
Systemic artery-pulmonary artery fistulae of adult bronchopulmonary dysplasia characterized with dynamic CT angiography and multiphase 3D-volumetric reconstruction: a case report
Introduction
Bronchopulmonary dysplasia (BPD), also known as the neonatal chronic lung disease, is the result of impaired alveolar and vascular development due to prematurity (1,2). Many adults affected by BPD as neonates suffer from chronic pulmonary airway disease which mimics adult onset asthma or smoking related chronic obstruction pulmonary disease. High-resolution non-contrast CT (HRCT) findings for such patients reveal various degrees of air-trapping and bronchiectasis, chronic subsegmental atelectasis, and fibrosis (3,4).
Altered angiogenesis in BPD leads to decreased pulmonary microvascular density, dysmorphic and sparse pulmonary vessels in the affected regions of the lung, and more abundant vessels in less affected areas (1,2). As patients age, pulmonary hypertension occurs, and microscopic intrapulmonary arteriovenous vascular anastomoses arise (5). These changes are followed by the development of macroscopic systemic artery-pulmonary artery fistulae (SAPAF) (5,6).
Herein, we present a case of an adult man with a known childhood history of BPD. In addition to the classic lung findings of BPD, an HRCT showed tortuous vessels within the mediastinum and the lungs. These vessels were confirmed to be related to multiple SAPAF with dynamic computed tomography angiography (DCTA) and multiphase 3D-volumetric reconstruction.
Case presentation
A 54-year-old man presented with progressive dyspnea. His past medical history was significant for chronic lung disease of prematurity (i.e., BPD) related to chronic ventilation and supplemental oxygen administration. The patient stated that late in adulthood he began to experience difficulty breathing during exercise and with cold exposure, with little or no relief of symptoms from inhaled beta agonist or steroid medications. He reported that although he had been a smoker in the past, he had quit this habit in his thirties. He denied any occupational exposure to lung irritants. A preliminary cardiac workup, including an electrocardiogram and an echocardiogram, was unremarkable. Pulmonary functional tests revealed an obstructive pattern upon bronchodilator provocative maneuvers.
A chest radiograph from 6 months prior revealed mild scarring in the inferior lung zones. A HRCT was obtained which showed diffuse emphysema, basilar scarring, mild cylindrical bronchiectasis, and tortuous vessels bilaterally within the areas of scaring and at the periphery of the lungs. In addition, tortuous enlarged vessels were noted throughout the mid mediastinum. DCTA and multiphase 3D-volumetric reconstruction was performed to further characterize these vascular abnormalities.
The DCTA scan was performed utilizing the radiation sparing adaptive 4D helical shuttle mode of a Somatom Force Scanner (Siemens Medical Solutions, Forchheim, Germany). The energy peak selected was 80 keV and the reference charge 150 mAs. Four temporal frames were obtained at an interval of 2.5 sec (the frame rate and frame number were selected based on the assumption that contrast transit time from the pulmonary artery to the aorta would be ~8 to 10 seconds). The dose length product for the scan was 548 mGy*cm. 100 mL of Iohexol 350 mgI/mL contrast media (Omnipaque, GE Healthcare Bio-Sciences Corp., Piscataway, New Jersey, USA) was injected at 5 mL/s, via a 20 gauge right antecubital intravenous angiocatheter, using a dual chamber automated programmable power injector (Medrad Stellant, Bayer Healthcare LLC, Whippany, USA), followed by a 50 mL saline flush at the same rate. The region of interest for bolus tracking was placed in the air above the patient so that the scan could be manually triggered at the first sight of contrast in the pulmonary artery during the monitoring phase. A four second delay was preprogramed into the scan protocol to allow for breath holding instructions.
Images were reconstructed using a soft vascular kernel (b36) and a model based iterative algorithm (ADMIRE) set at intermediate (i.e., “3”) strength. A displayed field of view (DFOV) of 294 mm × 294 mm was utilized to optimize in plane resolution. A stack of 0.5 mm thick slices reconstructed at an interval of 0.4 mm. The images were transferred to an advanced image post processing client/server (Aquarius Intuition version 4.4.12, Terarecon, Foster City, California, USA). In order to optimize the quality of volume rendering, segmentations of the pulmonary and bronchial arterial trees were created from different phases. The segmentations of the two phases were merged prior to volume rendering. Maximum intensity projection images of the phases were also created.
The post processed DCTA images showed that the tortuous pulmonary vessels seen on HRCT were pulmonary arteries and multiple SAPAF rather than a derangement of normal vascular architecture due to emphysema, scarring and atelectasis (Figures 1-3). In addition, marked enlargement of the bronchial arteries could be clearly seen, excluding the possibility of mediastinal varices. No right heart enlargement or central pulmonary artery abnormality was seen. However, several distal branches of the pulmonary arteries were noted to be subpleural, an unusual finding. The volume rendered images were instrumental to the comprehension of the relationship of the bronchial arteries to the pulmonary arteries.
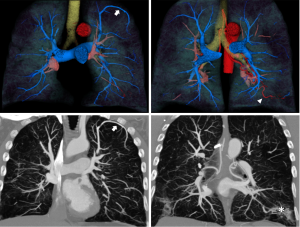
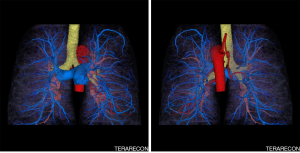

In light of the findings, the patient’s pulmonologist recommended further evaluation with echocardiographic bubble testing, shunt testing, and a quantitative ventilation perfusion scan. The pulmonologist also explained to the patient that ultimately right heart catheterization might be necessary to characterize the severity of his pulmonary hypertension. Unfortunately, the patient did not schedule these exams or return to the pulmonologist for follow-up.
Discussion
The root cause of bronchopulmonary dysplasia is disruption of the normal vasculogenic and angiogenic processes underpinning fetal lung development by the pathological processes of prematurity, such as decreased placental blood flow, hypoxia, and oxygen toxicity (2). Although the most evident manifestations of BPD are pulmonary (e.g., increased work of breathing and recurrent pneumonia), the vascular complication of pulmonary hypertension is a significant cause of mortality (5).
A number of pathological entities exhibit bronchial artery enlargement on CTA, including cystic fibrosis, post infectious bronchiectasis, and chronic pulmonary embolus, that later of which is also associated with SAPAF (8). In our case, we excluded these pathological processes from our differential diagnosis based on the patient’s clinical history and predominate HRCT finding of emphysema. We concluded that the presence of SAPAF was due to the underlying diagnosis of BPD. Unfortunately, the imaging literature related to adults with BPD focuses primarily on the bronchopulmonary manifestations of the disease (9,10). A search of the medical literature for communications regarding the CTA finding of SAPAF in the lungs of adult patients with BPD was unproductive.
With regard to the DCTA scan protocol, we designed it for optimal enhancement of both the pulmonary and the systemic circulation in order to achieve the best volume rendering possible during the post processing phase of interpretation. Region of interest growing for the purpose of segmentation depends on the presence of high contrast between the structure of interest and the surrounding tissues. In this case, it was not possible to optimize enhancement for all the vessels of interest on a single phase so a dynamic scanning mode (adaptive 4D) was utilized. Similar scanning protocols have been used for the assessment of aortic dissection (11). Multiphase imaging to evaluate SAPAF has been reported previously in the Chinese medical literature (12). The pulmonary artery segmentation was derived from a phase where pulmonary artery enhancement was ~820 Hounsfield units, and the bronchial artery segmentation was derived from a phase where aortic enhancement was ~580 Hounsfield units (Figure 4). The data-set-merging functionality of the Intuition postprocessing client/server allowed us to combine segmentations derived from two different phases, allowing for excellent visualization of both the bronchial and pulmonary arteries in a single volume rendering.
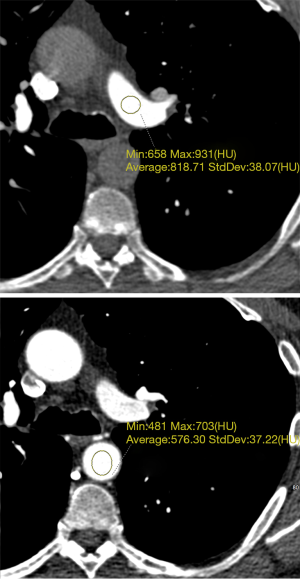
To the best of our knowledge, no case reports or series reporting CTA findings related to the vascular manifestation of BPD are present within the literature. The prevalence of SAPAF in the presence of adult BPD is unknown. Consequently, is important that interpreters of cardiovascular CT be alert to both the pulmonary and potential vascular manifestations of adult BPD, so that additional cases can be identified and studied. Eventually, the pathophysiological, prognostic, and therapeutic implications of the presence of SAPAR in the setting of adult BPD (e.g., the relationship of this finding to the incidence of pulmonary hypertension and ultimately right to left shunting) may be revealed.
DCTA, performed with radiation sparing technology, such as the Siemens adaptive 4D helical shuttle mode (as was utilized for this case), is an excellent tool to distinguish bronchial artery enlargement and SAPAF from other pathologies and will be of use during future investigations of the macroscopic vascular sequelae of adult BPD and other causes of pulmonary hypertension.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: For this case report, which utilizes deidentified radiographic images, the institutional review board did not require informed consent.
References
- Hilgendorff A, O’Reilly MA. Bronchopulmonary Dysplasia Early Changes Leading to Long-Term Consequences. Front Med (Lausanne) 2015;2:2. [Crossref] [PubMed]
- Alvira CM. Aberrant Pulmonary Vascular Growth and Remodeling in Bronchopulmonary Dysplasia. Front Med (Lausanne) 2016;3:21. [Crossref] [PubMed]
- Davidson LM, Berkelhamer SK. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J Clin Med 2017.6. [PubMed]
- van Mastrigt E, Logie K, Ciet P, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: A systematic review. Pediatr Pulmonol 2016;51:975-86. [Crossref] [PubMed]
- An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010;40:131-6. [Crossref] [PubMed]
- Lee JK, Park JH, Kim J, et al. Embolization of Multiple Systemic Artery to Pulmonary Artery Fistula with Recurrent Hemoptysis. Tuberc Respir Dis (Seoul) 2013;75:120-4. [Crossref] [PubMed]
- Zagurovskaya M, Issa M, Winkler M. Multi-phase volume rendering cine clip of pulmonary and bronchial arteries, showing prominent SAPAF in a dynamic CTA of a patient with bronchopulmonary dysplasia. Asvide 2019;6:025. Available online: http://www.asvide.com/article/view/29713
- Aluja Jaramillo F, Gutierrez FR, Díaz Telli FG, et al. Approach to Pulmonary Hypertension: From CT to Clinical Diagnosis. Radiographics 2018;38:357-73. [Crossref] [PubMed]
- Howling SJ, Northway WH Jr, Hansell DM, et al. Pulmonary sequelae of bronchopulmonary dysplasia survivors: high-resolution CT findings. AJR Am J Roentgenol 2000;174:1323-6. [Crossref] [PubMed]
- Wong P, Murray C, Louw J, et al. Adult bronchopulmonary dysplasia: computed tomography pulmonary findings. J Med Imaging Radiat Oncol 2011;55:373-8. [Crossref] [PubMed]
- Lu CY, Diao YK, Guo YQ, et al. Can multiphase dynamic CT angiography provide a better assessment of aortic dissection compared with the standard triphasic protocol? Acta Radiol 2018;59:58-64. [Crossref] [PubMed]
- Zhu Q, Wu X, Lin H, et al. The clinical and CT two-phase imaging features of bronchial-pulmonary arterial fistula. Zhonghua Jie He He Hu Xi Za Zhi 2014;37:687-93. [PubMed]


