
Echocardiographic guidance of interventions in adults with congenital heart defects
Introduction
Cardiac catheterization procedures have revolutionized the treatment of adults with congenital heart disease over the past six decades. Patients who previously would have required open heart surgery for various conditions can now undergo percutaneous cardiac catheter-based procedures to close intracardiac shunts, relieve obstructive valvular lesions, stent stenotic vessels, or even replace and repair dysfunctional valves (1). From the very first cardiac catheterization by Dr. Werner Forssmann in 1929 (2), to the early days of percutaneous treatments with balloon atrial septostomy by Dr. William Rashkind in 1966 (3), the imaging modality inherently tied to cardiac catheterization has been fluoroscopy and angiography. Over the past 20 years, however, as the complexity of percutaneous cardiac catheterization procedures has increased, the limitations of fluoroscopy and angiography have become apparent. Fluoroscopy of the heart only provides two-dimensional (2D) images of a three-dimensional (3D) structure, which can lead to incomplete spatial assessments (1). Many advanced imaging modalities, such as cardiac magnetic resonance (CMR) and cardiac computed tomography (CT), have emerged as invaluable tools for pre-procedural planning to delineate structural anatomy and function (4). Nevertheless, echocardiography, with its excellent temporal and spatial resolution (with the advent of 3D echocardiography), has become a very attractive adjunctive imaging modality during interventional procedures (1,5). Thus, as the field of interventional cardiology has grown, so has the use of echocardiography for interventional guidance in adults with congenital heart disease (1,6,7).
In this review, we aim to describe the different echocardiographic techniques and their role in various cardiac catheterization interventions specific to adults with congenital heart disease.
Transthoracic echocardiography (TTE)
TTE is the workhorse of cardiology and is integral to the pre-procedural assessment of any adult congenital heart disease (ACHD) patient. Given the various different anatomical anomalies and subsequent surgical repairs/palliations, a complete echocardiographic assessment via TTE should be performed before a cardiac catheterization and after catheter-based interventions. A TTE can be useful for preprocedural planning, gauging the risk of periprocedural complications, or assessing the need for procedural support with cardiac anesthesia or the need for mechanical support in patients with ventricular dysfunction. Furthermore, it can be used to confirm or refute diagnoses in patients who have been referred for catheter-based or surgical procedures. TTE has the favorable characteristics of being cost-effective, universally available, and does not subject the patient to radiation. The use of TTE to guide intraprocedural imaging has been limited given the superiority of transesophageal echocardiography and intracardiac echocardiography (ICE) for the visualization of atrial structures and left sided valves (Table 1).
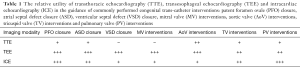
Full table
Transesophageal echocardiography (TEE)
Unlike the TTE, a TEE is widely considered the echocardiographic imaging modality of choice during catheterization interventions in patients with complex congenital heart disease. Cardiac fluoroscopy can be used concomitantly with TEE imaging (unlike TTE when the probe and the sonographer’s hand may be in the field of view) (1). Furthermore, TEE imaging has better image resolution compared to TTE and is superior to TTE when monitoring catheter position, imaging atrial and ventricular level defects, excluding atrial thrombus, or assessing left sided valvular anatomy (6). Unfortunately, periprocedural TEE does have some drawbacks. A cardiac catheterization procedure that utilizes TEE will require two operators (the catheterizer and the echocardiographer), and thus a successful procedure will require effective communication and a high level of coordination between the operators. Furthermore, a TEE usually requires deep sedation and may require endotracheal intubation for patient comfort. Finally, the proximity of the sonographer to the X-ray tube in most catheterization laboratories also leads to an increased radiation dose to the TEE sonographer (1).
Intracardiac echocardiography (ICE)
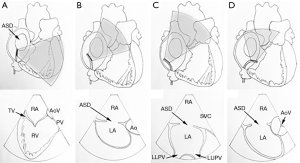
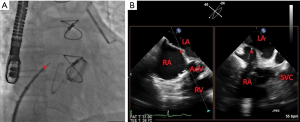
ICE is a newer imaging modality compared to TEE and TTE (8). ICE has great potential to be a procedural adjunct for monitoring and guiding interventions. First described in 1981, ICE is a catheter-based steerable ultrasound probe that is introduced into the right heart chambers and displays cardiac images from inside the heart (9). As phased-array probes were developed in the early 1990s, ICE quickly gained popularity throughout interventional and electrophysiology (EP) laboratories around the world, specifically for imaging the atrial septum from a right atrial location (Figure 1) (10). Compared to TEE, ICE obviates the need for a second operator. There may be a learning curve, however, to understand how to deftly manipulate the probe to visualize desired structures (9). Furthermore, general anesthesia is not required for ICE imaging, and some studies have shown that ICE can shorten procedure and fluoroscopy times (6). The cost seems to be also comparable with TEE interventions (11). In patients where TEE is contraindicated or may be dangerous (i.e., patients with esophagectomy, or large esophageal varices), ICE may be an attractive imaging modality as well. ICE can also be very useful in evaluating cardiac structures when TEE imaging is not optimal, such as in the setting of a mechanical valve (causing shadowing artifact), or for anterior right-sided cardiac structures (6). Nevertheless, ICE is not a benign procedure and does require a second venous puncture with at least an 8 or 10 French sheath (1). Other complications include retroperitoneal bleed, perforation of venous structures, pericardial effusion from a cardiac perforation, induced atrial/ventricular ectopy and arrhythmias, and thromboembolism (6).
3D echocardiography
With the development of matrix array transducers, both TTE and TEE probes have the ability to image in real-time 3D (12). ICE catheters have also recently been developed with 3D capabilities, but have limited distribution currently given their novelty (13,14). 3D technology can accurately depict cardiac structures and visualize long segments of catheters and entire devices in one view with limited manipulation, which makes it an ideal imaging modality during interventional procedures (15). Applying 3D echocardiography during interventional procedures has led to more effective myocardial biopsies in terms of safety and efficacy, improved analysis of mitral valve pathology, and improved planning of percutaneous approaches for complex procedures such as paravalvular leak occlusion, and improved sizing of intracardiac defects such as atrial septal defects (ASDs) or ventricular septal defects (VSDs) (6). Nevertheless, 3D imaging has not become ubiquitous during catheterization procedures. Many interventional cardiologists and echocardiographers have become accustomed to working with 2D images, and carefully standardized 2D views have been established for complex structural interventional cases (15). Furthermore, unlike 2D TTE and TEE imaging, 3D imaging does not have a standardized set of views during procedures, and requires real-time manipulation of the 3D dataset acquisition (cropping, rotating) during the procedure, which may increase complexity (15). 3D TEE can be helpful in monitoring right ventricular (RV) function before and after ASD closure, and can be used to predict which patient may need closer monitoring post closure due to reduced RV function (16). Recently, the American Society of Echocardiography (ASE) and the European Society of Cardiovascular Imaging (EACVI) have released a consensus document regarding the use of 3D echocardiography in congenital heart disease. This will hopefully lead to more standardized and broad use of this useful modality during catheter-based interventions, especially for closure of ASD/VSDs and visualization of catheters/devices (7).
Echocardiography and fluoroscopic fusion imaging “EchoNavigator”
More recently, with the advent of computerized software that can enable coregistration of two imaging modalities, operators in the catheterization lab can simultaneously visualize two imaging modalities superimposed on each other instead of on two separate monitors. EchoNavigatorTM (Philips Healthcare, Best, the Netherlands) is a software package that can fuse echocardiographic images (2D or 3D) with live fluoroscopy images. This allows for placement of markers on echocardiography images that can be shown on fluoroscopy images, which can help with complex structural cases and reducing radiation dose (1,17). Nevertheless, early studies using this technology have not shown a reduction in radiation dose or procedure time, but this may be biased given the initial learning curve required for this new technology (1).
Echocardiographic guidance of various ACHD interventions
ASD/PFO closure
When closing a secundum ASD or PFO, the operator can choose between TTE, TEE, and ICE for procedural guidance (6). TTE has been described for closing ASDs or PFOs in adult patients (18). TTE allows for imaging in multiple planes to evaluate the device and atrial septum, but has limited views of the inferior rim post-device deployment due to device interference. One center performed a randomized trial demonstrating that TTE was as effective as TEE in terms of procedural success and sizing of the ASD closure device in pediatric patients (19). Nevertheless, given the varying degree of body habitus and possibility of poor acoustic windows in adults, most ACHD centers rely on TEE or ICE for percutaneous closure of these defects (6).
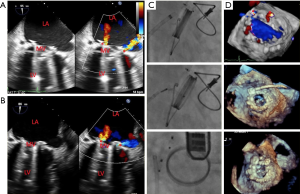
TEE is the main echocardiographic modality used for procedural guidance during ASD closures (Figure 2), and the pivotal trial for ASD closure device required TEE guidance for the procedure (20). TEE provides detailed and real-time imaging of the interatrial septum, surrounding cardiac structures, catheters, and the closure device. While TEE has many advantages, some disadvantages of TEE include the need for deep sedation or general anesthesia for patient comfort, as well as the need for a dedicated echocardiographer to perform the TEE (6). TEE has mostly been supplanted by ICE for PFO closure but retains an important role in the assessment of the atrial septum, atrial appendage, mitral and aortic valves as well as the aorta in patients with an embolic stroke.
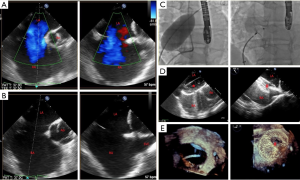
ICE is a newer imaging modality that also provides comparable imaging quality of the interatrial septum and surrounding structures as TEE (Figure 1). ICE does not require the use of deep sedation or general anesthesia, which obviates the need for a second operator for echocardiographic guidance, and has also been shown to reduce procedure and fluoroscopy times (11). ICE still requires additional expertise in manipulating the catheter, and thus some operators may not be as comfortable with the imaging, especially of large ASDs. However, it is ideal for guidance of PFO closure and has largely supplanted TEE for this indication (6).
3D imaging, with commercially available TEE, may also be more accurate in assessing ASD size and rim adequacy than 2D alone (1). It can be helpful for assessing multiple defects, such as in the setting of a fenestrated ASD (7). 3D imaging with ICE has been described, but it is still a new technology and has not been widely adopted at the time of this review (13).
VSD closure
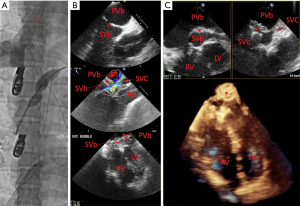
There are not as many studies highlighting the use of echocardiography during percutaneous closure of VSDs as compared to ASDs and PFOs. Studies detailing the efficacy of percutaneous VSD closure have described the use of both TEE and ICE during the procedure (21-24). One small study (12 patients in total) compared the use of TEE and ICE for guidance of percutaneous VSD closure and found no difference between modalities when measuring the size of the VSD or for facilitating correct device placement to close the defect (25). In our experience, the use of TEE may be limited in certain patients due to shadowing artifact from mechanical valves or other cardiac structures, and thus ICE is an attractive modality for periprocedural imaging guidance (Figure 3). 3D echocardiography with TEE can be used during closure procedures to monitor hardware and device deployment (7), and can also be used post-procedure to assess for any residual shunt.
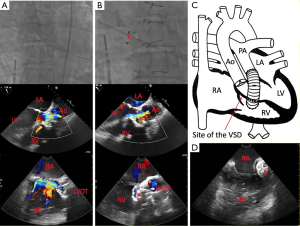
Transseptal puncture guidance
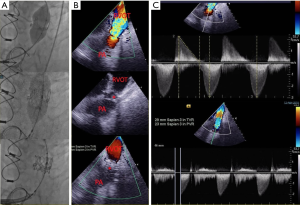
Transseptal punctures are required for many EP studies or left-sided interventions. While transseptal punctures can be done with fluoroscopy alone, most operators use imaging guidance to avoid inappropriate puncture sites (15). Traditionally TEE has been the imaging modality of choice (2D or 3D methods). Usually operators choose a simultaneous biplane view (X-plane) at the mid-esophageal level to visualize the bicaval (90–110°) and the short axis view at the aortic valve (30–60°) (Figure 4) (5,17). Visualizing the “tenting” seen with the puncture needle may be more difficult with 3D imaging (15). Since ICE does not need additional sedation or general anesthesia, many centers are adopting ICE as the primary imaging modality during transseptal punctures when imaging is only needed for the transseptal portion of the procedure (6).
Studies have shown that use of EchoNavigator, the fusion imaging software that integrates 2D/3D TEE with fluoroscopy, during transseptal puncture can lead to shorter procedure times and can be particularly helpful in cases where there is complex anatomy of the interatrial septum, such as with an interatrial septal aneurysm or lipomatous hypertrophy (26).
Paravalvular leak
Paravalvular regurgitation is a recognized complication that affects 5–17% of all surgically implanted prosthetic heart valves (17). Currently, TEE is the most widely utilized imaging modality to guide paravalvular leak closure (Figure 5). TEE is used to localize defects, describe the number and size of the hole(s), guide catheters and interventional devices across the defect, and to assess the degree of reduction of paravalvular leak after the intervention. TEE also can evaluate for possible complications (pericardial effusion, prosthetic leaflet entrapment) (17). 3D echocardiography is becoming the norm for echocardiographic guidance, and is especially useful in this regard for mitral valve procedures (5). Live 3D is excellent for guiding wires and catheters across a paravalvular defect (5). When closing periprosthetic aortic valve leaks, anterior defects are best seen with TTE, while posterior defects are seen best on TEE (5). ICE experience is limited during these types of procedures, but our experience has shown that anterior structures such as the tricuspid valve are more conducive to imaging with an ICE modality rather than TEE or TTE, and ICE can be effective at guiding paravalvular leak closures in the tricuspid position.
While no studies have compared the use of fusion imaging to standard TEE or ICE guidance for paravalvular leak procedures, expert operators have remarked on how beneficial having fused images and using 3D TEE-based markers can be for wiring paravalvular defects (26).
Baffle leak in Mustard/Senning atrial switch patients
In patients with dextro-transposition of the great arteries (D-TGA) status post an atrial switch operation, baffle leaks are common sequelae. Baffle leaks can be identified and characterized using many imaging modalities, such as angiography, echocardiography and cardiac MRI (27). One study determined that echocardiograms (TTE or TEE) with an agitated bubble study were more effective at detecting baffle leaks than echocardiographic studies without agitated saline or cardiac MRI (28). One case series detailed their experience with TEE for procedural guidance for baffle interventions, demonstrating it as a safe and effective modality (29). While most baffle leaks are percutaneously closed using TEE for procedural guidance (Figure 6), 3D TEE and 2D-ICE are becoming popular as well given the sometimes difficult process of visualizing these small leaks (27). The use of ICE has become more commonplace, and one interventional group described their experience with ICE as an excellent adjunct to fluoroscopy and angiography for localizing baffle leaks and guiding the closure, especially for defects in the inferior portion of the systemic venous baffle (30). Another case series documented three reports of instances when ICE was superior to TEE or TTE for real-time imaging guidance for baffle leak closure, since the baffle leaks occurred in unusual orientations or were small in size (31).
Percutaneous valve replacement
Over the past decade, the number of transcatheter valve replacements, mainly for the aortic position in adults with calcific aortic stenosis, has increased dramatically (32). There is an increasing number of implants in congenital aortic stenosis and regurgitation patients as well. The use of transcatheter valves in the pulmonary position has become commonplace in the congenital population with dysfunctional RV outflow tracts (33). There is also a growing number of implants in the tricuspid and mitral positions (34).
Transcatheter aortic valve replacement (TAVR)
When performing a TAVR, the use of multimodality imaging (echocardiography, angiography, fluoroscopy) during the procedure is key to a successful outcome (17). TTE plays an important role in establishing the need for a valve replacement and can help with preprocedural planning (35). Sizing of the aortic annulus is more accurate with TEE than with TTE, and is recommended when a computerized tomography angiogram (CTA) cannot be performed (i.e., chronic kidney disease) (35). Periprocedurally, TTE can be used for locating the apex of the heart and marking the optimum point of entry on the skin for a transapical approach, as well as post-implantation assessment of valve positioning and paravalvular or intravalvular regurgitation (5). TEE use for intraprocedural guidance, whether to position the balloon during valvuloplasty, help position the prosthesis during implantation, or to assess prosthesis function post-implantation, or to detect complications, is variable (5). Since TEE may require general anesthesia and can interfere with visualizing the aortic annulus during fluoroscopy, some operators prefer not to use TEE during the procedure and rely on TTE before and after valve implantation for assessing the prosthetic valve function (5,17,35). The benefits of TTE or TEE for imaging guidance during TAVR procedures are debated in the literature (36), but the decision should be made on an individual basis depending on operator preference and patient characteristics. The use of 3D echocardiography (mainly TEE) is also very helpful in sizing the aortic annulus and determining coronary heights pre-procedure and is very effective post-procedure for evaluating paravalvular or intravalvular regurgitation (35). The use of ICE for TAVRs is not as popular due to the challenge of securing a stable adequate window during the procedure, and currently is not a first-line imaging modality (35,37). Nevertheless, a small randomized study of 50 patients demonstrated that ICE is comparable to TEE in terms of image quality and hemodynamic assessment of the aortic prosthesis post-implantation (38). One of the downsides of periprocedural echocardiography during a TAVR, however, is the positioning of the machine and the echocardiographer to allow for free movement of the fluoroscopy cameras. Furthermore, the amount of radiation exposure to the echocardiographers is always a concern (35).
Right-sided valves
For patients requiring transcatheter valve replacement of right-sided valves, such as the pulmonary valve, the use of TEE may not be ideal given the anterior location of these structures. For these procedures, when imaging guidance is needed, the use of ICE has been demonstrated to be quite effective. The ICE catheter can be manipulated into the RV and directed coaxially with the RV outflow tract and pulmonary valve, leading to excellent images of the entire region and accurate measurements of the valve gradients pre and post-implantation (37,39-41). Furthermore, angiography of valve function is limited by catheter-induced regurgitation, which is not an issue with ICE (37,39-41). Nevertheless, operators vary widely in their use of periprocedural imaging for transcatheter valve implantations.
Valve-in-valve replacement
The use of transcatheter valve implantation in existing prosthetic valves, or “valve-in-valve”, technique, has also become more popular in patients who may no longer be a surgical candidate or who prefer a transcatheter procedure over open-heart surgery (Figure 7). One registry of 156 patients undergoing a procedure for a transcatheter valve-in-valve tricuspid valve replacement described a wide variability in the modality of intraprocedural echocardiography. Most patients (82%) had intraprocedural echocardiography, but patients receiving a Melody valve were mainly split between TEE and ICE (48% vs. 38%), while those receiving a Sapien valve mainly had TEE (88%) for procedural guidance, suggesting that the decision for procedural imaging depends largely on the operator and their comfort with the imaging modality used (42).
For the mitral position, since it is a left-sided structure, TEE is the imaging modality mainly used, especially since a transseptal puncture is usually needed (43). ICE has been described for mitral valve procedures, such as MitraClip, but it remains a suboptimal imaging modality since it does not allow for adequate left atrial visualization in order to guide catheters and wires during the intervention (44). The development of 3D ICE, however, may lead to a role for ICE to help with intraprocedural guidance during transcatheter mitral valve replacements in the future (13,14,44).
Triclip/Mitraclip
The development of the MitraClip for percutaneous mitral valve repair (and the off-label use of the MitraClip system in the tricuspid position) has been beneficial for select patients requiring atrioventricular valve (i.e., mitral or tricuspid) repair who are at high surgical risk.
Mitraclip
TTE and TEE have been used for preoperative assessment of mitral valve disease. During the procedure, however, TEE is essential for procedural guidance, from the transseptal puncture, introduction of the delivery sheath and clip system, positioning of the MitraClip, grasping the leaflets, deploying the clip, and assessing for residual regurgitation and measuring mitral valve gradients (35,45). Details of the procedure the necessary TEE views have been published previously (46,47), and the use of 3D TEE is highly recommended (7,46). When patients have a contraindication for TEE, such as esophageal stenosis, the use of ICE is usually not sufficient, but one example of a percutaneous mitral valve repair using 3D ICE technology for procedural guidance has been reported (13).
TriClip
The off-label use of the MitraClip system in the tricuspid position has been reported for patients with severe tricuspid regurgitation who are too high risk for surgical repair/replacement (48-50). TEE imaging was used to guide the operators when orienting the clip on the tricuspid valve, utilizing deep transgastric views, esophageal views, as well as 3D echocardiography (48,50). Patients in these studies were preselected for adequate visualization of the valve prior to the procedure however, and thus TEE may not be the best imaging modality for this type of procedure (49). Since the tricuspid valve is an anterior structure, TTE may be effectively used. In fact, 25% of patients in a large case series had received TTE in addition to TEE due to insufficient image quality for clip placement or grasping by TEE alone (49). One patient in a case series had to also receive ICE for procedural guidance (49,50).
EP procedures/lead extraction
For patients with atrial fibrillation undergoing pulmonary vein isolation, TEE is necessary to exclude the presence of a left atrial appendage thrombus prior to the procedure (17). During the procedure, TEE is very helpful for transseptal punctures and for delineating pulmonary vein anatomy and can help locate catheter position relative to important cardiac structures (17). Nevertheless, ICE has been demonstrated to be a safe and effective adjunct to fluoroscopy during transseptal puncture (17,37). Furthermore, for patients with complex congenital anatomy, ICE can help identify the pulmonary vein antrum and allow for more precise ablations and fewer complications, such as pulmonary vein stenosis (37). ICE can help improve the contact between the mapping/ablating catheter and the myocardial tissue, and help visualize cardiac structures that have an electrical interest as well (9). ICE can also monitor for procedural complications, such as cardiac tamponade, in a real-time fashion (9,37). When trying to diagnose right-sided pacemaker lead infections or thrombosis, ICE is superior to TEE since these are anterior cardiac structures. ICE can also be helpful during lead extractions as well (9).
Endomyocardial biopsy
As the ACHD population ages there is a growing need for heart (plus other organ) transplantation in those with irreversible heart failure. Following heart transplantation, endomyocardial biopsy to assess for rejection is indicated. While many endomyocardial biopsies are performed with fluoroscopy alone, some centers utilize 2D TTE to complement fluoroscopy (6). TEE or ICE can also be an optimal imaging tool to guide the procedure. With ICE, the operator can have direct visualization of the biopsy site and can monitor for procedural complications in real-time (9). If the operator is trying to biopsy a specific area like an intracardiac mass, ICE can be invaluable. All modalities, however, have their disadvantages. TTE may be difficult to perform on supine patients who have difficult windows due to body habitus or chronic lung disease. TEE may need extra sedation and anesthesia support. ICE has increased costs and requires a second vascular access site, typically in the femoral venous region (6). Thus, the choice of adjunctive imaging modality during an endomyocardial biopsy will depend on operator comfort, patient characteristics, and availability of ancillary support (6).
Pericardiocentesis
TTE is the main echocardiographic modality used for performing a pericardiocentesis. A large series out of the Mayo clinic has demonstrated its safety and efficacy, with major complications in only 1.2% of cases (51), and the ASE has recommended that a pericardiocentesis should be performed with TTE guidance (6). The proper technique for an echocardiography-guided pericardiocentesis is to locate the ideal needle entry site and trajectory for the needle. The entry point should be at the largest collection of fluid that is closest to the body surface where a straight needle trajectory would avoid vital structures (6). The typical pericardiocentesis sites are via the left chest wall or the subcostal approach.
Future directions/recommendations
The development of fusion imaging in ACHD has led to potentially safer and quicker procedures (26), but data are lacking evaluating its effectiveness compared to standard adjunctive imaging techniques at this time. As new technology emerges, such as the use of virtual or augmented reality in the catheterization lab (52), fusion imaging may become more commonplace.
The use of three-dimensional ICE is also a promising development in the field of interventional ACHD. The use of multiplanar ICE and full volume acquisitions with an ICE catheter may allow for lower sedation needs and improved spatial resolution, which would lead to more successful complex procedures (13,14). We are hopeful that with multi-planar and 3D ICE becoming more commercially available there will be less of a need to use TEE for closing large ASDs and paravalvular leaks.
The recent guidelines for 3D TEE for congenital heart disease acknowledge a lack of standardization for training in 3D echocardiography (7). They propose more formalized programs, including training courses in acquisition and post-processing of 3D echocardiographic images, as well as a minimum number of at least 50 3D volumes analyzed as a core competency requirement (7). Nevertheless, efforts are being made to try and codify and standardize proper 3D imaging techniques and create a standard set of 3D views, such as the “en face” views of the atrial septum or surgeon’s view of the mitral valve (7,15).
As structural and adult congenital catheter-based interventions become more complex, the reliance on echocardiography for real-time imaging guidance will only continue to increase. A dedicated echocardiographer who is willing to undergo rigorous training to become adept with these imaging techniques will become an integral team member in the catheterization laboratory. Being able to provide valuable information on the fly to the adult congenital interventional operator will be paramount to the success of these types of procedures.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hascoët S, Warin-Fresse K, Baruteau AE, et al. Cardiac imaging of congenital heart diseases during interventional procedures continues to evolve: Pros and cons of the main techniques. Arch Cardiovasc Dis 2016;109:128-42. [Crossref] [PubMed]
- Meyer JA. Werner Forssmann and catheterization of the heart, 1929. Ann Thorac Surg 1990;49:497-9. [Crossref] [PubMed]
- Rashkind WJ, Miller WW. Creation of an Atrial Septal Defect Without Thoracotomy. JAMA 1966;196:991. [Crossref] [PubMed]
- Burchill LJ, Huang J, Tretter JT, et al. Noninvasive Imaging in Adult Congenital Heart Disease. Circ Res 2017;120:995-1014. [Crossref] [PubMed]
- Pislaru SV, Michelena HI, Mankad SV. Interventional Echocardiography. Prog Cardiovasc Dis 2014;57:32-46. [Crossref] [PubMed]
- Silvestry FE, Kerber RE, Brook MM, et al. Echocardiography-Guided Interventions. J Am Soc Echocardiogr 2009;22:213-31. [Crossref] [PubMed]
- Simpson J, Lopez L, Acar P, et al. Three-dimensional echocardiography in congenital heart disease: an expert consensus document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2016;17:1071-97. [Crossref] [PubMed]
- Rigatelli G. Expanding the use of intracardiac echocardiography in congenital heart disease catheter-based interventions. J Am Soc Echocardiogr 2005;18:1230-1. [Crossref] [PubMed]
- Vitulano N, Pazzano V, Pelargonio G, et al. Technology update: intracardiac echocardiography - a review of the literature. Med Devices (Auckl) 2015;8:231-9. [PubMed]
- Wood FO, Hanzel GS. Intra-cardiac Echocardiography-Guided Interventional Imaging. In: Abbas A. editor. Interventional Cardiology Imaging. Springer, London, 2015:225-39.
- Boccalandro F, Baptista E, Muench A, et al. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am J Cardiol 2004;93:437-40. [Crossref] [PubMed]
- Celi S, Martini N, Pastormerlo LE, et al. Multimodality Imaging for Interventional Cardiology. Curr Pharm Des 2017;23:3285-300. [Crossref] [PubMed]
- Patzelt J, Schreieck J, Camus E, et al. Percutaneous Mitral Valve Edge-to-Edge Repair Using Volume Intracardiac Echocardiography—First in Human Experience. CASE (Phila) 2017;1:41-3. [PubMed]
- Fontes-Carvalho R, Sampaio F, Ribeiro J, et al. Three-dimensional intracardiac echocardiography: A new promising imaging modality to potentially guide cardiovascular interventions. Eur Heart J Cardiovasc Imaging 2013;14:1028. [Crossref] [PubMed]
- Faletra FF, Pedrazzini G, Pasotti E, et al. 3D TEE during catheter-based interventions. JACC Cardiovasc Imaging 2014;7:292-308. [Crossref] [PubMed]
- Kong D, Cheng L, Dong L, et al. Three-Dimensional Echocardiography in the Evaluation of Right Ventricular Global and Regional Systolic Function in Patients with Atrial Septal Defect before and after Percutaneous Closure. Echocardiography 2016;33:596-605. [Crossref] [PubMed]
- Patrianakos AP, Zacharaki AA, Skalidis EI, et al. The growing role of echocardiography in interventional cardiology: The present and the future. Hellenic J Cardiol 2017;58:17-31. [Crossref] [PubMed]
- Baruteau A-E, Petit J, Lambert V, et al. Transcatheter Closure of Large Atrial Septal Defects: Feasibility and Safety in a Large Adult and Pediatric Population. Circ Cardiovasc Interv 2014;7:837-43. [Crossref] [PubMed]
- Bartakian S, El-Said HG, Printz B, et al. Prospective randomized trial of transthoracic echocardiography versus transesophageal echocardiography for assessment and guidance of transcatheter closure of atrial septal defects in children using the Amplatzer septal occluder. JACC Cardiovasc Interv 2013;6:974-80. [Crossref] [PubMed]
- Du ZD, Hijazi ZM, Kleinman CS, et al. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: Results of a multicenter nonrandomized trial. J Am Coll Cardiol 2002;39:1836-44. [Crossref] [PubMed]
- Butera G, Carminati M, Chessa M, et al. Transcatheter Closure of Perimembranous Ventricular Septal Defects. Early and Long-Term Results. J Am Coll Cardiol 2007;50:1189-95. [Crossref] [PubMed]
- Holzer R, De Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the Amplatzer membranous VSD occluder: Immediate and midterm results of an international registry. Catheter Cardiovasc Interv 2006;68:620-8. [Crossref] [PubMed]
- Shabestari MM, Ghaderi F, Hamedanchi A. Transcatheter Closure of Postinfarction Ventricular Septal Defect: A Case Report and Review of Literature. J Cardiovasc Thorac Res 2015;7:75-7. [Crossref] [PubMed]
- Perez-David E, Garcia Fernandez MA, García E, et al. Successful Transcatheter Closure of a Postmyocardial Infarction Ventricular Septal Rupture in a Patient Rejected for Cardiac Surgery: Usefulness of Transesophageal Echocardiography. J Am Soc Echocardiogr 2007;20:1417.e9-12. [Crossref] [PubMed]
- Cao QL, Zabal C, Koenig P, et al. Initial clinical experience with intracardiac echocardiography in guiding transcatheter closure of perimembranous ventricular septal defects: Feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc Interv 2005;66:258-67. [Crossref] [PubMed]
- Ternacle J, Gallet R, Nguyen A, et al. Usefulness of echocardiographic-fluoroscopic fusion imaging in adult structural heart disease. Arch Cardiovasc Dis 2018;111:441-8. [Crossref] [PubMed]
- Klein AJ, Kim MS, Salcedo E, et al. The missing leak: a case report of a baffle-leak closure using real-time 3D transoesophageal guidance. Eur J Echocardiogr 2009;10:464-7. [Crossref] [PubMed]
- Wilhelm CM, Sisk TL, Roble SL, et al. Accuracy of Imaging Modalities in Detection of Baffle Leaks in Patients Following Atrial Switch Operation. Echocardiography 2016;33:437-42. [Crossref] [PubMed]
- Hill KD, Fleming G, Curt Fudge J, et al. Percutaneous interventions in high-risk patients following mustard repair of transposition of the great arteries. Catheter Cardiovasc Interv 2012;80:905-14. [Crossref] [PubMed]
- Kuppahally SS, Litwin SE, Green LS, et al. Utility of intracardiac echocardiography for atrial baffle leak closure in repaired transposition of the great arteries. Echocardiography 2010;27:E90-3. [Crossref] [PubMed]
- Armstrong EJ, Kwa AT, Bhat A, et al. Intracardiac Echocardiography to Guide Percutaneous Closure of Atrial Baffle Defects. J Invasive Cardiol 2012;24:473-6. [PubMed]
- Reinöhl J, Kaier K, Reinecke H, et al. Effect of Availability of Transcatheter Aortic-Valve Replacement on Clinical Practice. N Engl J Med 2015;373:2438-47. [Crossref] [PubMed]
- Wilson WM, Benson LN, Osten MD, et al. Transcatheter Pulmonary Valve Replacement with the Edwards Sapien System: The Toronto Experience. JACC Cardiovasc Interv 2015;8:1819-27. [Crossref] [PubMed]
- Ghobrial J, Aboulhosn J. Transcatheter valve replacement in congenital heart disease : the present and the future. Heart 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. J Am Soc Echocardiogr 2011;24:937-65. [Crossref] [PubMed]
- Kronzon I, Jelnin V, Ruiz CE, et al. Optimal imaging for guiding TAVR: Transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardiovasc Imaging 2015;8:361-70. [Crossref] [PubMed]
- Basman C, Parmar YJ, Kronzon I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr Cardiol Rep 2017;19:102. [Crossref] [PubMed]
- Bartel T, Bonaros N, Müller L, et al. Intracardiac echocardiography: A new guiding tool for transcatheter aortic valve replacement. J Am Soc Echocardiogr 2011;24:966-75. [Crossref] [PubMed]
- Awad SM, Masood SA, Gonzalez I, et al. The use of intracardiac echocardiography during percutaneous pulmonary valve replacement. Pediatr Cardiol 2015;36:76-83. [Crossref] [PubMed]
- Holzer RJ, Hijazi ZM. Transcatheter pulmonary valve replacement: State of the art. Catheter Cardiovasc Interv 2016;87:117-28. [Crossref] [PubMed]
- Whiteside W, Pasquali SK, Yu S, et al. The Utility of Intracardiac Echocardiography Following MelodyTM Transcatheter Pulmonary Valve Implantation. Pediatr Cardiol 2015;36:1754-60. [Crossref] [PubMed]
- McElhinney DB, Cabalka AK, Aboulhosn JA, et al. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Circulation 2016;133:1582-93. [Crossref] [PubMed]
- Mackensen GB, Lee JC, Wang DD, et al. Role of Echocardiography in Transcatheter Mitral Valve Replacement in Native Mitral Valves and Mitral Rings. J Am Soc Echocardiogr 2018;31:475-90. [Crossref] [PubMed]
- Natarajan N, Patel P, Bartel T, et al. Peri-procedural imaging for transcatheter mitral valve replacement. Cardiovasc Diagn Ther 2016;6:144-59. [Crossref] [PubMed]
- Katz WE, Conrad Smith AJ, Croc FW, et al. Echocardiographic evaluation and guidance for MitraClip procedure. Cardiovasc Diagn Ther 2017;7:616-32. [Crossref] [PubMed]
- Wunderlich NC, Siegel RJ. Peri-interventional echo assessment for the MitraClip procedure. Eur Heart J Cardiovasc Imaging 2013;14:935-49. [Crossref] [PubMed]
- Sherif MA, Paranskaya L, Yuecel S, et al. Mitraclip step by step; how to simplify the procedure. Neth Heart J 2017;25:125-30. [Crossref] [PubMed]
- Schofer J, Tiburtius C, Hammerstingl C, et al. Transfemoral tricuspid valve repair using a percutaneous mitral valve repair system. J Am Coll Cardiol 2016;67:889-90. [Crossref] [PubMed]
- Nickenig G, Kowalski M, Hausleiter J, et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge mitraclip technique. Circulation 2017;135:1802-14. [Crossref] [PubMed]
- Hammerstingl C, Schueler R, Malasa M, et al. Transcatheter treatment of severe tricuspid regurgitation with the MitraClip system. Eur Heart J 2016;37:849-53. [Crossref] [PubMed]
- Tsang TSM, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 2002;77:429-36. [Crossref] [PubMed]
- Silva JNA, Southworth M, Raptis C, et al. Emerging Applications of Virtual Reality in Cardiovascular Medicine. JACC Basic Transl Sci 2018;3:420-30. [Crossref] [PubMed]


