolras O. Classification and management of the various superficial vascular anomalies: hemangiomas and vascular malformations. J Dermatol 1997;24:701-10. [Crossref] [PubMed]
- Elluru RG, Azizkhan RG. Cervicofacial vascular anomalies. Vascular malformations. Semin Pediatr Surg 2006;15:133-39. [Crossref] [PubMed]
- Chang MW. Updated classification of hemangiomas and other vascular anomalies. Lymphat Res Biol 2003;1:259-65. [Crossref] [PubMed]
- Dubois J, Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 1999;29:879-93. [Crossref] [PubMed]
- Dubois J, Patriquin HB, Garel L, et al. Soft-tissue hemangiomas in infants and children: Diagnosis using Doppler sonography. AJR Am J Roentgenol 1998;171:247-52. [Crossref] [PubMed]
- Flors L, Leiva-Salinas C, Maged IM, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow up. RadioGraphics 2011;31:1321-40. [Crossref] [PubMed]
- Legiehn GM, Heran MK. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am 2006;37:435-74. [Crossref] [PubMed]
- Tekes A, Koshy J, Kalayci TO, et al. Mitchell Vascular Anomalies Flow Chart (SEMVAFC): A visual pathway combining clinical and imaging findings for classification of soft-tissue vascular anomaliesq. Clin Radiol 2014;69:443-57. [Crossref] [PubMed]
- Hyodoh H, Hori M, Akiba H, et al. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics 2005;25 Suppl 1:S159-71. [Crossref] [PubMed]
- Bashir U, Shah S, Jeph S, et al. Magnetic Resonance (MR) Imaging of Vascular Malformations. Pol J Radiol 2017;82:731-41. [Crossref] [PubMed]
- Lidsky ME, Spritzer CE, Shortell CK. The role of dynamic contrast-enhanced magnetic resonance imaging in the diagnosis and management of patients with vascular malformations. J Vasc Surg 2012;56:757-64.e1. [Crossref] [PubMed]
- Konez O, Burrows PE. An appropriate diagnostic workup for suspected vascular birthmarks. Cleve Clin J Med 2004;71:505-10. [Crossref] [PubMed]
- Abernethy LJ. Classification and imaging of vascular malformations in children. Eur Radiol 2003;13:2483-97. [Crossref] [PubMed]
- Vilgrain V, Boulos L, Vullierme MP, et al. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics 2000;20:379-97. [Crossref] [PubMed]
- Cavezzi A, Parsi K. Complications of foam sclerotherapy. Phlebology 2012;27:46-51. [Crossref] [PubMed]
- Lo K, Mihm M, Fay A. Current theories on the pathogenesis of infantile hemangioma. Semin Ophthalmol 2009;24:172-7. [Crossref] [PubMed]
- Eifert S, Villavicencio JL, Kao TC, et al. Prevalence of deep venous anomalies in congenital vascular malformations of venous predominance. J Vasc Surg 2000;31:462-71. [Crossref] [PubMed]
- McCafferty I. Management of Low-Flow Vascular Malformations: Clinical Presentation, Classification, Patient Selection, Imaging and Treatment. Cardiovasc Intervent Radiol 2015;38:1082-104. [Crossref] [PubMed]
- Mendonca DA, McCafferty I, Nishikawa H, et al. Venous malformations of the limbs: the Birmingham experience, comparisons and classification in children. J Plast Reconstr Aesthet Surg 2010;63:383-9. [Crossref] [PubMed]
- Hassanein AH, Mulliken J, Fishman S, et al. Venous malformations: risk of progression during childhood and adolescence. Ann Plast Surg 2012;68:198-201. [Crossref] [PubMed]
- Colletti G, Valassina D, Bertossi D, et al. Contemporary Management of Vascular malformations. J Oral Maxillofac Surg 2014;72:510-28. [Crossref] [PubMed]
- Smith RJ. Lymphatic malformations. Lymphat Res Biol 2004;2:25-31. [Crossref] [PubMed]
- Gloviczki P, editor. Summary of Guideline of American Venous Forum: Handbook of Venous Disorders, 3rd edn. London, UK: A Hodder Arnold, 2008:706-22.
- Lee BB. Venous malformation and haemangioma: differential diagnosis, diagnosis, natural history and consequences. Phlebology 2013;28:176-87. [Crossref] [PubMed]
- Emery PJ, Bailey CM, Evans JN. Cystic hygroma of the head and neck. A review of 37 cases. J Laryngol Otol 1984;98:613-9. [Crossref] [PubMed]
- Puig S, Casati B, Staudenherz A, et al. Vascular low-flow malformations in children:current concepts for classification, diagnosis and therapy. Eur J Radiol 2005;53:35-45. [Crossref] [PubMed]
- Zadvinskis DP, Benson MT, Kerr HH, et al. Congenital malformations of the cervicothoracic lymphatic system: embryology and pathogenesis. Radiographics 1992;12:1175-89. [Crossref] [PubMed]
- Legiehn GM, Heran MK. A step-by-step practical approach to imaging diagnosis and interventional radiologic therapy in vascular malformations. Semin Intervent Radiol 2010;27:209-31. [Crossref] [PubMed]
- de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck. A proposal for staging. Arch Otolaryngol Head Neck Surg 1995;121:577. [Crossref] [PubMed]
- Trenor CC 3rd, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg 2014;23:186-90. [Crossref] [PubMed]
- Stapf C, Mohr JP, Pile-Spellman J, et al. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus 2001;11:e1. [Crossref] [PubMed]
- Mulliken JB, Fishman SJ, Burrows PE. Vascular Anomalies. Curr Probl Surg 2000;37:517-84. [Crossref] [PubMed]
- Wójcicki P, Wójcickaa K. Epidemiology, Diagnostics and Treatment of Vascular Tumours and Malformations. Adv Clin Exp Med 2014;23:475-84. [Crossref] [PubMed]
- Gulati MS, Paul SB, Batra A, et al. Uterine arteriovenous malformations: the role of intravenous ‘dual-phase’ CT angiography. Clin Imaging 2000;24:10-4. [Crossref] [PubMed]
- Patel AS, Schulman JM, Ruben BS, et al. Atypical MRI features in soft-tissue arteriovenous malformation: a novel imaging appearance with radiologic-pathologic correlation. Pediatr Radiol 2015;45:1515-21. [Crossref] [PubMed]
- Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am 2001;39:701-20. [Crossref] [PubMed]
- Yakes WF, Krauth L, Ecklund J, et al. Ethanol endovascular management of brain arteriovenous malformations: initial results. Neurosurgery 1997;40:1145-52. [Crossref] [PubMed]
- Lee HJ, Kwon BS, Kwon JH, et al. Arteriovenous malformation incidentally found by ultrasonography in a thigh hematoma after contusion. Ann Rehabil Med 2012;36:287-90. [Crossref] [PubMed]
- Kron J, Sutherland D, Rosch J, et al. Arteriovenous fistula: a rare complication of arterial puncture for cardiac catheterization. Am J Cardiol 1985;55:1445-6. [Crossref] [PubMed]
- Glaser RL, McKellar D, Scher KS. Arteriovenous fistulas after cardiac catheterization. Arch Surg 1989;124:1313-5. [Crossref] [PubMed]
- Kim D, Orron DE, Skillman JJ, et al. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn 1992;25:91-7. [Crossref] [PubMed]
- Oweida SW, Roubin GS, Smith RB, et al. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg 1990;12:310-5. [Crossref] [PubMed]
- Christenson BM, Gipson MG, Smith MT. Pelvic Vascular Malformations. Semin Intervent Radiol 2013;30:364-71. [Crossref] [PubMed]


