
Need for active cardiovascular screening in HIV-infected children under antiretroviral therapy in Africa
Introduction
HIV prevalence in Mozambique is estimated at 1.8% among adolescents (12–14 years), 1.4% in children (0–11 years) and 2.3% in infants (under 1 year) (1). Women are more affected than men, with peak prevalence at ages occurring during reproductive years (2). A prevalence of 29.4% (95% CI: 27.0–31.8%) was found in a study of 1,429 sexually active women; the follow up of 479 uninfected women in the prospective phase found that the incidence of HIV was 4.8 per 100 woman-years (WY) (95% CI: 2.5–8.3) in women aged 18–24 years, 4.5 per 100 WY (95% CI: 1.2–11.4) in women aged 25–29 years and 3.2 per 100 WY (95% CI: 0.1–18.0) in the 30–35 years stratum (3). A high HIV incidence in the postpartum period has been shown to sustain vertical transmission in a cohort study in Southern Mozambique, which found an HIV incidence in postpartum women at 3.20/100 women-years (95% CI: 2.30–4.46), with the highest rate among 18- to 19-year-old (4.92 per 100 women-years; 95% CI: 2.65–9.15) (4). Thus, despite lack of data on the prevalence of HIV in Mozambican children, an unacceptably high rate of vertical transmission still occurs, with several thousand children starting antiretroviral therapy (ART) yearly.
The routine management of children under ART includes periodic assessment of its effects and complications, but screening for cardiovascular disease is not routinely done. We designed a pilot-study aiming at describing the occurrence of cardiovascular abnormalities in HIV-infected children under ART and assess major outcomes at 5 years.
Methods
We conducted a prospective observational study of HIV-infected children registered at an urban HIV center in Maputo City, Mozambique. Using systematic sampling from available electronic database, we randomly selected 10% of the registered patients. Recruitment took place between April/2012 and October/2012, since every child as a routine follow up appointment every six months. A structured questionnaire was used to collect socio-demographic characteristics, cardiovascular symptoms and signs, laboratory profile as well data on ART. The cardiovascular evaluation included transthoracic cardiac ultrasound. Venous blood was obtained to assess the hemoglobin level, CD4 count and HIV viral load. The clinical status was defined as per the World Health Organization criteria (5), anemia was defined according to age-appropriate reference standards (6), immunological and virological statuses were measured by CD4 and viral load, respectively. Severe left ventricular dysfunction was defined as shortening fraction below 30% (7). First line ART regimen comprised of two nucleoside reverse transcriptase inhibitors (zidovudine and lamivudine) and non-nucleoside reverse transcriptase inhibitors (nevirapine); stavudine replaced Zidovudine when hemoglobin was less than 8 gr/dL. For second line therapy Abacavir/lamivudine are used in fixed combined dosage. Patients found with significant cardiac abnormalities were offered treatment.
Five years after, we assessed patient’s vital status, occurrence of major clinical events, and performed transthoracic cardiac ultrasound in those who presented for evaluation. For patients who did not present for consultation we assessed the files at the health facility and contacted the family to obtain information on their vital status and history of hospital admissions.
Data were analyzed using the Statistical Package for Social Sciences (SPPS) version 20. The National Bioethics Review Board in Mozambique approved the study. Parents or guardians gave written informed consent and verbal assent was obtained for children above the age of seven.
Results
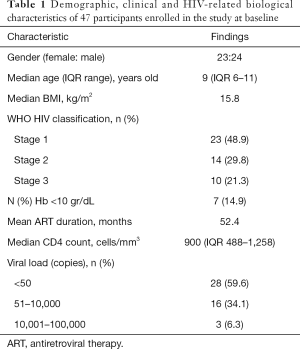
We studied 47 children patients [23 girls; median age 9 years (IQR 6–11); all black]. The body mass index (BMI) average was 16.2 (range, 10.1–22.8) kg/m2; 36 patients (80%) had BMI below 18.5 kg/m2. The mean time of treatment was 52.4 months. According to the WHO HIV clinical classification 37 (78.7%) patients were in stage 1–2; none were in stage IV. The median CD4 count was 900 cells/mm3 (IQR 488–1,258). Low HIV viral load (less than 50 copies) was found in only 28 (59.6%) patients. Five children (10.6%) were on second line treatment. The median hemoglobin level was 11.6 g/dL (IQR 10.6, 12.5) and 7 (14.9%) of the children had anemia. The characteristics of patients are summarized in Table 1.
Full table
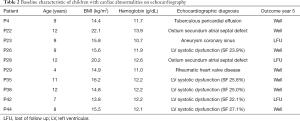
Five children had clinical features of heart failure. Cardiac ultrasound revealed cardiac abnormalities in 10 children: congenital heart defects (two atrial septal defect and one aneurysm of the coronary sinus); impaired systolic function (present in five children of which two had overt heart failure); severe aortic regurgitation of rheumatic origin (1 of 47) and tuberculous pericarditis (1 of 47) (Table 2). After medical therapy patients with heart failure, impaired systolic function, tuberculous pericarditis and anemia were treated, and all improved. The child with severe rheumatic disease underwent surgery with success. All children benefited from nutritional support and/or rehabilitation as part of the program at the institution.
Full table
The evaluation after five years revealed that we had six patients lost to follow-up, of which three with cardiac abnormalities. One patient died from malaria in year 2. No major cardiac events or hospital admissions occurred in the remaining 40.
New asymptomatic left ventricular systolic dysfunction with moderate mitral regurgitation appeared in three children (aged 9, 7 and 6) who had normal cardiac ultrasound at recruitment; one with mild, one with moderate and one with severe anemia.
Discussion and conclusions
Severe left ventricular dysfunction, malnutrition and anemia were uncovered in these HIV-infected children on ART, supporting the inclusion of systematic risk profiling and cardiovascular screening in routine care in Mozambique and underserved settings with high occurrence of cardiovascular disease. This is particularly important since adequate management of these abnormalities was associated with good outcomes, and survival free of major events. Only one child died due to a non-cardiac event.
The cardiac abnormalities found, although rare in pediatric HIV population with access to modern ART, correspond to the profile of cardiac disease in our setting (8,9). Similarly, cardiac abnormalities detected by cardiac ultrasound were found in 20 out of 285 HIV-infected Ugandan children on ART: left ventricular dysfunction in 6 (2.1%), dilated cardiomyopathy 1 child, congenital heart disease in 3 children (1%)—patent ductus arteriosus, secundum atrial septal defect and isolated cleft mitral valve—and acquired heart disease in 10 (pericardial disease in 8 and rheumatic heart disease in 2) (10).
We found patients with undiagnosed heart failure and congenital heart disease, reflecting both the occurrence of asymptomatic cardiac disease and issues of access to timely diagnosis. This further confirms the importance of using ultrasound for cardiovascular screening of HIV-infected children. A study of Indian children has shown that heart involvement in HIV/AIDS is mostly subclinical (11) and highlighted the role of cardiac ultrasound for identifying heart involvement in this age group. Since HIV infection is usually associated with heart failure, and because early diagnosis and intervention may halt the progression of cardiac disease, thereby preventing morbidity and mortality, these results call active cardiovascular screening in HIV-infected children from low-income countries with high occurrence of cardiac conditions.
A significant proportion of children had low BMI and anemia. Current knowledge suggests the need for aggressive approaches to correcting these conditions, since they increase the risk of left ventricular systolic dysfunction (12). We had three new cases of left systolic dysfunction on 5-year follow up, two of which had in children that had very low hemoglobin levels at recruitment. Although these children had recovered their anemia, it is well known that low hemoglobin levels partially mediate chamber dilation in ART-exposed children (13,14), and thus efforts should be made to correct it vigorously. On the other hand, despite controversy regarding the effect of ART on the levels of hemoglobin, a recent study of HIV-infected children with median age of 10 years in Ethiopia showed reduction on prevalence of anemia after ART initiation (15). Similarly, successful treatment can be achieved for HIV-infected children in Mozambique and in similar resource-limited settings (16).
Although overall encouraging and in line with the good results of ART in preventing cardiac events in adult population, our findings should be interpreted with caution due to the small size of our cohort and the loss to follow up of three patients with cardiac abnormalities.
The pathogenesis and long-term outcomes of ART in children are not well understood, but even in the context of viral suppression there seems to be an increased cardiovascular risk due to metabolic syndrome compared to that of uninfected children (13,17,18). One limitation of our study was the fact that we were not able to have the lipid profile done. We however acknowledge that in African children starting ART so early in life monitoring of metabolic markers and atherosclerotic risk, must be added to the usual screening of geographically relevant cardiovascular conditions.
Acknowledgements
We are grateful to the staff at the Non-Communicable Disease Division of Instituto Nacional de Saúde, Hospital da Polana Caniço, Community of S.Egidio-DREAM program Avenida Eduardo Mondlane, Maputo City, and to all the participants who gave their time, support and enthusiasm in making this study a success.
Funding: This research was funded in part by a 2014 grant from the University of California, San Diego, Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mozambique DHS 2015 (2018). IMASIDA - Malaria, HIV/AIDS, and Immunization Indicator Survey in Mozambique (2018). IMASIDA - Inquérito de Indicadores de Imunização, Malária e HIV/SIDA. Available online: https://www.researchgate.net/publication/323747814_Mozambique_DHS_2015_2018_IMASIDA
- Governo de Moçambique. Available online: http://www.portaldogoverno.gov.mz
- Feldblum PJ, Enosse S, Dubé K, et al. HIV Prevalence and Incidence in a Cohort of Women at Higher Risk for HIV Acquisition in Chókwè, Southern Mozambique. PLoS One 2014;9:e97547. [Crossref] [PubMed]
- De Schacht C, Mabunda N, Ferreira OC, et al. High HIV incidence in the postpartum period sustains vertical transmission in settings with generalized epidemics: a cohort study in Southern Mozambique. J Int AIDS Soc 2014;17:18808. [Crossref] [PubMed]
- World Health Organization. Haemoglobin levels to diagnose anaemia at sea level. In haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva, 2001.
- Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072-83. [Crossref] [PubMed]
- Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med 2013;173:614-22. [Crossref] [PubMed]
- Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med 2007;357:470-6. [Crossref] [PubMed]
- Mocumbi AO, Lameira E, Yaksh A, et al. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol 2011;148:285-8. [Crossref] [PubMed]
- Namuyonga J, Lubega S, Musiime V, et al. Cardiac Dysfunction Among Ugandan HIV-infected Children on Antiretroviral Therapy. Pediatr Infect Dis J 2016;35:e85-8. [Crossref] [PubMed]
- Singh P, Hemal A, Agarwal S, et al. Cardiac manifestations in HIV infected children. Indian J Pediatr 2015;82:230-4. [Crossref] [PubMed]
- Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc 2013;16:18597. [Crossref] [PubMed]
- Idris NS, Cheung MMH, Grobbee DE, et al. Cardiac Effects of Antiretroviral-Naïve versus Antiretroviral-Exposed HIV Infection in Children. PLoS One 2016;11:e0146753. [Crossref] [PubMed]
- Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-relate anemia in 2013. Hematology Am Soc Hematol Educ Program 2013;2013:377-81. [Crossref] [PubMed]
- Geletaw T, Tadesse MZ, Demisse AG. Hematologic abnormalities and associated factors among HIV infected children pre- and post-antiretroviral treatment, North West Ethiopia. J Blood Med 2017;8:99-105. [Crossref] [PubMed]
- Walter J, Molfino L, Moreno V, et al. Long-term outcomes of a pediatric HIV treatment program in Maputo, Mozambique: a cohort study. Glob Health Action 2015;8:26652. [Crossref] [PubMed]
- Eckard AR, Fowler SL, Haston JC, et al. Complications of Treatment in Youth with HIV. Curr HIV/AIDS Rep 2016;13:226-33. [Crossref] [PubMed]
- Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: the prospective P2C 2 HIV Multicenter Study. Am Heart J 2005;150:439-47. [Crossref] [PubMed]


